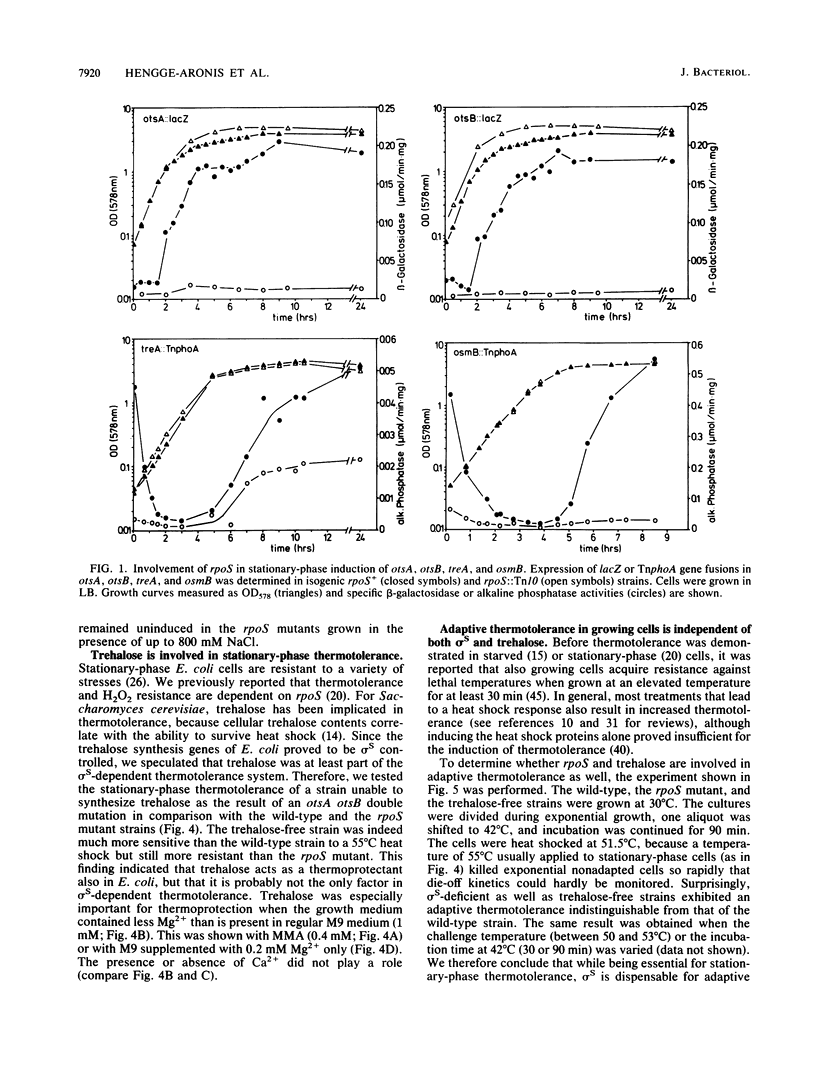
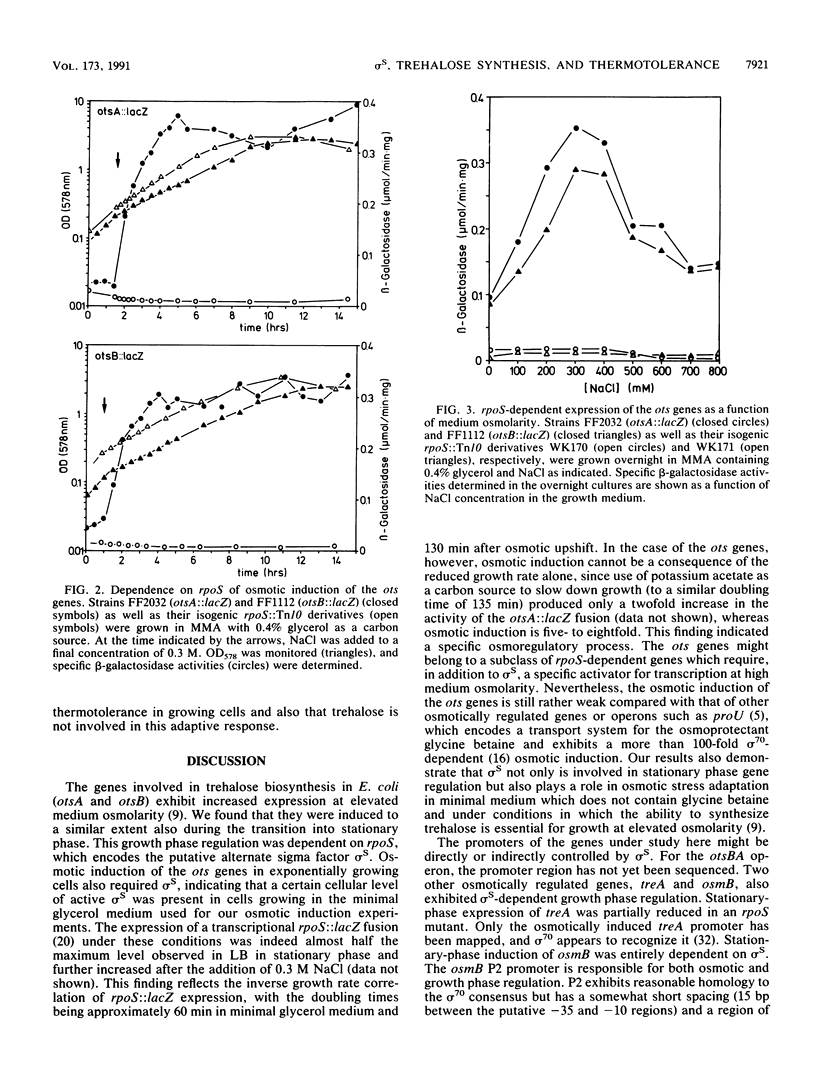
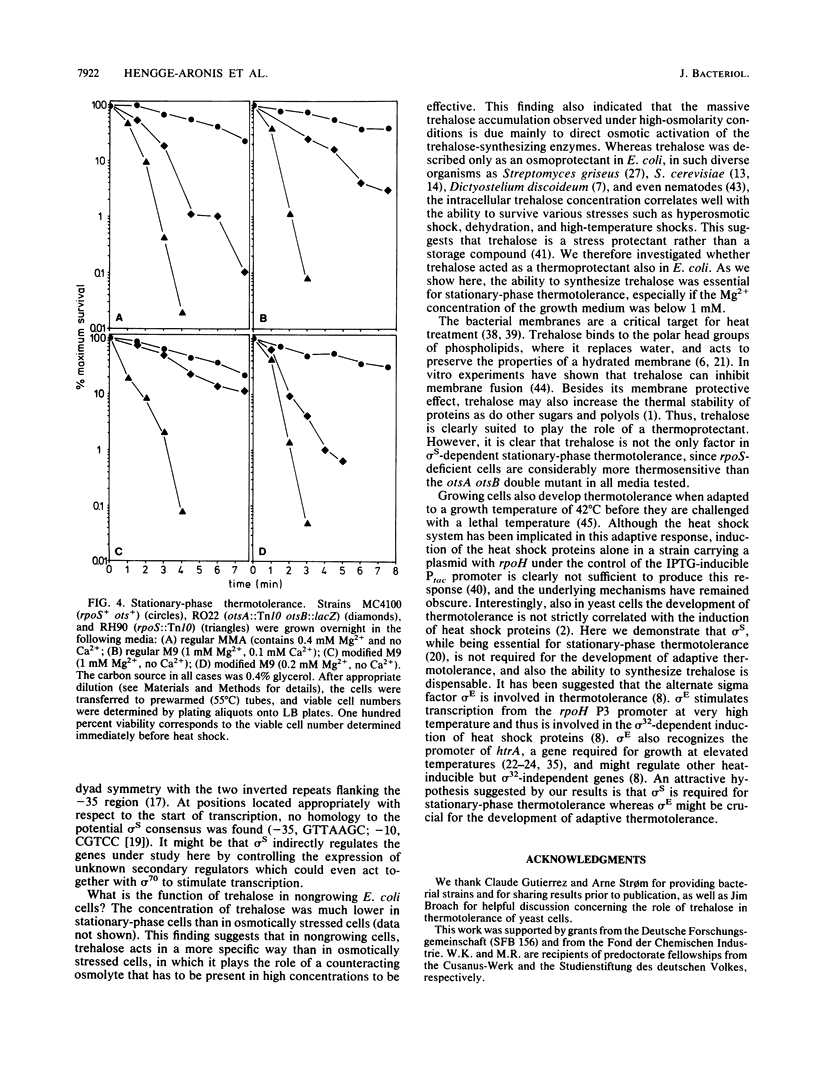
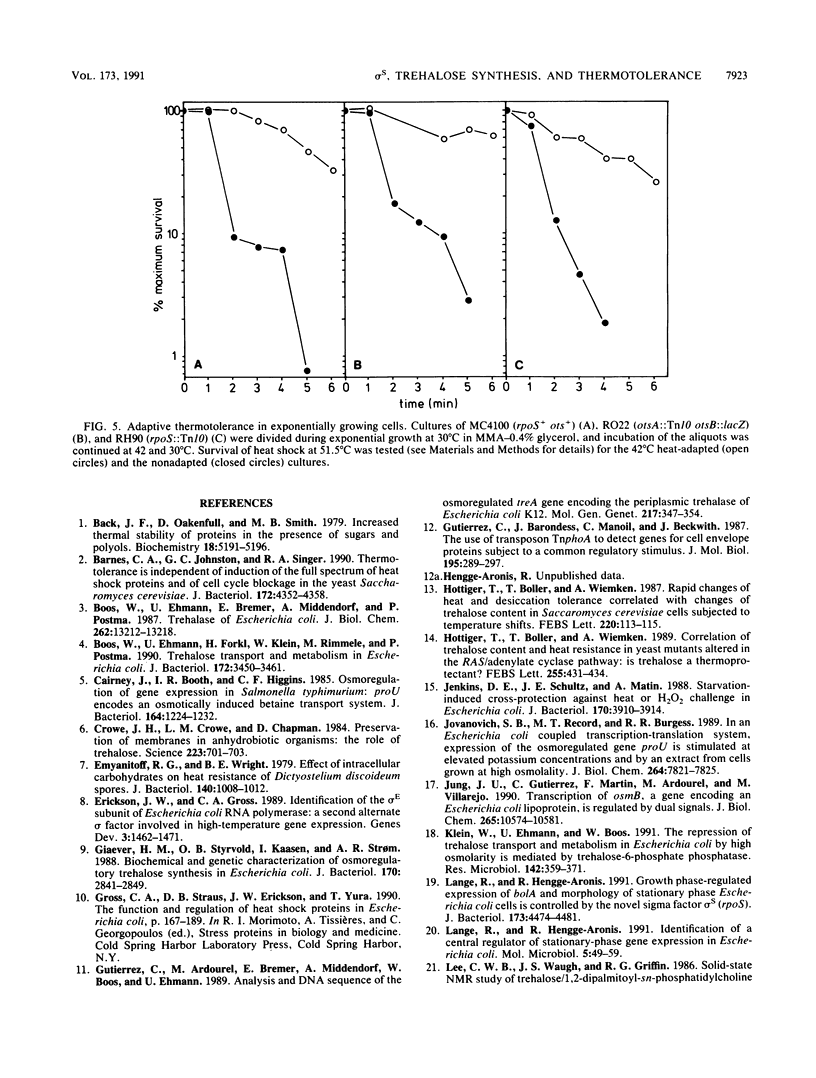
Abstract
The rpoS (katF) gene of Escherichia coli encodes a putative sigma factor (sigma S) required for the expression of a variety of stationary phase-induced genes, for the development of stationary-phase stress resistance, and for long-term starvation survival (R. Lange and R. Hengge-Aronis, Mol. Microbiol. 5:49-59, 1991). Here we show that the genes otsA, otsB, treA, and osmB, previously known to be osmotically regulated, are also induced during transition into stationary phase in a sigma S-dependent manner. otsA and otsB, which encode trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase, respectively, are involved in sigma S-dependent stationary-phase thermotolerance. Neither sigma S nor trehalose, however, is required for the development of adaptive thermotolerance in growing cells, which might be controlled by sigma E.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back J. F., Oakenfull D., Smith M. B. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979 Nov 13;18(23):5191–5196. doi: 10.1021/bi00590a025. [DOI] [PubMed] [Google Scholar]
- Barnes C. A., Johnston G. C., Singer R. A. Thermotolerance is independent of induction of the full spectrum of heat shock proteins and of cell cycle blockage in the yeast Saccharomyces cerevisiae. J Bacteriol. 1990 Aug;172(8):4352–4358. doi: 10.1128/jb.172.8.4352-4358.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Ehmann U., Bremer E., Middendorf A., Postma P. Trehalase of Escherichia coli. Mapping and cloning of its structural gene and identification of the enzyme as a periplasmic protein induced under high osmolarity growth conditions. J Biol Chem. 1987 Sep 25;262(27):13212–13218. [PubMed] [Google Scholar]
- Boos W., Ehmann U., Forkl H., Klein W., Rimmele M., Postma P. Trehalose transport and metabolism in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3450–3461. doi: 10.1128/jb.172.6.3450-3461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985 Dec;164(3):1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Emyanitoff R. G., Wright B. E. Effect of intracellular carbohydrates on heat resistance of Dictyostelium discoideum spores. J Bacteriol. 1979 Dec;140(3):1008–1012. doi: 10.1128/jb.140.3.1008-1012.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Gross C. A. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989 Sep;3(9):1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Giaever H. M., Styrvold O. B., Kaasen I., Strøm A. R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988 Jun;170(6):2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C., Ardourel M., Bremer E., Middendorf A., Boos W., Ehmann U. Analysis and DNA sequence of the osmoregulated treA gene encoding the periplasmic trehalase of Escherichia coli K12. Mol Gen Genet. 1989 Jun;217(2-3):347–354. doi: 10.1007/BF02464903. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Barondess J., Manoil C., Beckwith J. The use of transposon TnphoA to detect genes for cell envelope proteins subject to a common regulatory stimulus. Analysis of osmotically regulated genes in Escherichia coli. J Mol Biol. 1987 May 20;195(2):289–297. doi: 10.1016/0022-2836(87)90650-4. [DOI] [PubMed] [Google Scholar]
- Hottiger T., Boller T., Wiemken A. Correlation of trehalose content and heat resistance in yeast mutants altered in the RAS/adenylate cyclase pathway: is trehalose a thermoprotectant? FEBS Lett. 1989 Sep 25;255(2):431–434. doi: 10.1016/0014-5793(89)81139-1. [DOI] [PubMed] [Google Scholar]
- Hottiger T., Boller T., Wiemken A. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 1987 Aug 10;220(1):113–115. doi: 10.1016/0014-5793(87)80886-4. [DOI] [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovich S. B., Record M. T., Jr, Burgess R. R. In an Escherichia coli coupled transcription-translation system, expression of the osmoregulated gene proU is stimulated at elevated potassium concentrations and by an extract from cells grown at high osmolality. J Biol Chem. 1989 May 15;264(14):7821–7825. [PubMed] [Google Scholar]
- Jung J. U., Gutierrez C., Martin F., Ardourel M., Villarejo M. Transcription of osmB, a gene encoding an Escherichia coli lipoprotein, is regulated by dual signals. Osmotic stress and stationary phase. J Biol Chem. 1990 Jun 25;265(18):10574–10581. [PubMed] [Google Scholar]
- Klein W., Ehmann U., Boos W. The repression of trehalose transport and metabolism in Escherichia coli by high osmolarity is mediated by trehalose-6-phosphate phosphatase. Res Microbiol. 1991 May;142(4):359–371. doi: 10.1016/0923-2508(91)90105-j. [DOI] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J Bacteriol. 1991 Jul;173(14):4474–4481. doi: 10.1128/jb.173.14.4474-4481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Waugh J. S., Griffin R. G. Solid-state NMR study of trehalose/1,2-dipalmitoyl-sn-phosphatidylcholine interactions. Biochemistry. 1986 Jul 1;25(13):3737–3742. doi: 10.1021/bi00361a001. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Sharma S., Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988 Nov 11;16(21):10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Zylicz M., Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990 Apr;172(4):1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984 Nov;160(2):668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- McBride M. J., Ensign J. C. Effects of intracellular trehalose content on Streptomyces griseus spores. J Bacteriol. 1987 Nov;169(11):4995–5001. doi: 10.1128/jb.169.11.4995-5001.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Loewen P. C. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. doi: 10.1093/nar/17.23.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Switala J., Borys A., Loewen P. C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repoila F., Gutierrez C. Osmotic induction of the periplasmic trehalase in Escherichia coli K12: characterization of the treA gene promoter. Mol Microbiol. 1991 Mar;5(3):747–755. doi: 10.1111/j.1365-2958.1991.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Sak B. D., Eisenstark A., Touati D. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc Natl Acad Sci U S A. 1989 May;86(9):3271–3275. doi: 10.1073/pnas.86.9.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrvold O. B., Strøm A. R. Synthesis, accumulation, and excretion of trehalose in osmotically stressed Escherichia coli K-12 strains: influence of amber suppressors and function of the periplasmic trehalase. J Bacteriol. 1991 Feb;173(3):1187–1192. doi: 10.1128/jb.173.3.1187-1192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati E., Dassa E., Dassa J., Boquet P. L., Touati D. Are appR and katF the same Escherichia coli gene encoding a new sigma transcription initiation factor? Res Microbiol. 1991 Jan;142(1):29–36. doi: 10.1016/0923-2508(91)90094-q. [DOI] [PubMed] [Google Scholar]
- Tsuchido T., Aoki I., Takano M. Interaction of the fluorescent dye 1-N-phenylnaphthylamine with Escherichia coli cells during heat stress and recovery from heat stress. J Gen Microbiol. 1989 Jul;135(7):1941–1947. doi: 10.1099/00221287-135-7-1941. [DOI] [PubMed] [Google Scholar]
- Tsuchido T., Katsui N., Takeuchi A., Takano M., Shibasaki I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl Environ Microbiol. 1985 Aug;50(2):298–303. doi: 10.1128/aem.50.2.298-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Acton M. A., Neidhardt F. C. Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1987 Aug;1(6):525–531. doi: 10.1101/gad.1.6.525. [DOI] [PubMed] [Google Scholar]
- Womersley C., Uster P. S., Rudolph A. S., Crowe J. H. Inhibition of dehydration-induced fusion between liposomal membranes by carbohydrates as measured by fluorescence energy transfer. Cryobiology. 1986 Jun;23(3):245–255. doi: 10.1016/0011-2240(86)90050-7. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ossowski I., Mulvey M. R., Leco P. A., Borys A., Loewen P. C. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol. 1991 Jan;173(2):514–520. doi: 10.1128/jb.173.2.514-520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



