Abstract
Superoxide (O2⨪) and nitric oxide (NO) act to kill invading microbes in phagocytes. In macrophages NO is synthesized by inducible nitric oxide synthase (iNOS, NOS 2) from l-arginine (l-Arg) and oxygen; however, O2⨪ was thought to be produced mainly by NADPH oxidase. Electron paramagnetic resonance (EPR) spin trapping experiments performed in murine macrophages demonstrate a novel pathway of O2⨪ generation. It was observed that depletion of cytosolic l-Arg triggers O2⨪ generation from iNOS. This iNOS-mediated O2⨪ generation was blocked by the NOS inhibitor N-nitro-l-arginine methyl ester or by l-Arg, but not by the noninhibitory enantiomer N-nitro-d-arginine methyl ester. In l-Arg-depleted macrophages iNOS generates both O2⨪ and NO that interact to form the potent oxidant peroxynitrite (ONOO−), which was detected by luminol luminescence and whose formation was blocked by superoxide dismutase, urate, or l-Arg. This iNOS-derived ONOO− resulted in nitrotyrosine formation, and this was inhibited by iNOS blockade. iNOS-mediated O2⨪ and ONOO− increased the antibacterial activity of macrophages. Thus, with reduced l-Arg availability iNOS produces O2⨪ and ONOO− that modulate macrophage function. Due to the existence of l-Arg depletion in inflammation, iNOS-mediated O2⨪ and ONOO− may occur and contribute to cytostatic/cytotoxic actions of macrophages.
Keywords: l-arginine, nitrotyrosine, NADPH oxidase, cytotoxicity, electron paramagnetic resonance
Both superoxide (O2⨪) and nitric oxide (NO) are important mediators of cellular immune response. While macrophages possess a potent O2⨪-generating enzyme, NADPH oxidase, after cytokine stimulation inducible nitric oxide synthase (iNOS, NOS 2) is also expressed in large amounts (1–3). iNOS uses l-arginine (l-Arg), NADPH, and oxygen as substrates along with the cofactors FAD, FMN, calmodulin, and tetrahydrobiopterin to synthesize NO and l-citrulline. Although the catalytic mechanism of iNOS is hypothesized to be similar to that of constitutive neuronal NOS (nNOS) and endothelial NOS isoforms, iNOS has important differences, including its tightly bound calmodulin, which results in calcium-independent activation, and its high-output and long-lasting NO generation (4). Another unique characteristic of this isoform is that it is not constitutively expressed but requires cytokine or microbial product stimulation for induction of its expression.
In addition to synthesizing NO, purified nNOS generates O2⨪ at low levels of l-Arg (5). Recently, we demonstrated that nNOS produces both O2⨪ and NO in l-Arg-depleted cells, leading to peroxynitrite (ONOO−)-mediated cellular injury (6). However, important questions still remain regarding whether iNOS is also capable of producing O2⨪ and if so how this process is triggered. It has been reported that purified iNOS is less prone to oxidize NADPH in the absence of l-Arg than nNOS; however, it was also reported that 16% of NADPH consumption from iNOS is l-Arg independent (7, 8). Therefore it is unclear whether iNOS can generate significant amounts of O2⨪, and whether this O2⨪ generation would be sufficient to influence cellular function. It is also not known if O2⨪ and NO generated by iNOS combine to form the potent oxidant ONOO−. Furthermore, the biological significance of iNOS-mediated formation of O2⨪ and ONOO− in the immune function of macrophages has not been established.
While l-Arg is essential for NO production from iNOS and for the NO-mediated immune response of macrophages (9–11), l-Arg depletion was also reported to be involved in the mechanism of macrophage cytotoxicity (12, 13). There are studies demonstrating that low l-Arg levels can enhance oxygen radical generation and cytotoxicity in macrophages (14, 15). The reasons for these paradoxical observations have remained a mystery. We hypothesize that this paradox is due to O2⨪ production from iNOS at low l-Arg concentrations, where balanced synthesis of O2⨪ and NO would lead to the generation of ONOO−. Therefore, we performed studies in macrophages to measure O2⨪ generation from iNOS, as well as the formation of ONOO−, and the functional significance of these oxidants in the antibacterial activity of these cells.
MATERIALS AND METHODS
Cell Culture and l-Arg Depletion.
A mouse macrophage cell line (RAW 264.7, American Type Culture Collection) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (GIBCO). To deplete intracellular l-Arg, confluent cells were incubated in medium containing all amino acids except l-Arg. Cells were stimulated to express iNOS by addition of Escherichia coli serotype O26:B6 lipopolysaccharide (LPS, 2 μg/ml; Sigma) and recombinant mouse interferon-γ (IFN-γ, 100 units/ml; Sigma).
Electron Paramagnetic Resonance (EPR) Spectroscopy and Spin Trapping.
Spin trapping measurements of oxygen radicals were performed on 107 cells per ml in phosphate-buffered saline (PBS) with 50 mM 5,5-dimethyl-1-pyrroline N-oxide (DMPO; Aldrich). EPR spectra were recorded in a flat cell at room temperature with a Bruker ER 300 spectrometer operating at X-band with a TM 110 cavity using a modulation frequency of 100 kHz, modulation amplitude of 0.5 G, microwave power of 20 mW, microwave frequency of 9.77 GHz, and acquisition of ten 1-min scans as described (6). The microwave frequency and magnetic field were precisely measured using an EIP 575 microwave frequency counter and Bruker ER035M NMR gaussmeter. Quantitation of the free radical signals was performed by comparing the double integral of the observed signal with that of a known concentration of the 2,2,6,6,-tetramethylpiperidinoxy free radical in aqueous solution (16).
HPLC.
Cultured cells were washed and harvested in PBS. Intracellular free amino acids were extracted by using ice-cold 0.3 M perchloric acid, and the extract was neutralized by 3 M KHCO3. The protein and cellular debris in the preparations were pelleted by centrifugation (10,000 × g for 20 min) at 4°C, and the supernatant was recovered for HPLC analysis. The samples were then dried under vacuum and derivatized by a reagent containing methanol, triethylamine, water, and phenylisothiocyanate at a volume ratio of 7:1:1:1. Reversed-phase HPLC amino acid separation was performed using a Waters Pico-tag column, and the amino acid peaks from the samples were identified and quantitated by comparing with those from a standard containing each amino acid at known concentrations (Pierce) (6).
Western Blotting.
Confluent cells were lysed in a boiling lysis buffer containing 1% SDS, 1 mM sodium vanadate, and 10 mM Tris⋅HCl (pH 7.4), and centrifuged at 8,000 × g for 20 min at 4°C. Cytosolic proteins (7.5 μg per lane) were electrophoresed on an SDS/7.5% polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with a mouse anti-iNOS monoclonal antibody (1:2,500 dilution; Transduction Laboratories, Lexington, KY). Sheep antibody to mouse IgG, conjugated to horseradish peroxidase, was used as a secondary antibody (1:1,000 dilution; Amersham). Antibodies on blots were detected with an enhanced chemiluminescence technique (ECL, Amersham).
Chemiluminescence Measurement.
Luminescence measurements of ONOO− were performed in Earl’s balanced salt solution (GIBCO) containing 107 cells per ml and 500 μM luminol (5-amino-2,3-dihydro-1,4-phthalazinedione; Sigma) at 37°C by using a Berthold LB9505C luminometer (17).
Immunocytochemistry.
Cells were plated on chamber slides that were coated with 0.01% polylysine. After 24-hr stimulation with LPS and IFN-γ in the presence or absence of l-Arg, cells were fixed with 4% paraformaldehyde in 0.01 M phosphate-buffered saline (pH 7.4) at room temperature for 30 min. The slides were incubated with affinity-purified mouse monoclonal anti-nitrotyrosine antibody (1:500 dilution; Upstate Biotechnology, Lake Placid, NY) (18). The immunostaining was accomplished with an Extravidin peroxidase staining kit (Sigma) using 3-amino-9-ethylcarbazole as a chromogen (6).
Antibacterial Assay.
Bacteria (E. coli strain JM109; Promega) were grown in Luria–Bertani (LB) medium in a shaker incubator at 37°C. For assay, aliquots of exponentially growing bacteria (OD600 = 0.2) were incubated with macrophages in flasks, and the growth of bacteria was monitored as the optical density at 600 nm (19).
Statistical Analysis.
Results are expressed as mean ± SEM. Student’s unpaired t test was used to determine the statistical significance of differences between the means, and a P value of <0.05 was considered as significant.
RESULTS AND DISCUSSION
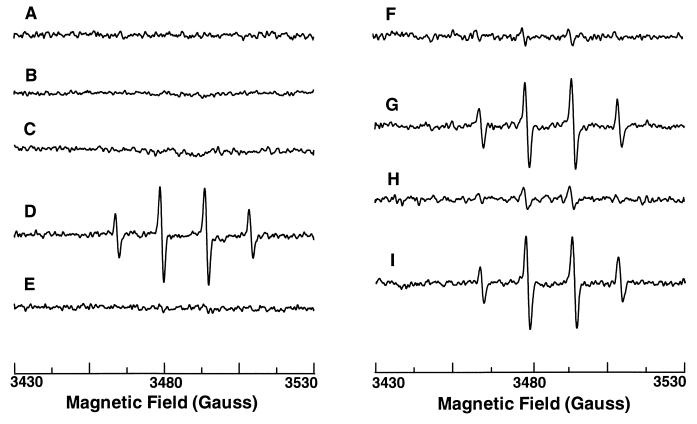
To determine if iNOS produces O2⨪, EPR spectroscopy was used to directly measure oxygen radicals in murine macrophages (RAW 264.7) with the oxygen radical trap DMPO. Cells were stimulated to express iNOS by 24-hr activation with bacterial LPS and mouse IFN-γ. No oxygen radical signals were seen in either nonactivated or activated cells (Fig. 1, traces A and B), whereas abundant NO generation occurs in activated cells as previously shown (20). To determine if iNOS generates O2⨪ in l-Arg-depleted cells, RAW 264.7 cells were incubated in l-Arg-free medium to deplete intracellular l-Arg. With cells activated in l-Arg-free medium, prominent oxygen radical signals were observed (Fig. 1, trace D), consisting of a 1:2:2:1 quartet with hyperfine splitting constants aH = aN = 14.9 G, indicative of DMPO-OH (6, 21). These signals were totally quenched by superoxide dismutase (SOD) but were not affected by catalase (Fig. 1, traces E and I), demonstrating that the signals were derived from trapping of O2⨪. l-Arg-free incubation of nonstimulated cells did not cause O2⨪ formation (Fig. 1, trace C). Thus, O2⨪ generation occurred in l-Arg-depleted macrophages, but this required cellular activation.
Figure 1.
iNOS-mediated oxygen radical generation in macrophages measured by EPR spin trapping. EPR spectra were obtained in the presence of 50 mM DMPO from the following preparations. Trace A, normal cultured cells. Trace B, cells after 24-hr activation with LPS and IFN-γ in DMEM. Trace C, cells incubated in l-Arg-free medium for 24 hr. Trace D, cells after 24-hr activation with LPS and IFN-γ in l-Arg-free medium (l-Arg-depleted cells). Trace E, l-Arg-depleted cells with SOD (200 units/ml). Trace F, l-Arg-depleted cells with 1 mM l-NAME. Trace G, l-Arg-depleted cells with 1 mM d-NAME. Trace H, l-Arg-depleted cells with 1 mM l-NMMA. Trace I, l-Arg-depleted cells with catalase (300 units/ml). Representative spectra were shown from triplicate measurements.
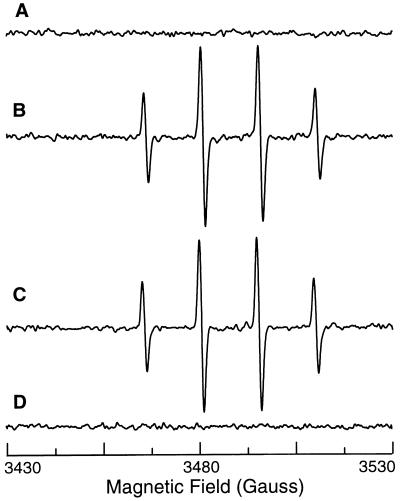
To confirm that O2⨪ was generated by iNOS, l-Arg-depleted cells were pretreated with a specific NOS blocker N-nitro-l-arginine methyl ester (l-NAME). The DMPO-OH signals were more than 90% blocked by 1 mM l-NAME but not affected by its noninhibitory enantiomer, d-NAME (1 mM) (Fig. 1, traces F and G). O2⨪ production was also largely inhibited by another NOS blocker, NG-monomethyl-l-arginine (l-NMMA, 1 mM) (Fig. 1, trace H), whereas l-NMMA has previously been shown not to block O2⨪ from nNOS (5). Since NADPH oxidase is the principal source of O2⨪ in phagocytic cells, control experiments were performed to confirm that NOS blockers do not affect O2⨪ generation from NADPH oxidase. Neither l-NAME nor l-NMMA affected O2⨪ generation from NADPH oxidase in macrophages activated with protein kinase C activator phorbol 12-myristate 13-acetate (PMA) (Fig. 2, traces A, B, and C). Since O2⨪ formation was totally blocked by NOS inhibitors, iNOS was the main source of O2⨪ generation in l-Arg-depleted macrophages. Furthermore, in cells activated with LPS and IFN-γ, PMA-stimulated O2⨪ generation was abolished, suggesting that cytokine pretreatment with induction of iNOS inhibited NADPH oxidase (Fig. 2, trace D).
Figure 2.
Effects of l-NAME or iNOS induction on NADPH oxidase-mediated oxygen radical generation in macrophages. EPR spin trapping was performed in the presence of 50 mM DMPO. Trace A, control macrophages. Trace B, after activation of NADPH oxidase with 200 ng/ml PMA. Trace C, activation of NADPH oxidase with 200 ng/ml PMA in the presence of 1 mM l-NAME. Trace D, macrophages prestimulated for 24 hr with LPS and IFN-γ with addition of 200 ng/ml PMA. The NOS inhibitor l-NAME did not alter NADPH oxidase-mediated radical generation, but prestimulation with LPS and IFN-γ totally blocked this radical generation.
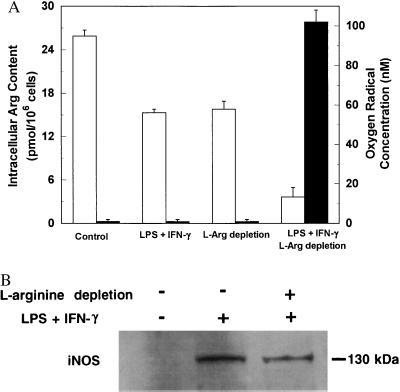
To define the role of cytosolic l-Arg concentrations in controlling O2⨪ generation from iNOS, we examined the relationship of O2⨪ production to intracellular l-Arg levels. In control nonstimulated cells, intracellular l-Arg was saturated (25.9 ± 0.8 pmol per 106 cells) and no O2⨪ was detected (Fig. 3A). After 24-hr activation with LPS and IFN-γ in l-Arg-containing medium, cytosolic l-Arg level decreased to 15.3 ± 0.5 pmol per 106 cells (P < 0.05 versus control, n = 3), which may be due to the consumption by both iNOS and arginase. l-Arg-free incubation itself also markedly reduced cytosolic l-Arg (15.8 ± 1.1 pmol per 106 cells, P < 0.05, n = 3); however, no O2⨪ was seen from cells under either of these conditions. When cells were activated in l-Arg-free medium, cytosolic l-Arg was further depleted to 3.6 ± 0.6 pmol per 106 cells (P < 0.01), and prominent O2⨪ generation was observed. These data indicated that substantial cytosolic l-Arg depletion was required for iNOS-mediated O2⨪ generation. To further establish the role of cytosolic l-Arg levels in controlling iNOS-mediated O2⨪ generation, we determined if restoring cytosolic l-Arg abolished this O2⨪ generation. l-Arg-depleted cells were incubated in PBS containing 2 mM l-Arg to replete the cytosolic l-Arg pool. After 45 min of incubation, O2⨪ formation no longer occurred. These results unambiguously demonstrated that iNOS-catalyzed O2⨪ generation was triggered by low intracellular l-Arg concentrations.
Figure 3.
Effects of l-Arg on oxygen radical formation and iNOS expression. (A) Relationship between intracellular l-Arg levels (open bars) and oxygen radical production (filled bars) in macrophages. Control, normal cultured cells; LPS + IFN-γ, cells after 24-hr activation in DMEM; l-Arg depletion, cells incubated in l-Arg-free medium for 24 hr; LPS + IFN-γ and l-Arg depletion, cells after 24-hr activation in l-Arg-free medium. Data are expressed as mean ± SEM obtained from three experiments. (B) Western blot analysis of iNOS protein in nonactivated cells and cells activated in the presence or absence of l-Arg. While no iNOS could be detected in nonactivated cells, identical amounts of iNOS protein were observed in cells activated by LPS and IFN-γ in either normal DMEM or l-Arg-free medium.
To determine whether l-Arg depletion alters cellular iNOS expression, Western blotting was performed. While no iNOS protein could be detected in nonstimulated cells, iNOS was present in the cells after 24-hr activation with LPS and IFN-γ (Fig. 3B). With cells activated in l-Arg-free medium, an identical amount of iNOS protein was observed. Thus, l-Arg depletion did not alter IFN-γ- and LPS-induced iNOS expression.
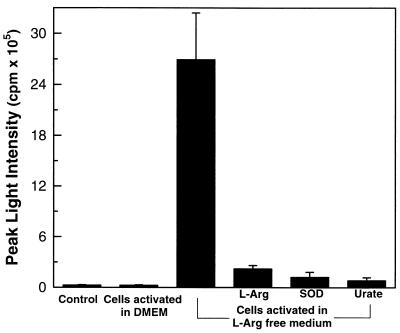
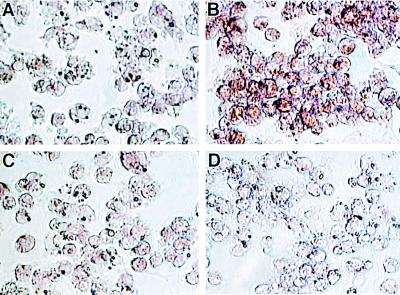
O2⨪ reacts with NO to form ONOO−, a potent cytotoxic oxidant (22). It was previously proposed that production of O2⨪ and NO in macrophages are separately regulated and do not occur simultaneously (1). However, we observe that iNOS generates O2⨪ as well as NO in l-Arg-depleted cells. To determine if iNOS directly generates ONOO−, chemiluminescence measurements were performed using the ONOO− enhancer luminol (17). Although no luminescence was detected in control cells or cells activated in normal medium, strong luminescence was seen in l-Arg-depleted macrophages (Fig. 4). This luminescence was blocked by SOD or the ONOO− scavenger urate, confirming that it was derived from ONOO− (17). Restoring l-Arg abolished this ONOO− generation. To further confirm that ONOO− was formed, immunocytochemistry measurements of the ONOO− specific nitration product nitrotyrosine were performed. While in control activated macrophages no nitrotyrosine was seen, in l-Arg-depleted cells prominent staining was present (Fig. 5 A and B). l-NAME largely abolished this nitrotyrosine staining, and specificity of the antibody used was demonstrated by the complete block of staining seen in the presence of excess free nitrotyrosine (Fig. 5 C and D).
Figure 4.
iNOS-mediated ONOO− generation in l-Arg-depleted macrophages. Although no luminescence was detected in control cells or cells activated in normal DMEM, strong luminescence was seen in l-Arg-depleted macrophages. This luminescence was blocked by l-Arg (1 mM), SOD (200 units/ml), or urate (1 mM).
Figure 5.
Nitrotyrosine formation in l-Arg-depleted macrophages. Prominent immunostaining of nitrotyrosine was observed in the cells activated in l-Arg-free medium (B) but not in the cells activated in normal DMEM (A). This staining was blocked by 1 mM l-NAME (C) and preincubation of the primary antibody with 1 mM nitrotyrosine (D). (×400.)
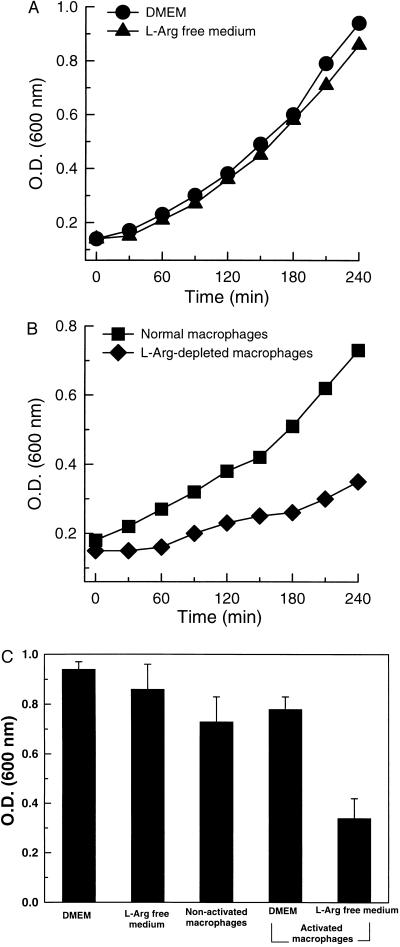
To explore the functional significance of iNOS-mediated O2⨪ and ONOO− in macrophage immune function, we assessed the effects of this oxidant formation on bacterial growth. It is known that ONOO− inhibits the growth of the important and ubiquitous bacterial pathogen E. coli (23). Experiments were performed comparing the inhibitory effects of normal macrophages and those preactivated in l-Arg-containing or l-Arg-free medium on the growth of E. coli. In control experiments in the absence of macrophages, similar bacterial growth patterns were seen in either normal or l-Arg-free medium, indicating that elimination of l-Arg did not significantly alter bacterial growth (Fig. 6A). In the presence of cells preactivated in l-Arg-free medium which generate O2⨪ and ONOO−, bacterial growth was inhibited by more than 2-fold compared with that in the presence of normal cells or cells preactivated in the presence of l-Arg, which do not generate O2⨪ or ONOO− (P < 0.01) (Fig. 6 B and C). Thus, O2⨪ and ONOO− generated by macrophage iNOS depress bacterial growth, indicating that iNOS-catalyzed O2⨪ and ONOO− can exert immune defense function.
Figure 6.
Inhibitory effects of iNOS-mediated O2⨪ and ONOO− on the growth of E. coli. (A) Growth of E. coli in DMEM and l-Arg-free medium. (B) Growth of E. coli in the presence of normal RAW 264.7 cells and cells preactivated in l-Arg-free medium (l-Arg-depleted macrophages). Results are the average of three experiments. (C) E. coli levels in the medium after 4-hr incubations.
NOS is a cytochrome P450 reductase-like hemoprotein containing NADPH, FAD, FMN, calmodulin, and heme binding sites (24). The catalytic mechanism of iNOS involves flavin-mediated electron transport from C-terminal bound NADPH to N-terminal heme iron, where oxygen is reduced to form NO in the presence of l-Arg (7). iNOS shares 50–60% homology in amino acid sequence with nNOS. In the absence of l-Arg, it has been demonstrated that nNOS generates O2⨪ (5, 6). While the catalytic mechanisms of NO formation are thought to be similar for these two enzymes, it was previously reported from studies of the isolated enzyme that iNOS is less prone to oxidize NADPH in the absence of l-Arg, and therefore it was inferred that iNOS would not be a significant source of O2⨪ (7, 8). However, our studies directly demonstrate that iNOS in macrophages does generate functionally important amounts of O2⨪, and this is triggered by l-Arg depletion. Furthermore, we found that with l-Arg depletion iNOS produces both O2⨪ and NO, leading to ONOO− formation. This iNOS-mediated ONOO− generation is unique because in these cells O2⨪ and NO arise from the same enzyme, not from different sources as previously reported (22). Since O2⨪ rapidly dismutates either spontaneously or by the action of SOD, the formation of both O2⨪ and NO by the same enzyme may enable more efficient production of ONOO− than possible with generation of these species by different enzymes.
The production of O2⨪ by macrophages is critical for host defense. The cytotoxic nature of O2⨪ and its associated oxidants not only contributes to the killing of invading microbes but also causes tissue damage in inflammation (1). Previously, NADPH oxidase was considered the main source of O2⨪ in macrophages. Our findings reveal a novel O2⨪ generation pathway from iNOS in activated macrophages, which is controlled by cytosolic l-Arg. In cytokine-stimulated cells O2⨪ generation from NADPH oxidase was blocked, rendering these cells immunocompromised. These observations are in agreement with past reports suggesting that cytokine stimulation or NO can inhibit O2⨪ production from the NADPH oxidase (25–27). However, when l-Arg was depleted O2⨪ generation was restored but iNOS became the primary source of this important oxidant.
Our findings provide a potential explanation of why macrophage cytotoxicity is l-Arg dependent but paradoxically can be enhanced by l-Arg depletion. We observe that while l-Arg is required for NO generation from iNOS, partial l-Arg depletion is required to trigger O2⨪ and ONOO− generation. Since l-Arg is depleted in inflammatory sites during macrophage infiltration and wound healing (14, 15), under these circumstances iNOS-mediated O2⨪ and ONOO− could be particularly important in the cytotoxic actions of macrophages. Although we observe that l-Arg depletion enhanced the antibacterial effects of macrophages, this pathway of O2⨪ and ONOO− formation could also cause subsequent autotoxicity. While iNOS-mediated formation of O2⨪ and ONOO− may initially enhance macrophage immune function, overproduction of these oxidants could also trigger cell death. Since iNOS-mediated O2⨪ and ONOO− generation were controlled by l-Arg concentrations, modulating cytosolic l-Arg may provide a therapeutic approach to influence macrophage immune function in inflammatory disease.
Acknowledgments
This work was supported by National Institutes of Health Grants HL-38324 and HL-52315.
ABBREVIATIONS
- NOS
nitric oxide synthase
- iNOS
inducible NOS
- nNOS
neuronal NOS
- O2⨪
superoxide
- ONOO−
peroxynitrite
- l-Arg
l-arginine
- LPS
lipopolysaccharide
- IFN-γ
interferon-γ
- EPR
electron paramagnetic resonance
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- l-NAME
N-nitro-l-arginine methyl ester
- l-NMMA
NG-monomethyl-l-arginine
- SOD
superoxide dismutase
- PMA
phorbol 12-myristate 13-acetate
References
- 1.Bastian N R, Hibbs J B., Jr Curr Opin Immunol. 1994;6:131–139. doi: 10.1016/0952-7915(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C, Xie Q-w. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 3.Rosen G M, Pou S, Ramos C L, Cohen M S, Britigan B E. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C, Xie Q-w. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 5.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 6.Xia Y, Dawson V L, Dawson T M, Snyder S H, Zweier J L. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith O W, Stuehr D J. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Soud H M, Stuehr D J. Proc Natl Acad Sci USA. 1993;90:10769–10771. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibbs J B, Jr, Taintor R R, Vavrin Z. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 10.Stuehr D J, Nathan C F. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marletta M A, Yoon P S, Iyengar R, Leaf C D, Wishonk J S. Biochemistry. 1988;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- 12.Currie G A. Nature (London) 1978;273:758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- 13.Currie G A, Gyure L, Cifuentes L. Br J Cancer. 1979;39:613–620. doi: 10.1038/bjc.1979.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albina J E, Mills C D, Barbul A, Thirkill C E, Henry W L, Jr, Mastrofrancesco B, Caldwell M D. Am J Physiol. 1988;254:E459–E467. doi: 10.1152/ajpendo.1988.254.4.E459. [DOI] [PubMed] [Google Scholar]
- 15.Albina J E, Caldwell M D, Henry W L, Jr, Mills C D. J Exp Med. 1989;169:1021–1029. doi: 10.1084/jem.169.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zweier J L, Kuppusamy P, Lutty G A. Proc Natl Acad Sci USA. 1988;85:4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radi R, Cosgrove T P, Beckman J S, Freeman B A. Biochem J. 1993;290:51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckman J S, Ye Y Z, Anderson P G, Chen J, Accavetti M A, Tarpey M M, White C R. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 19.Hausladen A, Privalle C T, Keng T, DeAngelo J, Stamler J S. Cell. 1996;86:719–729. doi: 10.1016/s0092-8674(00)80147-6. [DOI] [PubMed] [Google Scholar]
- 20.Xie Q-w, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein E, Rosen G M, Rauckman E J. Mol Pharmacol. 1982;21:262–265. [PubMed] [Google Scholar]
- 22.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Gunn C, Beckman J S. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 24.Bredt D S, Snyder S H. Annu Rev Biochem. 1991;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 25.Szuro-Sudol A, Murray H W, Nathan C F. J Immunol. 1983;131:384–387. [PubMed] [Google Scholar]
- 26.Ding A, Nathan C F. J Immunol. 1987;139:1971–1977. [PubMed] [Google Scholar]
- 27.Clancy R M, Leszczynska-Piziak J, Abramson S B. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]