Abstract
Mouse cartilage matrix deficiency (cmd) is an autosomal recessive disorder caused by a genetic defect of aggrecan, a large chondroitin sulfate proteoglycan in cartilage. The homozygotes (−/−) are characterized by cleft palate and short limbs, tail, and snout. They die just after birth because of respiratory failure, and the heterozygotes (+/−) appear normal at birth. Here we report that the heterozygotes show dwarfism and develop spinal misalignment with age. Within 19 months of age, they exhibit spastic gait caused by misalignment of the cervical spine and die because of starvation. Histological examination revealed a high incidence of herniation and degeneration of vertebral discs. Electron microscopy showed a degeneration of disc chondrocytes in the heterozygotes. These findings may facilitate the identification of mutations in humans predisposed to spinal degeneration.
Aggrecan, a large chondroitin sulfate proteoglycan, is one of the major structural macromolecules in cartilage. Aggrecan forms huge aggregates of ≈0.2 μm by binding to both hyaluronate and link protein whereas collagens, such as types II, IX, and XI, form a fiber structure in cartilage (1). The protein core of aggrecan consists of three globular domains and two glycosaminoglycan attaching domains (chondroitin and keratan sulfates). Extensive hydration of chondroitin sulfate chains attached to the protein core results in the unique gel-like property and resistance to deformation characteristic of cartilage (2). Thus, aggrecan plays a major role in the maintenance of cartilage structure and functions (3–5).
Cartilage matrix deficiency (cmd) (6) is the first known genetic disorder of proteoglycans identified in mammals and is caused by an autosomal recessive mutation in the aggrecan gene (7). The phenotype of homozygous cmd mice is characterized by dwarfism and cleft palate, and death occurs shortly after birth, presumably due to immature formation of tracheal cartilage. A 7-bp deletion in exon 5 of the aggrecan gene leads to a premature termination codon in exon 6 (8). Although a severely truncated protein without glycosaminoglycan-attachment domains is synthesized, immunohistochemical and biochemical analyses have revealed no evidence of the existence of the stable truncated aggrecan in cartilaginous tissues of the homozygotes (9). cmd heterozygotes are apparently normal at birth. Here, we report that the heterozygous cmd mice show two characteristic phenotypes, a slight dwarfism and age-associated hyperlordosis, the anterior concavity in the curvature of the spine. Although mutations in collagens and other extracellular matrix molecules cause cartilage-associated genetic disorders (10, 11), no human genetic diseases caused by an aggrecan gene defect have been reported. Our findings support aggrecan as a candidate gene predisposing individuals to spinal problems.
EXPERIMENTAL PROCEDURES
Animals.
cmd mice (6) have been maintained on regular mouse chow at the Craniofacial Developmental Biology and Regeneration Branch, National Institute of Dental Research, National Institutes of Health, since 1978.
Genotype Analysis.
A set of primers was synthesized from the sequence of the mouse aggrecan gene (12, 13) to distinguish genotypes. These primers are W33 (5′-CCATCTCCTCAGCGAAGCAG-3′) from exon 4 and W74 (5′-CTACAAGGACAGTGACTTTG-3′) from intron 5 of the aggrecan gene. Genomic DNA was prepared from either tails or livers of mice using the QIAamp tissue kit (Qiagen, Chatsworth, CA). Typically, 50 ng of DNA in each reaction was preincubated for 5 min at 94°C to disrupt possible secondary or ternary structure. PCR was then performed using Taq DNA polymerase (Perkin–Elmer) with a program of 15 s at 94°C, 30 s at 58°C, and 1 min at 72°C for 30 cycles (Gene Amp PCR System 9600, Perkin–Elmer). The PCR products were analyzed on a 5% polyacrylamide gel in 45 mM Tris⋅borate buffer (pH 8.3) and 1 mM EDTA (45 mM Tris/45 mM boric acid/1 mM EDTA, pH 8.3), and the gel was stained with ethidium bromide. The PCR products were digested by restriction enzyme BpmI (New England Biolabs) for 3 h at 37°C and were analyzed on a 10% polyacrylamide gel. The PCR products were confirmed by DNA sequencing using an automated sequencer (Applied Biosystems).
Quantitative Reverse Transcriptase-PCR (RT-PCR) Analysis of Aggrecan and Type II Collagen (Col2a1) mRNA in cmd Mice.
mRNA was extracted from cartilaginous tissue of day 15 embryonic limbs of the cmd homozygotes, heterozygotes, and wild-type mice using the Micro Fast Track kit (Invitrogen). Two hundred nanograms of mRNA was reverse-transcribed to generate cDNA using random hexamer primers and the first-strand cDNA Synthesis Kit (CLONTECH). Quantitative RT-PCR was performed using the PCR MIMIC Construction kit (CLONTECH). A 2-μl aliquot of the random-primed cDNA reaction and known amounts of the mimic DNA (two-fold serial dilutions of the 10 attomol mimic DNA stock) were coamplified by PCR in a 50-μl reaction mixture containing 0.7 μM of primers for either aggrecan or Col2a1, 0.2 mM each of dATP, dCTP, dGTP, and dTTP, 0.05 mCi of [32P]dCTP (3000 mmol/Ci), and 1 unit of ExTaq DNA polymerase (Takara, Otsu, Japan) with a program of 94°C for 20 s, 57°C for 20 s, and 72°C for 30 s for 35 cycles. The primers used were: 5′-CACGCTACACCCTGGACTTTG-3′ and 5′-CCATCTCCTCAGCGAAGCAGT-3′ for mouse aggrecan mRNA (12, 13); 5′-CACGCTACACCCTGGACTTTGcaagtttcgtgagctgattg-3′ and 5′-CCATCTCCTCAGCGAAGCAGTatttgattctggaccatggc-3′ for the two composite primers of aggrecan mimic DNA; 5′-GTGGAGCAGCAAGAGCAAGGA-3′ and 5′-CTTGCCCCACTTACCAGTGTG-3 for mouse Col2a1 mRNA; and 5′-GTGGAGCAGCAAGAGCAAGGAcgcccaagtgaaatctcctccg-3′ and 5′-CTTGCCCCACTTACCAGTGTGatttgattctggaccatggc-3′ for the Col2a1 mimic DNA. PCR products were electrophoresed on a 8% polyacrylamide gel, and the gel was exposed to x-ray film. The intensities of amplified cDNA and mimic DNA were measured using a densitometer (PDSI-PC, Molecular Dynamics).
Quantitative Analysis of Chondroitin Sulfate.
Proteoglycans were extracted using the 4 M guanidine·hydrochloride method (14). The spine was sectioned from either 6 month- or 14 day-old mice. After removal of surrounding connective tissues, the specimens were lyophilized. After determining the dry weight, the samples were ground into powders by mortar and pestle in liquid nitrogen. Extraction was carried out twice using 10 volumes (vol/wt) of 4 M guanidine·hydrochloride in 50 mM Tris⋅HCl (pH 8.0). Half of the extract was then precipitated twice with three volumes of 93% ethanol in 1.3% potassium acetate. The precipitate was resuspended in 0.2 M NaOH for 24 h at room temperature. After neutralization with 2 M Tris⋅HCl (pH 8.0) the sample was incubated with 100 μg/ml proteinase K (Merck) for 16 h and 2500 units Benzonase (Merck) for 4 h. The extract was then treated for 1 h with chondroitinase ABC (Seikagaku America, Ijamsville, MD). The supernate containing chondroitinase-sensitive glycosaminoglycan was used for the measurement of absorbance at 232 nm. The amount of chondroitin sulfate was calculated using the molar extinction coefficient of unsaturated disaccharide of chondroitin sulfate as 5500.
X-Ray Roentgenography.
Formalin-fixed mice or those anesthetized by avertin (Aldrich) were used for x-ray roentgenography. Mice were placed onto a film cassette (24 × 30 cm) that contained a sheet of Kodak film (Min-R) and a single Kodak Lanex Fine Screen. X-ray films were taken using a Kodak CGC model SPG-S (500 MA) machine at a distance of 40 inches with a current of 200 MA at 60 for 1/40 s.
Histological Analysis.
Mice killed by CO2 gas were sectioned and fixed with 10% formalin in 0.1 M PBS for 2 days. The samples with hard tissues were then decalcified with 0.1 M EDTA for 2 weeks at room temperature and were embedded into paraffin (Paraplast X-tra, Oxford Labware, St. Louis, MO). Sections (≈3 μm) were prepared, and they were stained by either hematoxylin/eosin, Alcian blue at pH 2.5, toluidine blue at pH 6.5, or toluidine blue at pH 2.5.
Electron Microscopy.
Samples were obtained from cervical spine and/or trachea and were dissected into cubes ≈0.5 mm in size. The tissue samples were then fixed for 60 min at 4°C in 4% paraformaldehyde and 0.4% glutaraldehyde in 0.1 M sodium cacodylate (pH 7.4) followed by postfixation for 90 min at 4°C in 2% osmium tetroxide in 0.1 M sodium cacodylate (pH 7.4). After fixation, the specimens were dehydrated with ethanol and embedded in Epon 812. Ultrathin sections were obtained with an LKB ultratome using a diamond knife and were stained with uranyl acetate and lead citrate. The sections were observed in an H-600 electron microscope at an accelerating voltage of 75 kV.
RESULTS
Genotype Analysis by Genomic PCR.
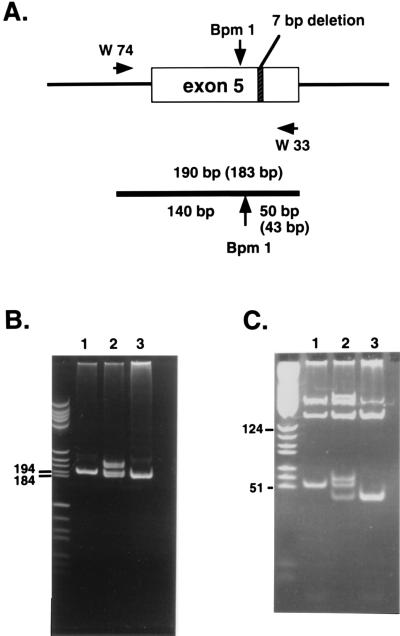
The heterozygous cmd mice and the wild-type mice were distinguished by genomic PCR with two primers from intron 4 and exon 5 of the aggrecan gene (Fig. 1). These primers produced one 190-bp product for the wild-type allele and one 183-bp product for the cmd allele. The PCR products from the heterozygotes showed an additional band representing a heteroduplex between the wild type and cmd strands, which migrated slightly slower than the 190-bp fragment (Fig. 1B, lane 2). To confirm the identity of the PCR products, the products were digested with BpmI, whose cleavage sequence is located within exon 5. BpmI digestion generated a 50-bp fragment for the wild-type allele and a 43-bp fragment for the cmd allele. The 50-bp fragment contained part of the sequence from intron 4 and exon 5 of the wild-type aggrecan allele, and the 43-bp fragment was generated from the cmd allele (7-bp deletion). The band migrating around 60 bp from the heterozygotes was confirmed as a product annealed with the normal and mutant DNA strand. The identity of these PCR products was confirmed by DNA sequencing.
Figure 1.
Analysis of genotypes by genomic PCR. Genomic DNA was prepared from the tails or livers of mice using the QIAamp tissue kit. PCRs were performed using a set of primers, W74, and W33 with genomic DNA as a template. The reaction products were electrophoresed on 5% or 8% polyacrylamide gels. (A) Schematic diagram of a part of the aggrecan gene containing exon 5. The expected sizes of the PCR products are indicated. (B) The gel electrophoresis patterns of the PCR products with DNA from a wild-type mouse (1), heterozygote (2), or homozygote (3). (C) The patterns of the gel electrophoresis of the PCR products after digestion by BpmI.
Reduced Levels of Aggrecan Expression in Cartilage of the cmd Heterozygotes.
The levels of mRNA for aggrecan and the α1 chain of type II collagen (Col2a1) in embryonic limb cartilage of cmd homozygotes and heterozygotes were measured by quantitative RT-PCR. Quantitation of mRNA was performed using various amounts of a competitor DNA fragment (mimic DNA) that was unrelated to the aggrecan gene or Col2a1 gene except for the primer sequences for these specific genes at the 5′ and 3′ ends. The quantitative RT-PCR experiments showed that the levels of aggrecan mRNA in cmd heterozygotes and homozygotes decreased to 81% and 41%, respectively, compared with that in the wild-type mice (Fig. 2). The levels of Col2a1 mRNA of both the heterozygote and the homozygote were similar to that in the wild-type mice. The level of chondroitin sulfate in the spinal cartilage from cmd heterozygous mice was reduced to 87% of the wild type. The amount of chondroitin sulfate (μmol/g, mean ± SD) in the spine of mice at 6 months was 2.30 ± 0.04 for the wild type and 2.00 ± 0.11, respectively (Table 1). According to Student’s t test, this difference was statistically significant (P < 0.0001). For the spine from mice at day 14, a similar decrease in the heterozygotes was found.
Figure 2.
Quantitative RT-PCR analyses of mRNA for aggrecan (A) and type II collagen (Col2a1) (B) in the cmd mice. mRNA was prepared from cartilaginous tissue of day 15 embryonic limbs of cmd homozygote, cmd heterozygote, and wild-type mice. Quantitative RT-PCR analysis was performed using the PCR MIMIC Construction kit (CLONTECH). The RT-PCR mixture contained a constant amount of cDNA, various amounts of the neutral mimic DNA as a competitor, and two oligonucleotide primers for aggrecan or Col2a1. The competitor DNA fragment (mimic DNA) was unrelated to the aggrecan gene or to Col2a1 except for containing the same short sequences for aggrecan or Col2a1 at both ends. mRNA levels for wild-type mice (Top), cmd heterozygotes (Middle), and cmd homozygotes (Bottom) were determined. Mimic DNA added was 10 or 80 attomoles (amol) (1 amol = 10−18 mol) (lane 1); 5 or 40 amol (lane 2); 2.5 or 20 amol (lane 3); 1.25 or 10 amol (lane 4); 0.63 or 5 amol (lane 5); 0.31 or 2.5 amol (lane 6); and 0.15 or 1.5 amol (lane 7) for aggrecan or Col2a1, respectively. Open arrowheads indicate mimic PCR product. Closed arrowheads indicate PCR product for aggrecan or Col2a. (Lower graphs) Ratios of cDNA for aggrecan or Col2a1 to PCR mimic vs. input mimic DNA amounts are shown.
Table 1.
Quantitation of chondroitin sulfate (CS) in the spine
| Case | Gender | Genotype | CS/dry weight, μmol/g |
|---|---|---|---|
| 6-month-old mice | |||
| 1 | M | +/+ | 2.27 |
| 2 | M | +/+ | 2.35 |
| 3 | F | +/+ | 2.29 |
| Average | 2.3 ± 0.04 | ||
| 4 | M | +/− | 2.04 |
| 5 | M | +/− | 1.88 |
| 6 | F | +/− | 2.10 |
| Average | 2.0 ± 0.11 | ||
| 14-day-old mice | |||
| 7* | NA | +/+ | 14.92 |
| 8* | NA | +/− | 13.21 |
NA, not applicable.
Cases 7 and 8: Samples from each mouse were combined and measured. Case 7, n = 4; Case 8, n = 3.
Slight Dwarfism.
Aggrecan is important in cartilage development, so we determined whether a reduced level of aggrecan may affect the growth of the heterozygotes. Approximately 28 days after birth, the heterozygotes exhibited proportional dwarfism. The body weights measured on day 90 after birth showed a significant difference between that of the wild type and the heterozygote. The body weights (mean ± SD) of the heterozygotes were 27.6 ± 2.1 for male (n = 14) and 24.9 ± 0.9 for female (n = 10), and those of the wild-type animals were 35.8 ± 3.0 for males (n = 13) and 29.8 ± 2.1 for females (n = 10). The difference between the heterozygotes and the wild-type mice was statistically significant according to Student’s t test (P < 0.0001).
Early Death Caused by Spinal Misalignment in Heterozygotes.
We found that the heterozygotes died younger than the wild-type mice, which lived for 2–2.5 years. No heterozygotes (n = 17) were found to live longer than 19 months whereas all of the wild-type mice (n = 20) lived for more than 2 years (Fig. 3). No gender differences were found. Close observations of the heterozygotes revealed that they developed a marked lordosis in the cervical spine and kyphosis in the thoraco-lumbar spine (Fig. 4). The mice with such spinal distortions developed spastic gait disturbance and showed decreased movement with a sudden onset. All of these mice were unable to eat and starved to death within 1 month of acquisition of the gait disturbance. X-ray analysis was performed on 1-year-old mice from wild-type to heterozygote. From a total of five mice of each genotype, two heterozygotes showed misalignment between C7 and Th1 vertebrae (Fig. 4), and another showed a compression fracture of C4. No wild-type mice showed any misalignment in their spine.
Figure 3.
Survival rate of the heterozygotes and wild-type mice. Survival rate of the heterozygotes (n = 17, closed circle) is significantly less than that of the wild-type mice (n = 20, open circle).
Figure 4.
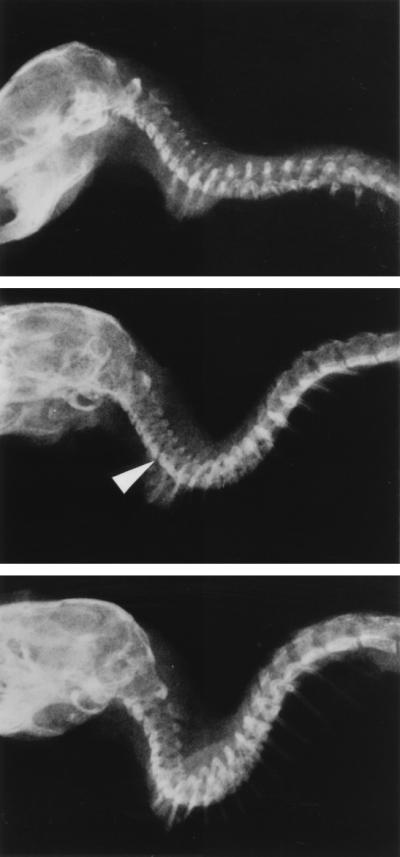
Radiographs of 1-year-old wild-type and heterozygous cmd mice. Head and spine were taken from mice killed with CO2 gas and were fixed by 10% buffered formalin. (Top) Control wild-type mouse. (Middle and Bottom) Two different heterozygous cmd mice. Arrow in the Middle indicates misalignment at C7∼Th1. In the lower panel, vertebral bodies show deformation.
Histology and Electron Microscopic Examinations.
Histological examination of the spines of 10 1-year old heterozygous mice was performed. Eight of 10 mice had visually recognizable spinal misalignment at this age. Tissue staining with hematoxylin/eosin (Fig. 5 A–C), Alcian blue staining (Fig. 5 E and F), and toluidine blue staining at pH 6.5 (Fig. 5D) revealed herniation of vertebral discs and deformation of vertebral bodies in the heterozygotes (Fig. 5 B and C). Oppression of the spinal cord by herniated disc also was observed in these mice (Fig. 5 B and D, arrows). Disappearance of apophysis of vertebral bodies also was noticed (Fig. 5 B and C). In contrast, we found no specific changes in the spine from 1-year-old wild-type mice (n = 10). Alcian blue staining (n = 10) in the heterozygotes showed reduction of glycosaminoglycan content (Fig. 5 E and F). Toluidine blue staining at pH 2.5 also confirmed a significant reduction in glycosaminoglycans (data not shown). Alcian blue stained matrix surrounding chondrocytes in the disc cartilage of the heterozygotes, and diffuse staining was observed in the wild type (Fig. 5 E and F). The vertebral disc of the heterozygotes was analyzed by electron microscopy. Chondrocytes of the heterozygotes were packed together abnormally and contained degenerative vacuoles in their cytoplasm, and rough fibers in the matrix were organized in concentric circles that surrounded the chondrocytes. Chondrocytes in the wild-type mice were distributed as individual cells in a fine extracellular matrix. (Fig. 6). Histological examination of the knee joint and other cartilaginous tissues demonstrated no particular changes in the heterozygotes (data not shown). Thus, the defects in these mice appeared to be restricted to the spinal column.
Figure 5.
Histological analysis of the spine from 1-year-old wild-type and heterozygous cmd mice. Hematoxylin/eosin-stained samples from the wild type (A) and heterozygotes (B and C), toluidine blue-stained sample from a heterozygote (D), and Alcian blue-stained samples from the wild type (E) and heterozygotes (F) are shown. Spines of heterozygotes show protrusion of discs [Th3∼4 (B) and Th4∼5 (C)] or marked herniation at C4∼5 (D). Note disappearance of apophyseal cartilage and deformation of vertebral bodies in heterozygotes (B-D) while the spines of the wild type show no remarkable changes (A). Note staining around chondrocytes in the cartilage from heterozygotes (F) while diffuse staining patterns are observed in that from the wild type (E). (A–C, ×10; D, ×25; E and F, ×125.)
Figure 6.
Transmission electron micrographs of vertebral discs from 1-year old wild type (A) and age-matched heterozygote (B), stained by uranyl acetate. The chondrocytes of the heterozygote show degeneration and are packed together. The extracellular matrix shows concentric dense bundle patterns whereas that of the wild type shows fine diffuse patterns (×4500).
DISCUSSION
We have identified two phenotypes of the cmd heterozygote mice: dwarfism and delayed onset of spinal misalignment. The cmd mutation creates a frame shift at the N terminus of aggrecan and potentially produces a severely truncated protein. Reduced stability of mRNA in such mutations is often found. Expression of aggrecan was reduced in the heterozygous cmd mice at both the mRNA and chondroitin sulfate levels. Quantitative RT-PCR analysis indicated that the level of aggrecan mRNA was reduced to 41% and 81% in the homozygotes and heterozygotes, respectively, compared with the wild-type mice. Significant reduction in aggrecan mRNA levels in heterozygous cmd mice is likely due to instability of aggrecan mRNA. Analysis of chondroitin sulfate contents suggested that aggrecan deposition in the spine of the heterozygotes was ≈87% of the wild type (Table 1). There is an apparent compensatory increase in aggrecan levels produced from the normal allele in cartilage of the heterozygous mice. However, this small reduction of aggrecan expression appears significant enough to cause slight dwarfism and spinal disorder.
The most notable phenotype of the cmd heterozygotes is misalignment of the cervical and thoracic spine at ≈1 year after birth. Until this time, the heterozygotes appear to be normal and show no signs of pathological changes. In mice, the cervical spine is the region of the spine that is the most susceptible to the gravitational load because the animals have to support their head. The histological examinations revealed not only misalignment of the cervical spine but also degenerative changes of the spine. Disc herniation found in the cervico-thoracic spine of the heterozygotes explains their spastic gait disturbance that results in death due to starvation. Noticeable morphological changes included erosive vertebral discs and disappearance of apophysis. No osteophyte formation was found. In spinal degeneration, pathological changes are usually found in both intervertebral discs and facet joints (15). In the cmd heterozygotes, pathological changes were found entirely in intervertebral discs, and facet joints apparently were normal. These histological findings indicate that the primary lesion lies in discs and that degeneration characteristic for reactive bone growth had not occurred.
Although the turnover of collagen within the disc is estimated to be very slow (>100 years), aggrecan turnover is more rapid, with the half-life of 8–300 days in rabbits (16). Because of the relatively rapid turnover of aggrecan, decreased synthesis of aggrecan significantly affects its deposition in the tissue. A certain level of aggrecan deposition in the disc may be critical for the maintenance of disc function. Aggrecan is thought to play a role in maintaining the collagen network (17). Electron microscopy of the cartilage of the cmd homozygotes showed abnormal collagen fibrils with an increase in the diameter, appearance of periodic banding patterns, and bundling formation (18). These results support a role for aggrecan in collagen fibrillogenesis. Similar changes such as rough fiber distribution with concentric patterns were found in discs of the cmd heterozygotes, likely because of reduced deposition of aggrecan in discs. It has been reported that aggrecan levels in the cartilage decrease in normal individuals with aging (15), so the cmd heterozygotes with the reduced aggrecan levels may suffer onset of spinal degeneration more quickly than the wild type. It is interesting to note that the pathological changes in the cmd heterozygotes were found mainly in the specific portions of the spine that are supposedly most susceptible to gravitational load.
cmd has been classified as an autosomal recessive disorder. Autosomal recessive inheritance is defined as inheritance in which a clinical phenotype occurs only when both alleles are defective. Nevertheless, heterozygotes with some recessive disorders may have subtle differences in phenotype that may be accentuated by environmental factors. The pathologies reported here for cmd heterozygote mice suggest that defects in a single copy of this gene also can have clinical manifestations.
cmd was the first mutation of proteoglycan genes identified in mammals (8). Although several genetic disorders of collagens, such as osteogenesis imperfecta (19–21) and chondrodysplasia (22–27), have been reported, human genetic diseases caused by a defect of the aggrecan gene have not yet been identified. The cmd heterozygous mice showed a high incidence of spinal misalignment and movement problems with age, primarily spastic paralysis of hind limbs. The phenotype of the heterozygous cmd mice is similar to that of early onset disc herniation, suggesting that aggrecan gene defects may be involved in genetic predisposition to disc herniation in humans.
Acknowledgments
We thank Masaki Yanagishita for technical comments and Hynda Kleinman, Peter Burbelo, Robert Lafrenie, and Sharon Powell for critical reading of the manuscript. We also thank John Backer, Nikki Hayes, Ryoko Tokuda, and Joanne Seinsheimer for technical assistance. Koji Kimata is a Visiting Scholar-in Residence at the Fogarty International Center, National Institutes of Health. This research was partially supported by a grant from Seikagaku Corporation.
ABBREVIATIONS
- cmd
cartilage matrix deficiency
- RT-PCR
reverse transcriptase-PCR
References
- 1.Muir H. BioEssays. 1995;17:1039–1048. doi: 10.1002/bies.950171208. [DOI] [PubMed] [Google Scholar]
- 2.Hardingham T E, Fosang A J. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- 3.Lark M W, Bayne E K, Lohmander L S. Acta Orthop Scand Suppl. 1995;266:92–97. [PubMed] [Google Scholar]
- 4.Poole A R, Ionescu M, Swan A, Dieppe P A. J Clin Invest. 1994;94:25–33. doi: 10.1172/JCI117314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratcliffe A, Beauvais P J, Saed-Nejad F. J Orthop Res. 1994;12:464–473. doi: 10.1002/jor.1100120403. [DOI] [PubMed] [Google Scholar]
- 6.Rittenhouse E, Dunn L C, Cookingham J, Calo C, Spiegelman M, Dooher G B, Bennett D. J Embryol Exp Morphol. 1978;43:71–84. [PubMed] [Google Scholar]
- 7.Kimata K, Barrach H J, Brown K S, Pennypacker J P. J Biol Chem. 1981;256:6961–6968. [PubMed] [Google Scholar]
- 8.Watanabe H, Kimata K, Line S, Strong D, Gao L Y, Kozak C A, Yamada Y. Nat Genet. 1994;7:154–157. doi: 10.1038/ng0694-154. [DOI] [PubMed] [Google Scholar]
- 9.Kimata K, Takeda M, Suzuki S, Pennypacker J P, Barrach H J, Brown K S. Arch Biochem Biophys. 1983;226:506–516. doi: 10.1016/0003-9861(83)90320-x. [DOI] [PubMed] [Google Scholar]
- 10.Olsen B R. Bone. 1995;17:45S–49S. doi: 10.1016/8756-3282(95)00208-u. [DOI] [PubMed] [Google Scholar]
- 11.Williams C J, Jimenez S A. J Rheumatol Suppl. 1995;43:28–33. [PubMed] [Google Scholar]
- 12.Walcz E, Deak F, Erhardt P, Coulter S N, Fulop C, Horvath P, Doege K J, Glant T T. Genomics. 1994;22:364–371. doi: 10.1006/geno.1994.1396. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe H, Gao L, Sugiyama S, Doege K, Kimata K, Yamada Y. Biochem J. 1995;308:433–440. doi: 10.1042/bj3080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hascall V C, Kimura J H. In: Proteoglycans: Isolation and Characterization. Cunningham L W, Frederiksen D W, editors. Vol. 82. New york: Academic; 1982. pp. 769–800. [DOI] [PubMed] [Google Scholar]
- 15.Frymoyer J W, Moskowitz R W. In: Spinal Degeneration. Frymoyer J W, Ducker T B, Hadler N M, Kostuik J P, Weinstein J N, Whitecloud T S III, editors. New York: Raven; 1991. pp. 611–634. [Google Scholar]
- 16.Mankin H J, Lippiello L. J Bone Jt Surg Am Vol. 1969;51:1591–1600. [PubMed] [Google Scholar]
- 17.Schmidt M B, Mow V C, Chun L E, Eyre D R. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 18.Kobayakawa M, Iwata H, Brown K S, Kimata K. Coll Rel Res. 1985;5:137–147. doi: 10.1016/s0174-173x(85)80035-2. [DOI] [PubMed] [Google Scholar]
- 19.Prockop D J, Colige A, Helminen H, Khillan J S, Pereira R, et al. J Bone Miner Res. 1993;8:S489–S492. doi: 10.1002/jbmr.5650081311. [DOI] [PubMed] [Google Scholar]
- 20.Prockop D J, Kuivaniemi H, Tromp G. Clin Plast Surg. 1994;21:407–413. [PubMed] [Google Scholar]
- 21.Romanic A M, Spotila L D, Adachi E, Engel J, Hojima Y, Prockop D J. J Biol Chem. 1994;269:11614–11619. [PubMed] [Google Scholar]
- 22.Warman M L, Abbott M, Apte S S, Hefferon T, McIntosh I, Cohn D H, Hecht J T, Olsen B R, Francomano C A. Nat Genet. 1993;5:79–82. doi: 10.1038/ng0993-79. [DOI] [PubMed] [Google Scholar]
- 23.Vissing H, D’Alessio M, Lee B, Ramirez F, Godfrey M, Hollister D W. J Biol Chem. 1989;264:18265–18267. [PubMed] [Google Scholar]
- 24.Winterpacht A, Hilbert M, Schwarze U, Mundlos S, Spranger J, Zabel B U. Nat Genet. 1993;3:323–326. doi: 10.1038/ng0493-323. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Vissing H, Ramirez F, Rogers D, Rimoin D. Science. 1989;244:978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad N N, Ala-Kokko L, Knowlton R G, Jimenez S A, Weaver E J, Maguire J I, Tasman W, Prockop D J. Proc Natl Acad Sci USA. 1991;88:6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad N N, Dimascio J, Knowlton R G, Tasman W S. Arch Ophthalmol. 1995;113:1454–1457. doi: 10.1001/archopht.1995.01100110114034. [DOI] [PubMed] [Google Scholar]