Abstract
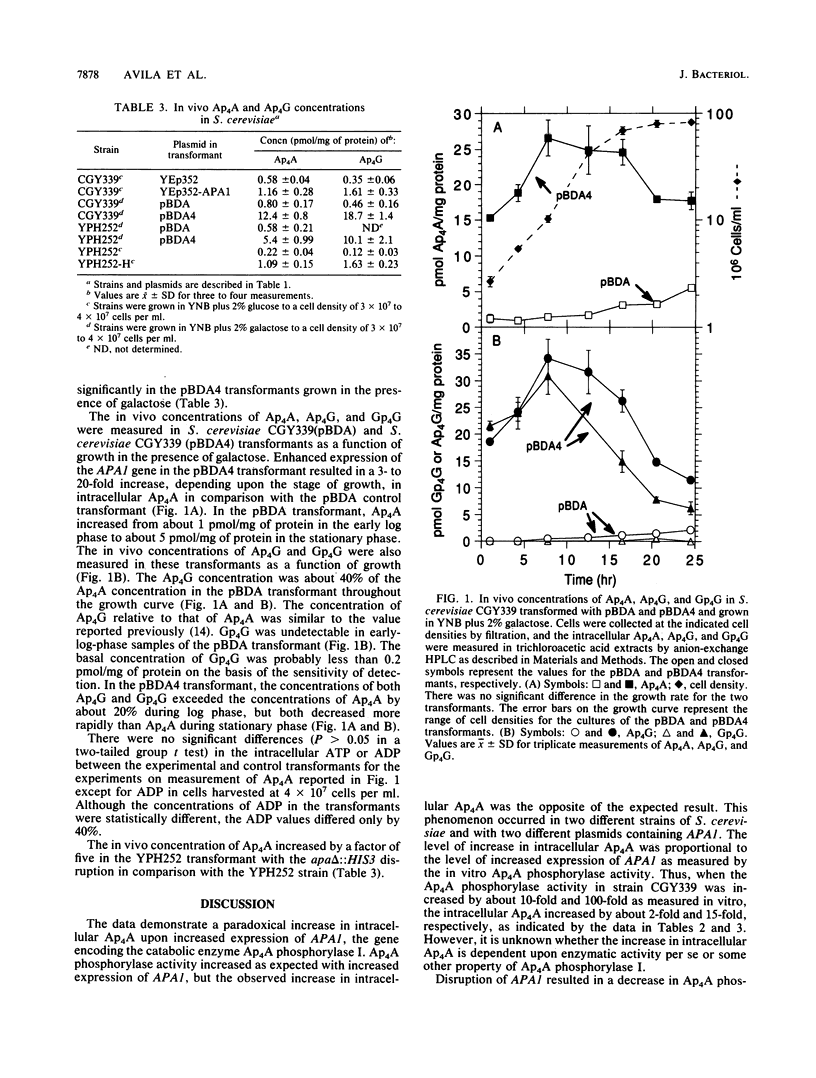
The APA1 gene in Saccharomyces cerevisiae encodes Ap4A phosphorylase I, the catabolic enzyme for diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A). APA1 has been inserted into a multicopy plasmid and into a centromeric plasmid with a GAL1 promoter. Enhanced expression of APA1 via the plasmids resulted in 10- and 90-fold increases in Ap4A phosphorylase activity, respectively, as assayed in vitro. However, the intracellular concentration of Ap4A exhibited increases of 2- and 15-fold, respectively, from the two different plasmids. Intracellular Ap4A increased 3- to 20-fold during growth on galactose of a transformant with APA1 under the control of the GAL1 promoter. Intracellular adenosine 5'-P1-tetraphospho-P4-5"'-guanosine (Ap4G) and diguanosine 5',5"'-P1,P4-tetraphosphate (Gp4G) also increased in the transformant under these conditions. The chromosomal locus of APA1 has been disrupted in a haploid strain. The Ap4A phosphorylase activity decreased by 80% and the intracellular Ap4A concentration increased by a factor of five in the null mutant. These results with the null mutant agree with previous results reported by Plateau et al. (P. Plateau, M. Fromant, J.-M. Schmitter, J.-M. Buhler, and S. Blancquet, J. Bacteriol. 171:6437-6445, 1989). The paradoxical increase in Ap4A upon enhanced expression of APA1 indicates that the metabolic consequences of altered gene expression may be more complex than indicated solely by assay of enzymatic activity of the gene product.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila D. M., Kaushal V., Barnes L. D. Immunoaffinity chromatography of diadenosine 5',5'''-P1,P4-tetraphosphate phosphorylase from Saccharomyces cerevisiae. Biotechnol Appl Biochem. 1990 Jun;12(3):276–283. [PubMed] [Google Scholar]
- Barnes L. D., Robinson A. K., Mumford C. H., Garrison P. N. Assay of diadenosine tetraphosphate hydrolytic enzymes by boronate chromatography. Anal Biochem. 1985 Jan;144(1):296–304. doi: 10.1016/0003-2697(85)90120-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet S., Plateau P., Brevet A. The role of zinc in 5',5'-diadenosine tetraphosphate production by aminoacyl-transfer RNA synthetases. Mol Cell Biochem. 1983;52(1):3–11. doi: 10.1007/BF00230583. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Brevet A., Chen J., Lévêque F., Plateau P., Blanquet S. In vivo synthesis of adenylylated bis(5'-nucleosidyl) tetraphosphates (Ap4N) by Escherichia coli aminoacyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8275–8279. doi: 10.1073/pnas.86.21.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet A., Coste H., Fromant M., Plateau P., Blanquet S. Yeast diadenosine 5',5'''-P1,P4-tetraphosphate alpha,beta-phosphorylase behaves as a dinucleoside tetraphosphate synthetase. Biochemistry. 1987 Jul 28;26(15):4763–4768. doi: 10.1021/bi00389a025. [DOI] [PubMed] [Google Scholar]
- Coste H., Brevet A., Plateau P., Blanquet S. Non-adenylylated bis(5'-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J Biol Chem. 1987 Sep 5;262(25):12096–12103. [PubMed] [Google Scholar]
- Farr S. B., Arnosti D. N., Chamberlin M. J., Ames B. N. An apaH mutation causes AppppA to accumulate and affects motility and catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5010–5014. doi: 10.1073/pnas.86.13.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freist W., Sternbach H., Cramer F. Survey on substrate specificity with regard to ATP analogs of aminoacyl-tRNA synthetases from E. coli and from Baker's yeast. Correlation to synthetase families. Hoppe Seylers Z Physiol Chem. 1981 Sep;362(9):1247–1254. doi: 10.1515/bchm2.1981.362.2.1247. [DOI] [PubMed] [Google Scholar]
- Garrison P. N., Barnes L. D. Assay of adenosine 5'-P1-tetraphospho-P4-5"'-adenosine and adenosine 5'-P1-tetraphospho-P4-5"'-guanosine in Physarum polycephalum and other eukaryotes. An isocratic high-pressure liquid-chromatography method. Biochem J. 1984 Feb 1;217(3):805–811. doi: 10.1042/bj2170805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich O., Foeckler R., Holler E. Mechanism of synthesis of adenosine(5')tetraphospho(5')adenosine (AppppA) by aminoacyl-tRNA synthetases. Eur J Biochem. 1982 Aug;126(1):135–142. doi: 10.1111/j.1432-1033.1982.tb06757.x. [DOI] [PubMed] [Google Scholar]
- Guranowski A., Blanquet S. Phosphorolytic cleavage of diadenosine 5',5'''-P1,P4-tetraphosphate. Properties of homogeneous diadenosine 5',5'''-P1,P4-tetraphosphate alpha, beta-phosphorylase from Saccharomyces cerevisiae. J Biol Chem. 1985 Mar 25;260(6):3542–3547. [PubMed] [Google Scholar]
- Guranowski A., Just G., Holler E., Jakubowski H. Synthesis of diadenosine 5',5'''-P1,P4-tetraphosphate (AppppA) from adenosine 5'-phosphosulfate and adenosine 5'-triphosphate catalyzed by yeast AppppA phosphorylase. Biochemistry. 1988 Apr 19;27(8):2959–2964. doi: 10.1021/bi00408a044. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal V., Avila D. M., Hardies S. C., Barnes L. D. Sequencing and enhanced expression of the gene encoding diadenosine 5',5'''-P1, P4-tetraphosphate (Ap4A) phosphorylase in Saccharomyces cerevisiae. Gene. 1990 Oct 30;95(1):79–84. doi: 10.1016/0378-1119(90)90416-o. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftfield R. B. The mechanism of aminoacylation of transfer RNA. Prog Nucleic Acid Res Mol Biol. 1972;12:87–128. doi: 10.1016/s0079-6603(08)60660-1. [DOI] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Blanquet S. Heat shock and hydrogen peroxide responses of Escherichia coli are not changed by dinucleoside tetraphosphate hydrolase overproduction. J Bacteriol. 1987 Aug;169(8):3817–3820. doi: 10.1128/jb.169.8.3817-3820.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Schmitter J. M., Blanquet S. Catabolism of bis(5'-nucleosidyl) tetraphosphates in Saccharomyces cerevisiae. J Bacteriol. 1990 Dec;172(12):6892–6899. doi: 10.1128/jb.172.12.6892-6899.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Schmitter J. M., Buhler J. M., Blanquet S. Isolation, characterization, and inactivation of the APA1 gene encoding yeast diadenosine 5',5'''-P1,P4-tetraphosphate phosphorylase. J Bacteriol. 1989 Dec;171(12):6437–6445. doi: 10.1128/jb.171.12.6437-6445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Presence of diadenosine 5',5''' -P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive "pleiotypic activator". Proc Natl Acad Sci U S A. 1976 Nov;73(11):3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. A physical, genetic and transcriptional map of the cloned his3 gene region of Saccharomyces cerevisiae. J Mol Biol. 1980 Jan 25;136(3):309–332. doi: 10.1016/0022-2836(80)90376-9. [DOI] [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Janeway C. M., Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966 Jul 6;24(1):91–97. doi: 10.1016/0006-291x(66)90415-3. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. Diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem. 1983 Oct 1;134(1):1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Brown T. R., Shulman R. G. Phosphorus-31 nuclear magnetic resonance studies of the effect of oxygen upon glycolysis in yeast. Biochemistry. 1981 Sep 29;20(20):5871–5880. doi: 10.1021/bi00523a034. [DOI] [PubMed] [Google Scholar]



