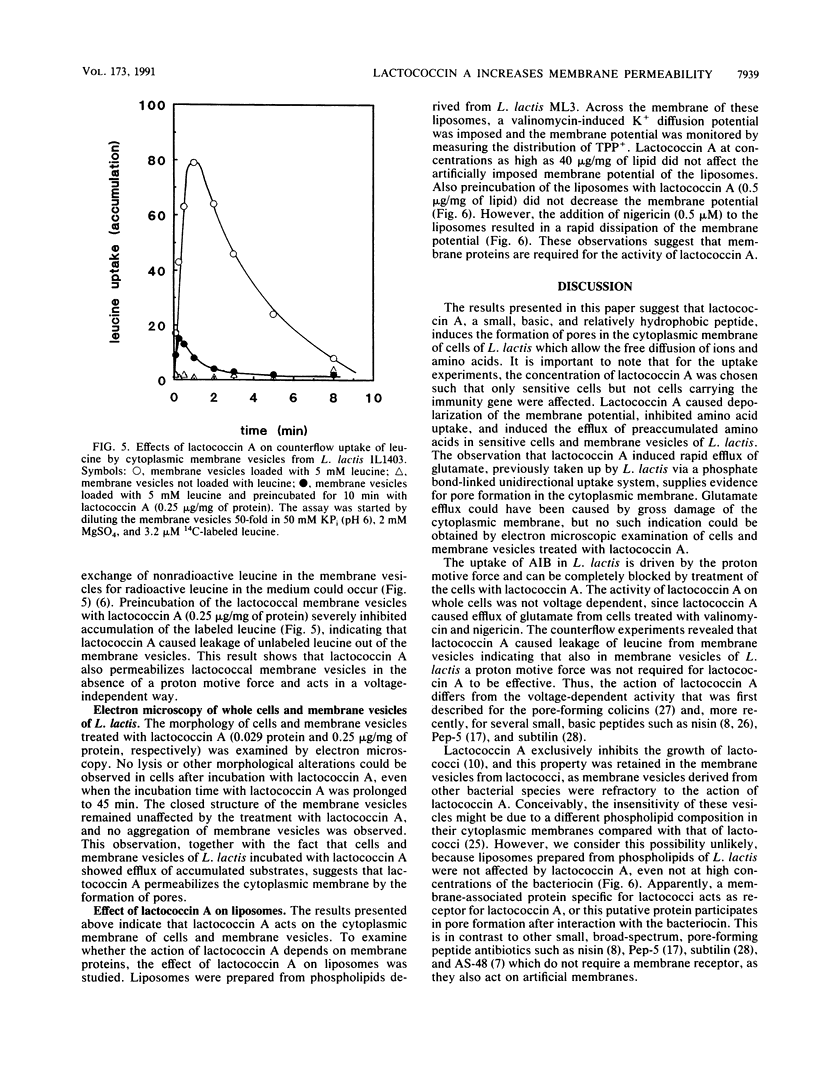
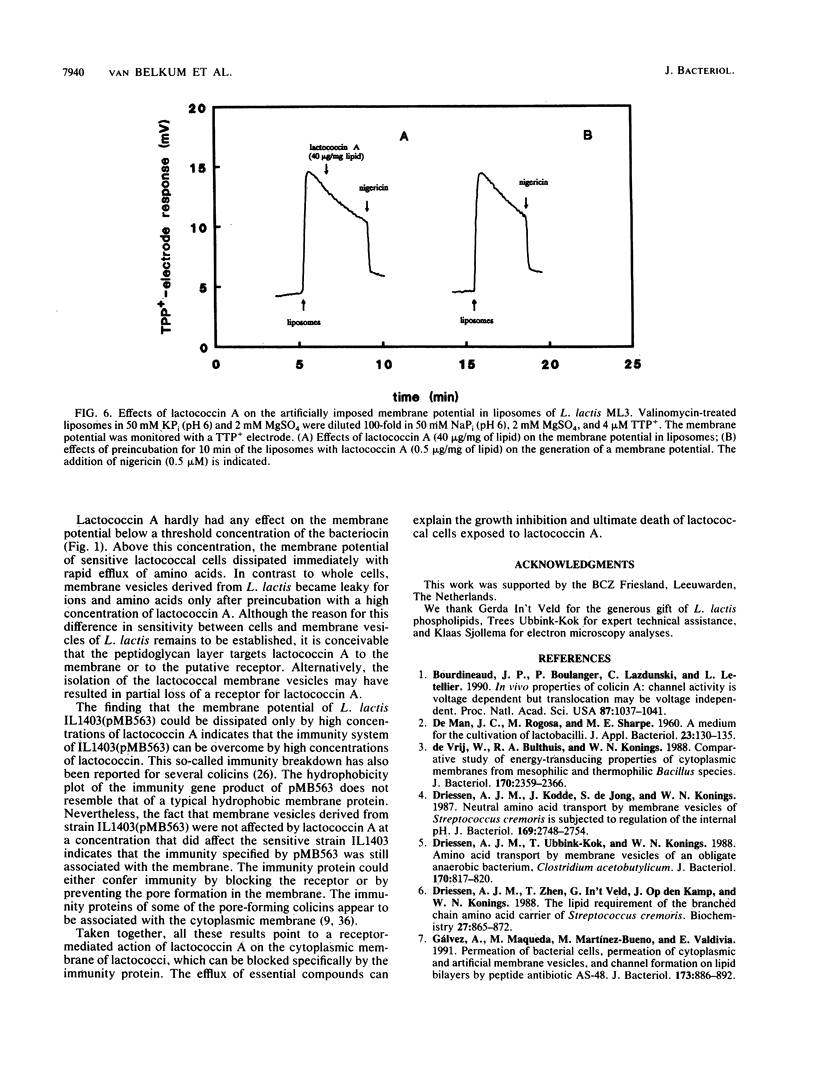
Abstract
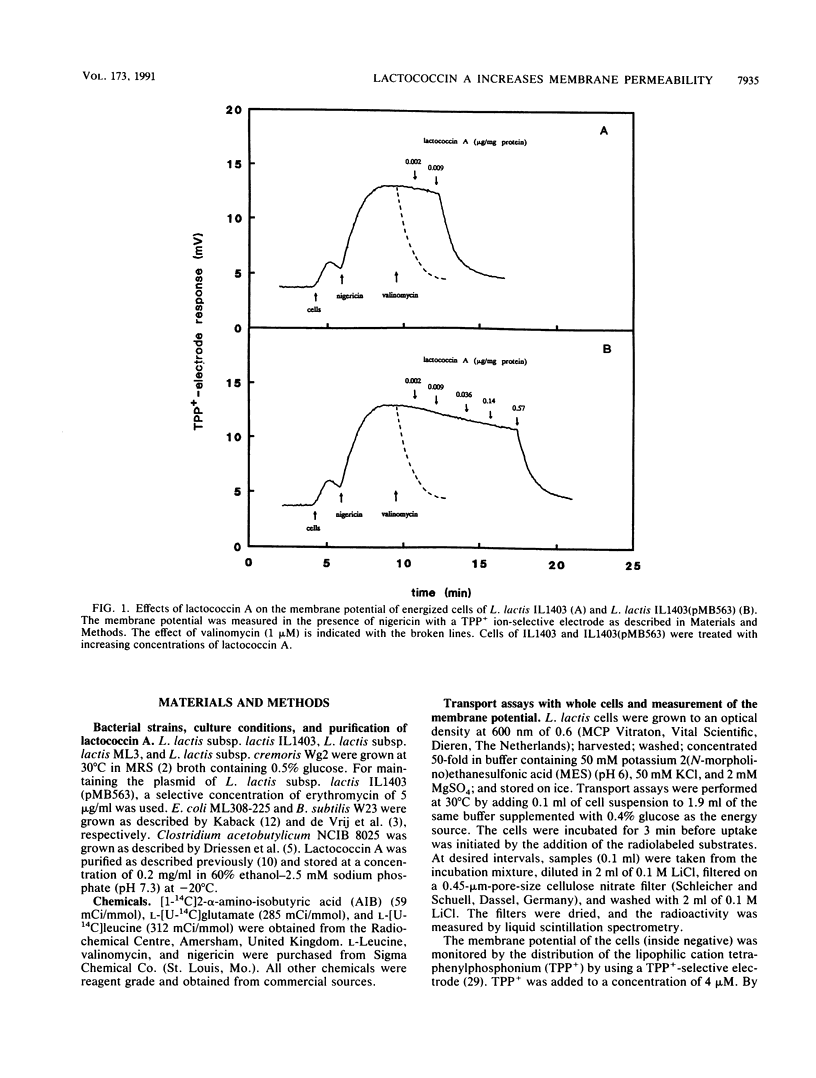
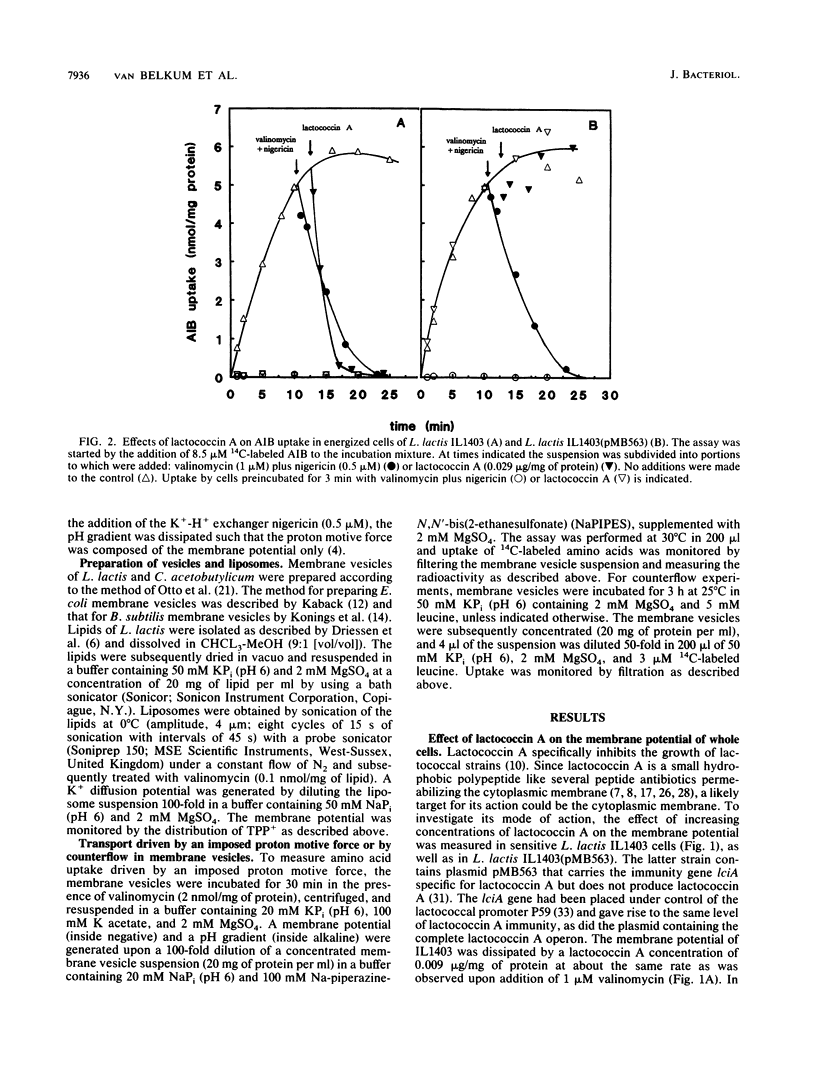
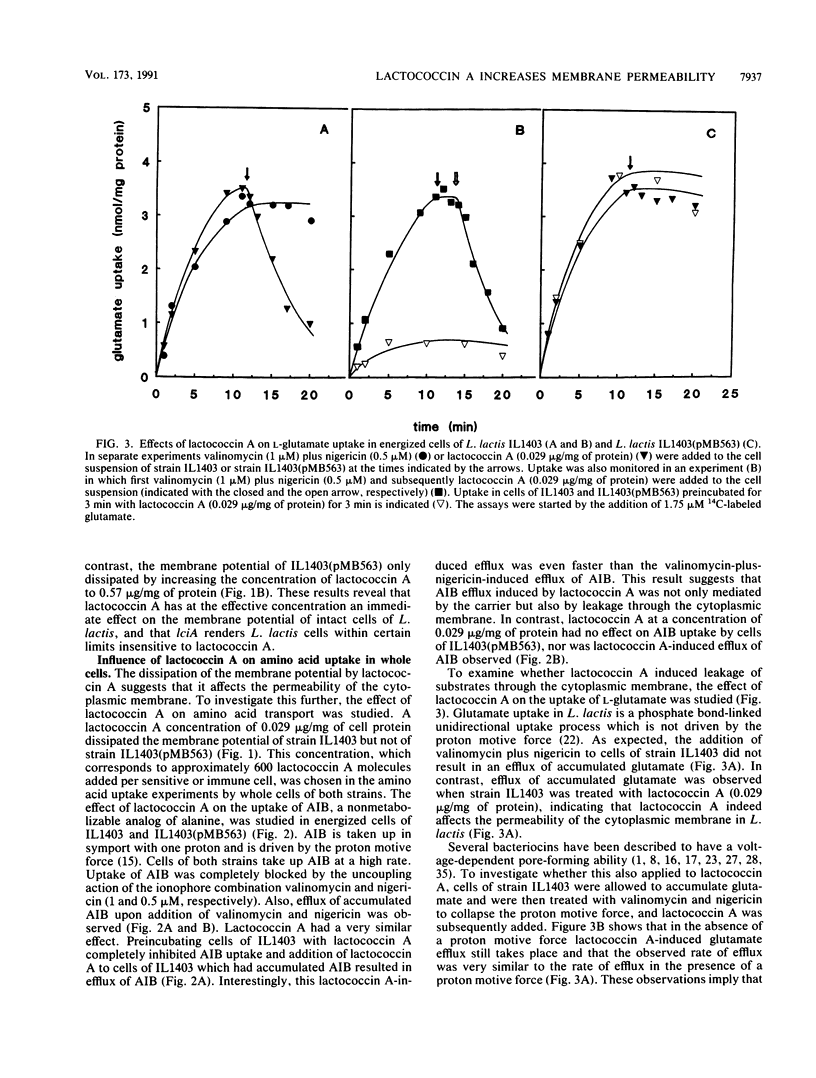
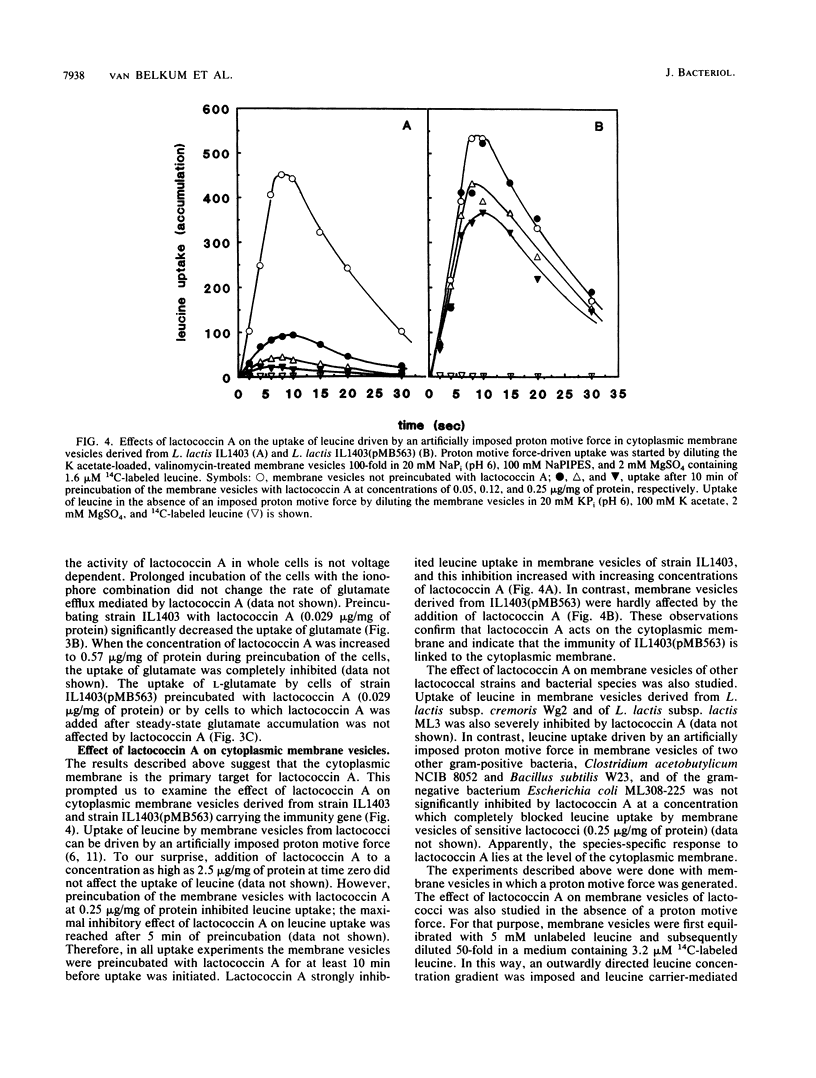
Lactococcin A is a bacteriocin produced by Lactococcus lactis. Its structural gene has recently been cloned and sequenced (M. J. van Belkum, B. J. Hayema, R. E. Jeeninga, J. Kok, and G. Venema, Appl. Environ. Microbiol. 57:492-498, 1991). Purified lactococcin A increased the permeability of the cytoplasmic membrane of L. lactis and dissipated the membrane potential. A significantly higher concentration of lactococcin A was needed to dissipate the membrane potential in an immune strain of L. lactis. Lactococcin A at low concentrations (0.029 microgram/mg of protein) inhibited secondary and phosphate-bond driven transport of amino acids in sensitive cells and caused efflux of preaccumulated amino acids. Accumulation of amino acids by immune cells was not affected by this concentration of lactococcin A. Lactococcin A also inhibited proton motive force-driven leucine uptake and leucine counterflow in membrane vesicles of the sensitive strain but not in membrane vesicles of the immune strain. These observations indicate that lactococcin A makes the membrane permeable for leucine in the presence or absence of a proton motive force and that the immunity factor(s) is membrane linked. Membrane vesicles of Clostridium acetobutylicum, Bacillus subtilis, and Escherichia coli were not affected by lactococcin A, nor were liposomes derived from phospholipids of L. lactis. These results indicate that lactococcin A acts on the cytoplasmic membrane and is very specific towards lactococci. The combined results obtained with cells, vesicles, and liposomes suggest that the specificity of lactococcin A may be mediated by a receptor protein associated with the cytoplasmic membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdineaud J. P., Boulanger P., Lazdunski C., Letellier L. In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1037–1041. doi: 10.1073/pnas.87.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vrij W., Bulthuis R. A., Konings W. N. Comparative study of energy-transducing properties of cytoplasmic membranes from mesophilic and thermophilic Bacillus species. J Bacteriol. 1988 May;170(5):2359–2366. doi: 10.1128/jb.170.5.2359-2366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Ubbink-Kok T., Konings W. N. Amino acid transport by membrane vesicles of an obligate anaerobic bacterium, Clostridium acetobutylicum. J Bacteriol. 1988 Feb;170(2):817–820. doi: 10.1128/jb.170.2.817-820.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Zheng T., In't Veld G., Op den Kamp J. A., Konings W. N. Lipid requirement of the branched-chain amino acid transport system of Streptococcus cremoris. Biochemistry. 1988 Feb 9;27(3):865–872. doi: 10.1021/bi00403a005. [DOI] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli V., Baty D., Lazdunski C. Use of a foreign epitope as a "tag" for the localization of minor proteins within a cell: the case of the immunity protein to colicin A. Proc Natl Acad Sci U S A. 1988 Feb;85(3):689–693. doi: 10.1073/pnas.85.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Permeation of bacterial cells, permeation of cytoplasmic and artificial membrane vesicles, and channel formation on lipid bilayers by peptide antibiotic AS-48. J Bacteriol. 1991 Jan;173(2):886–892. doi: 10.1128/jb.173.2.886-892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holo H., Nilssen O., Nes I. F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991 Jun;173(12):3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In 't Veld G., Driessen A. J., Op den Kamp J. A., Konings W. N. Hydrophobic membrane thickness and lipid-protein interactions of the leucine transport system of Lactococcus lactis. Biochim Biophys Acta. 1991 Jun 18;1065(2):203–212. doi: 10.1016/0005-2736(91)90231-v. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Poolman B., Driessen A. J. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16(6):419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S. E., Dobrogosz W. J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol Rev. 1990 Sep;7(1-2):149–163. doi: 10.1111/j.1574-6968.1990.tb04885.x. [DOI] [PubMed] [Google Scholar]
- Neve H., Geis A., Teuber M. Conjugal transfer and characterization of bacteriocin plasmids in group N (lactic acid) streptococci. J Bacteriol. 1984 Mar;157(3):833–838. doi: 10.1128/jb.157.3.833-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler U., Braun V., Wittmann-Liebold B., Benz R. Structural and functional properties of colicin B. J Biol Chem. 1986 Feb 25;261(6):2654–2659. [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part II. Lethal action, immunity and ecological implications. Microbiol Sci. 1984 Nov;1(8):203–205. [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Schüller F., Benz R., Sahl H. G. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur J Biochem. 1989 Jun 1;182(1):181–186. doi: 10.1111/j.1432-1033.1989.tb14815.x. [DOI] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Redborg A. H., Konisky J. Plasmid-determined immunity of Escherichia coli K-12 to colicin Ia Is mediated by a plasmid-encoded membrane protein. J Bacteriol. 1981 Dec;148(3):817–828. doi: 10.1128/jb.148.3.817-828.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmsen H. U., Pugsley A. P., Pattus F. Colicin N forms voltage- and pH-dependent channels in planar lipid bilayer membranes. Eur Biophys J. 1990;18(3):149–158. doi: 10.1007/BF02427374. [DOI] [PubMed] [Google Scholar]
- Zajdel J. K., Ceglowski P., Dobrazański W. T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985 Apr;49(4):969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Geis A., Kok J., Venema G. Cloning of two bacteriocin genes from a lactococcal bacteriocin plasmid. Appl Environ Microbiol. 1989 May;55(5):1187–1191. doi: 10.1128/aem.55.5.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Jeeninga R. E., Kok J., Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991 Feb;57(2):492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., van der Lelie D., Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987 Oct;53(10):2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


