Abstract
An invertebrate intestinal mucin (IIM) was identified from a lepidopterous insect, Trichoplusia ni. The IIM is a major protein constituent of the peritrophic membrane that facilitates the digestive process, as well as protecting invertebrate digestive tracts from microbial infections. The IIM demonstrated biochemical characteristics similar to vertebrate mucins, but exhibited strong association with the chitin-containing peritrophic membrane matrix. We have demonstrated that a baculovirus enhancin, which is encoded and carried by specific baculoviruses, has mucin-degrading activity both in vitro and in vivo. The in vivo degradation of IIM by enhancin was correlated with the enhancement of baculovirus infections in insects. These findings have shown that viruses have evolved a novel strategy to overcome intestinal mucinous barriers against microorganisms by utilizing a mucin-degrading enzyme.
Microbial pathogenesis involves numerous cellular and molecular interactions between microbes and their host organisms. Animals have developed various defense mechanisms against pathogens and, conversely, pathogenic microorganisms have evolved strategies to overcome these barriers to the infection process. Epithelial cells in mammalian digestive tracts, for example, are covered with a protective mucus layer which plays a crucial role in protecting these cells (1). The protective functions of the mucosal layer rely on a major proteinaceous component that is heavily glycosylated and known as mucin (1–3). Mucins are reported to play an active role in preventing bacterial, viral, and other pathogens from interacting with vertebrate intestinal epithelia (1, 4–6).
In invertebrate species, the intestinal tracts do not have a mucus layer similar to that found in mammals; however, in insects the intestine is commonly lined and shielded by a unique noncellular matrix known as the peritrophic membrane (PM) (7, 8). PMs are semipermeable structures that are composed primarily of chitin, protein, and glycoproteins. Although there are few studies on the interaction between microbial pathogens and PMs, these structures are proposed to serve as a physical barrier to pathogenic microorganisms (see ref. 8 for a comprehensive overview). The chitin component of PMs is normally present as a network of chitin fibrils in which proteins and glycoproteins are present. The chitin can be a potential target substrate for intestinal pathogens, and it was demonstrated that degradation of chitin in the PM by a pathogen-encoded chitinase allowed an avian malaria parasite to overcome its mosquito vector intestinal PM barrier (9). Proteins are the major PM component; however, their functions in the PM are unknown. Studies on the PM proteins are primarily limited to several preliminary analyses on the amino acid composition of total PM proteins (10–12) and PM protein profiles as determined by electrophoresis (12–14). The only PM protein characterized to date, peritrophin-44, was isolated from Lucilia cuprina larvae (15), but its biological function is unclear. To date, studies on the interaction of PM proteins with microbial pathogens are limited to the effect of a baculovirus enhancin on lepidopteran PM proteins (16, 17).
Previous studies have demonstrated that a Trichoplusia ni granulosis virus (TnGV) encodes an enhancin, a viral enhancing protein, that was identified as a metalloprotease (18, 19). Enhancin degrades high molecular weight PM proteins in vivo and in vitro. In addition, the protein degradation is correlated with the disruption of the structural integrity of the PM (16) and enhanced viral infection (20). It was recently demonstrated that enhancin could degrade high molecular weight PM proteins from several lepidopterous species (17); however, the chemical nature and function of these proteins in baculovirus pathogenesis are unknown. (16, 17, 19).
In this paper, we report the identification of an intestinal mucin from the PM of T. ni larvae and the observation that TnGV encodes a mucin-degrading enzyme to overcome its host’s mucinous intestinal defense barrier.
MATERIALS AND METHODS
Insect Rearing and Viruses.
T. ni larvae from a laboratory colony were reared on a high wheat-germ-based artificial diet. An isolate of Autographa californica multiple nuclear polyhedrosis virus (AcMNPV), strain 1A, was used for infection (21). Production of TnGV and the isolation of TnGV enhancin were as described by Wang et al. (17).
Preparation of T. ni PMs.
Midgut PMs were dissected from mid-fifth instar T. ni larvae, thoroughly rinsed with de-ionized water, and stored at −70°C.
SDS/PAGE Analyses.
In experiments designed to determine the apparent molecular weight of invertebrate intestinal mucin (IIM), the continuous SDS/PAGE procedure was used as described by Weber and Osborn (22). For other electrophoresis analyses, Laemmli’s discontinuous SDS/PAGE system was used (23).
Treatment of PMs.
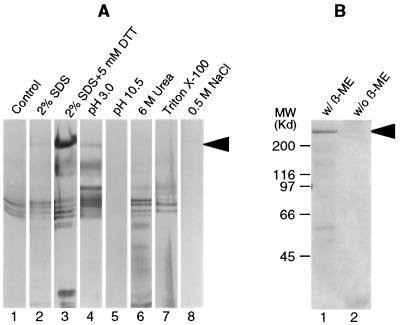
To test the association of IIM with the PM, the treatments were varied in an attempt to dissociate IIM from the PMs. Briefly, PMs were incubated at 28°C for 1 h under different conditions, including the presence of a denaturing or reducing agent, extreme pH range, and high salt strength. After incubation the PMs were pelleted by centrifugation and the supernatants collected. The released IIM in the supernatant was examined by SDS/PAGE. The incubation solutions for each treatment are described in detail in the legend for Fig. 2.
Figure 2.
(A) Silver-stained SDS/PAGE gel showing proteins released from the chitin-containing PM matrix after incubation of PMs at 28°C for 1 h in water (lane 1), 2% SDS (lane 2), 2% SDS plus 5 mM DTT (lane 3), 0.1 M sodium citrate buffer, pH 3.0 (lane 4), 0.1 M sodium carbonate buffer, pH 10.5 (lane 5), 6 M urea (lane 6), 0.1% Triton X-100 (lane 7), and 0.5 M NaCl (lane 8). (B) Coomassie blue-stained SDS/PAGE gel showing release of IIM after boiling the PMs for 10 min in 2% SDS in the presence (lane 1) and absence (lane 2) of 5% 2-mercaptoethanol (β-ME). The arrows in both A and B indicate IIM.
Preparation of IIM from T. ni PMs.
IIM was isolated from PMs and purified by preparative SDS/PAGE. To isolate IIM, PM proteins were solubilized by boiling PMs in SDS/PAGE sample buffer, and then separated by SDS/PAGE (23). To prepare IIM for antiserum production, protein bands were first visualized by staining the gel with 0.05% Coomassie blue R-250 in 40% methanol followed by destaining with de-ionized water; this procedure was followed by excision of the IIM band. After equilibration in a SDS/PAGE running buffer (23), the IIM in the gel slice was electroeluted, and the preparation was concentrated and resuspended in PBS by ultrafiltration using a centriprep-30 concentrator (Amicon).
For general biochemical analyses, PM protein bands on the SDS/PAGE gel were initially visualized by copper staining (24), which facilitated the excision of the IIM band. IIM from this gel slice was also electroeluted after copper ions were removed by washing the gel slice several times in 0.2 M EDTA. Subsequently, the eluted protein preparation was desalted by ultrafiltration.
To isolate IIM for amino acid composition analysis, the sodium phosphate-buffered SDS/PAGE system was used (22). The gel was stained with copper chloride after equilibration of the gel in 0.375 M Tris⋅HCl (pH 8.8) with 0.1% SDS. The IIM band was excised and the IIM was recovered by electroelution as described above; the preparation was further desalted by extensive dialysis against de-ionized water and then lyophilized.
Antiserum.
An antiserum to IIM was generated by immunizing a Flemish Giant/Chinchilla Cross rabbit with purified IIM from T. ni PMs. Preimmune serum from the rabbit was collected and used as a control for immunodetection of IIM.
Amino Acid Composition Analysis.
Purified IIM was hydrolyzed with HCl/propionic acid (1:1) at 150°C for 90 min. The amino acid composition was analyzed at the Analytical Chemistry and Peptide/DNA Synthesis Facility of the Biotechnology Program, Cornell Center for Advanced Technology (Ithaca, NY) with a Pico-Tag amino acid analysis system. The content of tryptophane was estimated spectrophotometrically by determination of absorbance at 280 and 288 nm in 6.0 M guanidine-HCl solution (25).
Quantification of Protein and Carbohydrates on IIM.
Aliquots of purified IIM preparations were used for both carbohydrate and protein quantification. Carbohydrates were quantified by an Anthrone assay as described by Calza et al. (26), using mannose and galactose (1:1) as standards. The protein content was determined by quantitative amino acid composition analysis as described above.
Analyses of Carbohydrate Moieties on IIM.
Carbohydrate moieties on IIM were characterized by structure-specific lectin binding assays. Purified IIM was electrophoresed on a 7.5% SDS/PAGE gel and transferred onto Immobilon-P transfer membrane (Millipore). The carbohydrate structures were detected in the membrane using a set of digoxigenin-labeled lectins (Boerhinger Mannheim), as described by the manufacturer. To confirm the results from positive lectin-binding assays, controls which consisted of O-glycosidase and N-glycosidase F (Boerhinger Mannheim) pretreated IIMs were included in the lectin binding assays. Treatment of IIM with O-glycosidase was conducted by incubating 0.1 μg of IIM in 15 μl of 0.02 M sodium phosphate buffer (pH 6.0) containing 0.1% SDS, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, and 0.25 milliunit O-glycosidase at 37°C for 24 h. Treatment of IIM with N-glycosidase F was performed by incubating 40 ng of IIM in 20 μl of 50 mM sodium phosphate buffer (pH 7.0) containing 0.1% SDS and 2% 2-mercaptoethanol at 100°C for 5 min. When cooled, 2 μl of Nonidet P-40 and 0.6 unit of N-glycosidase F were added, followed by incubation at 37°C for 24 h. The apparent molecular weight reduction after O-glycosidase treatment was determined by SDS/PAGE analysis.
In Situ Deglycosylation of IIM with O-Glycosidase.
To confirm that resistance of IIM to digestive protease hydrolysis is primarily conferred by O-linked carbohydrates, PMs, which normally contain endogenous digestive proteases, were treated with O-glycosidase to remove the disaccharide, galactose β(1–3) N-acetylgalactosamine from IIM. The PMs were incubated in 0.05 M sodium phosphate buffer (pH 6.0) containing 15 milliunits/ml O-glycosidase at 37°C for 4 h, and following the incubation, IIM from the treated PMs was analyzed by SDS/PAGE. A parallel treatment of PMs in the absence of O-glycosidase was included as a control. In addition, two other controls using protease-inactivated PMs were included in these analyses. In one control, intestinal proteases associated with the PM were inhibited by a cocktail of protease inhibitors (1 mM phenylmethylsulfonyl fluoride/1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml pepstatin/10 μg/ml E64/10 mM EDTA). In the other control, the intestinal proteases were inactivated and removed by boiling the PMs in 2% SDS for 5 min; this procedure was followed by extensive washing with deionized water.
Digestion of IIM with TnGV Enhancin.
To demonstrate proteolytic activity by TnGV enhancin against IIM, purified IIM was incubated with 1.25 μg/ml TnGV enhancin in 0.05 M Tris⋅HCl buffer (pH 7.5) containing a cocktail of protease inhibitors minus the metalloprotease inhibitor, EDTA, at 37°C for 3 h or overnight. The degradation of IIM was examined by SDS/PAGE analysis. A parallel treatment of IIM without enhancin was included as a control. To confirm the metalloprotease nature of enhancin, IIM was incubated with TnGV enhancin in the presence of 10 mM EDTA.
In Vivo Assays for IIM Degradation by Enhancin.
Two in vivo assays were developed to include neonate and fifth instar T. ni larvae, based on the methods employed to determine the efficacy of an enhancin on virus infections (20). The in vivo neonate IIM assay and a concomitant virus bioassay were conducted by feeding T. ni neonate larvae with inoculum droplets containing 105 occlusion bodies/ml of AcMNPV, and varying doses of TnGV enhancin, as described by Wang et al. (17). Following ingestion of the inoculum, 25 larvae from each treatment were transferred onto artificial diet, incubated at 28°C for 90 min, and collected for Western blot analysis using an antiserum specific to IIM. For Western blot analysis, the larvae were homogenized in 100 μl of SDS/PAGE sample buffer. Subsequently, 4 μl of each sample was electrophoresed through a 7.5% SDS/PAGE gel, blotted, and then probed with anti-IIM antiserum. To assess the correlation between the extent of IIM degradation in living insects and the degree of enhanced AcMNPV infection by TnGV enhancin, 60 neonate larvae from each feeding group were also collected and individually reared on artificial diet. Viral infections were monitored and examined throughout the whole insect larval developmental stages, as described by Wang et al. (17).
The in vivo IIM degradation assay was also conducted by feeding fifth instar T. ni larvae with TnGV enhancin and analyzing the residual IIM in the fecal pellets. Early fifth instar T. ni larvae were fed 10 μl of inoculum containing 5% sucrose, 10 μg/ml blue food coloring, and 5 μg TnGV enhancin in 25 mM sodium carbonate buffer (pH 10.5). Afterward, the larvae were transferred to individual rearing cups containing artificial diet and incubated at 28°C. During the incubation period, enhancin will digest the IIM present in the PM. PMs are secreted within the intestine and later excreted with fecal pellets, which are normally ensheathed within the remnants of a PM (27). The first three fecal pellets marked with blue food coloring therefore were collected and subjected to Western blot analysis using the IIM-specific antiserum.
RESULTS
IIM has an Apparent Molecular Weight of 400 kDa and Is Tightly Associated with PMs.
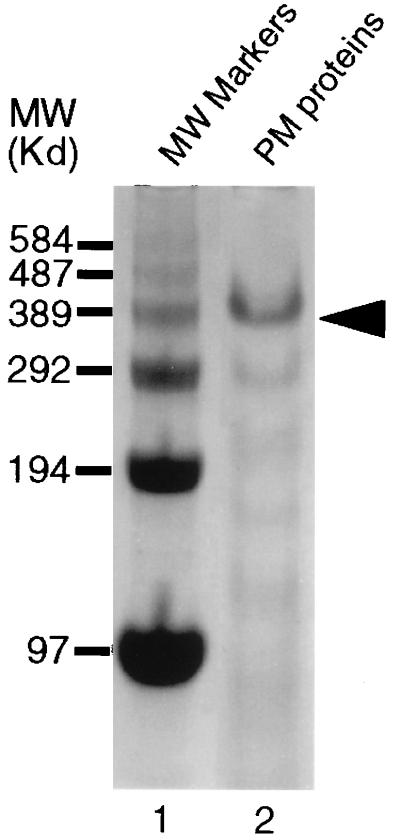
IIM from T. ni PMs appears as a 400-kDa protein on 3.5% SDS/PAGE gels (Fig. 1). The association of the IIM with PMs is stable over a wide range of pHs, in the presence of nonionic and ionic detergents, and in the presence of protein denaturing reagents. Therefore, very little, or no IIM was present in the supernatants from these treatments (Fig. 2A). IIM, the predominant PM protein, could be released from the PM by a combination of 2% SDS plus 5 mM DTT (Fig. 2A, lane 3), confirming that it was strongly associated with the chitin-containing PM matrix. The IIM was not extracted from the PM by boiling in 2% SDS for 10 min unless a reducing agent was included (Fig. 2B), suggesting the presence of intermolecular disulfide bonding in native IIM.
Figure 1.
SDS/PAGE analysis of T. ni larval PM proteins with a 3.5% gel stained with Coomassie blue. IIM on the gel (lane 2) is indicated by the arrow.
IIM is Rich in Threonine, Proline, and Alanine, But Low in Aromatic Amino Acids.
Amino acid composition analysis of IIM (Table 1), indicated that IIM was rich in threonine (18.7%), proline (16.9%), and alanine (15.9%). These three amino acids accounted for 51.5% of the total amino acid residues in the protein, while aromatic amino acids accounted for less than 5% of the amino acid residues in the protein. The IIM amino acid composition profile resembles that of a typical vertebrate mucin that is commonly rich in threonine, serine, proline, alanine, and glycine, and rare in aromatic amino acids (28, 29).
Table 1.
Amino acid composition of IIM from T. ni larvae
| Residue | Mol, % |
|---|---|
| Ala | 15.9 |
| Arg | 1.5 |
| Asp + Asn | 8.1 |
| Gly | 4.5 |
| Glu + Gln | 9.3 |
| Cys | 2.5 |
| His | 2.5 |
| Ile | 2.4 |
| Leu | 3.8 |
| Lys | 0.8 |
| Met | 0.2 |
| Phe | 2.1 |
| Pro | 16.9 |
| Ser | 3.4 |
| Thr | 18.7 |
| Trp | 1.4 |
| Tyr | 1.4 |
| Val | 4.7 |
The values represent averages from three analyses.
IIM Is Highly Glycosylated and Has Both N- and O-Linked Glycosylation.
Quantification of the protein and carbohydrate content of IIM indicated that it was highly glycosylated. Carbohydrate content on IIM accounted for 56% of the total IIM mass, with protein accounting for 44%.
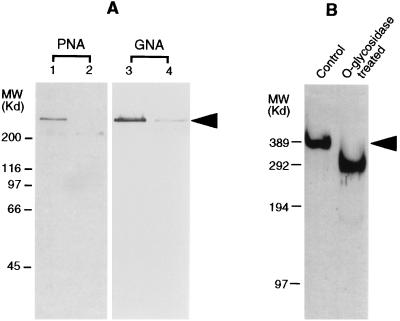
Terminal mannose residues and galactose β(1–3) N-acetylgalactosamine were detected on IIM by the specific binding of peanut agglutinin and Galanthus nivalis agglutinin (GNA) (Fig. 3A, lanes 1 and 3, respectively). The lectin binding assays using IIM samples pretreated with either O-glycosidase or N-glycosidase showed no binding or significantly reduced binding of the lectins (Fig. 3A, lanes 2 and 4), confirming the positive recognition of G. nivalis agglutinin and peanut agglutinin to IIM. These results demonstrated that IIM has both N-glycosylation and O-glycosylation, since terminal mannose is present in N-linked carbohydrate moieties and galactose β(1–3) N-acetylgalactosamine is one type of O-linked carbohydrate moiety found in glycoproteins. In addition, removal of the disaccharide, galactose β(1–3) N-acetylgalactosamine by O-glycosidase treatment, resulted in significant reduction (≈100 kDa) in the molecular weight of the IIM (Fig. 3B), further confirming the heavy O-glycosylation on IIM.
Figure 3.
Identification of carbohydrate structures on the IIM by lectin binding assays and glycosidase treatments. (A) Lectin binding analysis. IIM was electrophoresed by SDS/PAGE, transferred onto blotting membrane and assessed for terminal mannose and galactose β(1–3) N-acetylgalactosamine with G. nivali agglutinin (GNA) (lane 3) and peanut agglutinin (PNA) (lane 1), respectively. Lane 2 shows binding of PNA to IIM pretreated with O-glycosidase. Lane 4 shows binding of GNA to IIM pretreated with N-glycosidase F. (B) SDS/PAGE analysis of the IIM treated with O-glycosidase, showing an apparent molecular weight reduction from 400 to 300 kDa (left lane, control; right lane, treated) (silver-stained gel). The position of IIM in both A and B are indicated by an arrow.
O-Glycosylation Plays an Important Role in the Protease Resistance of IIM.
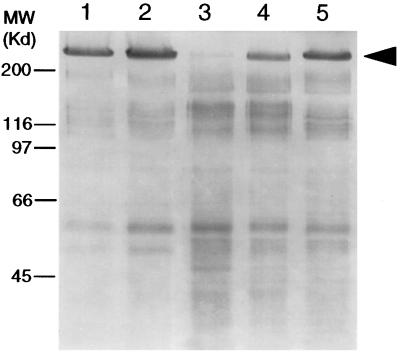
Experiments were conducted to demonstrate the highly protease-resistant nature of a mucin, the stability of IIM in digestive enzyme-rich PMs, and the contribution of the O-linked carbohydrates to the stability of IIM. The IIM was highly resistant to endogenous digestive proteases after a 4-h-long incubation (Fig. 4, lane 5). Even after a 16-h-long incubation, no degradation of IIM in PMs was observed (data not shown). However, in the presence of O-glycosidase, IIM was quickly degraded (Fig. 4, lane 3). Control treatments using PMs with inactivated (Fig. 4, lane 2) or inhibited (Fig. 4, lane 4) endogenous midgut proteases, confirmed that the degradation of IIM in the presence of O-glycosidase was a result of hydrolysis by endogenous digestive proteases, following removal of the protective carbohydrate moiety, galactose β(1–3) N-acetylgalactosamine.
Figure 4.
Silver-stained SDS/PAGE gel showing the role of O-glycosylation on IIM. Lanes: 1, PMs were pretreated by boiling in 2% SDS to inactivate the endogenous proteases, followed by extensive washing to remove inactivated proteins and residual SDS; the treated PMs were incubated with buffer only; 2, same as lane 1, except 15 milliunits/ml of O-glycosidase was included in the incubation buffer; 3, nonpretreated PMs incubated with 3 milliunits/ml of O-glycosidase; 4, same as lane 3, except protease inhibitors were included in the incubation buffer; 5, same as lane 3, except no O-glycosidase was present in the incubation buffer. The arrow indicates IIM.
TnGV Enhancin Degrades IIM.
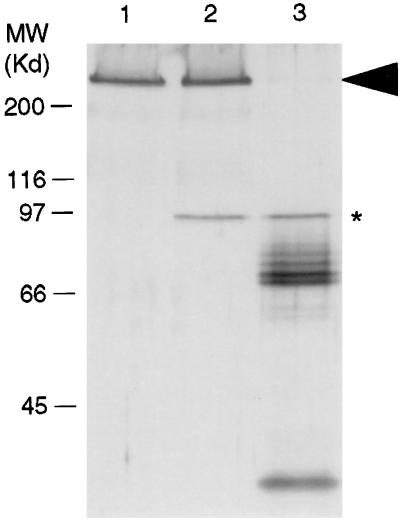
Incubation of IIM with TnGV enhancin showed that the enhancin had proteolytic activity against IIM (Fig. 5). The degradation products of IIM (Fig. 5, lane 3) displayed a banding pattern similar to that observed during incubation of intact PMs with enhancin (17). The addition of 10 mM EDTA to the incubation buffer blocked the digestion of the IIM and confirmed the metalloprotease nature of enhancin (Fig. 5, lane 2).
Figure 5.
SDS/PAGE analysis showing digestion of IIM with TnGV enhancin. Lanes: 1, IIM control; 2, IIM incubated with TnGV enhancin in the presence of the metalloprotease inhibitor, EDTA; 3, IIM incubated with TnGV in the absence of EDTA. IIM is indicated by an arrow. Enhancin is indicated with an asterisk. Multiple bands below the enhancin band are degradation products of IIM (silver stained gel).
TnGV Enhancin Degrades IIM in Living Insect Larvae.
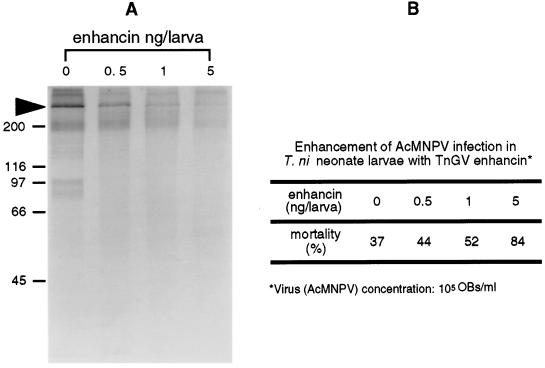
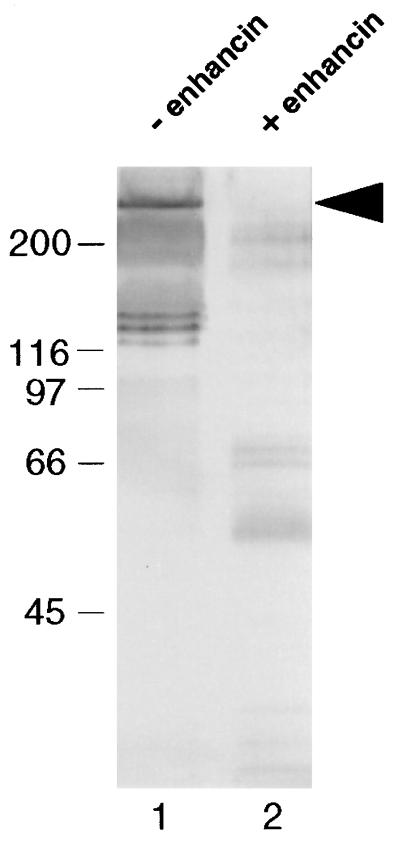
In vivo IIM degradation assays with T. ni neonate larvae demonstrated that enhancin degraded IIM in the midgut of living insects and that the degree of degradation appeared to be dose-dependent (Fig. 6A). In addition, the extent of degradation of IIM was correlated with increased AcMNPV infection in larvae (Fig. 6B). This enhanced mortality was statistically significant and can be presented by the regression analysis: probit mortality = 4.72 + 0.256 × enhancin dose (ng/larva) (R2 = 99.2; P = 0.004). The in vivo IIM-degradation assay using fifth instar larvae showed that IIM was present in the control fecal pellets and exhibited some minor degradation (Fig. 7, lane 1). However, no IIM was detected in the fecal pellets collected from the TnGV enhancin-fed larvae (Fig. 7, lane 2), confirming that enhancin completely degraded IIM in the digestive tract of living insects.
Figure 6.
(A) In vivo T. ni neonate IIM assay for degradation by TnGV enhancin. The degradation of IIM in the larval intestine by feeding increasing concentrations of enhancin was analyzed by Western blot analysis using anti-IIM antiserum. The arrow indicates IIM. (B) Result from a parallel neonate larval bioassay showing increased AcMNPV infection with increased enhancin concentration.
Figure 7.
In vivo IIM assay with fifth instar T. ni larvae showing degradation by TnGV enhancin. Lanes: 1, Western blot analysis of the fecal pellets from control insects; 2, Western blot analysis of the fecal pellets from insects that ingested TnGV enhancin. The arrow indicates undegraded IIM.
DISCUSSION
PMs have long been implicated as selective physical barriers in invertebrate intestines (7, 8). The primary components of PMs include chitin, protein and glycoprotein, but only one PM protein has been isolated and characterized thus far (15). PMs in invertebrates appear to be analogous to vertebrate intestinal mucus layers that are also secreted by epithelial cells. These vertebrate mucus secretions are composed primarily of one major constituent, intestinal mucin. Intestinal mucins from humans have been broadly studied, and the major human intestinal mucin (MUC2) was fully sequenced (1, 3, 29). To date, no intestinal mucin has been previously identified from invertebrates.
The IIM described from the T. ni PM resembles mammalian secretory mucins in several characteristics, including high O-glycosylation, possible intermolecular cross-linking disulfide bonds, high concentrations of threonine, alanine, and proline, and resistance to proteases. Selective removal of galactose β(1–3) N-acetylgalactosamine resulted in greatly increased susceptibility to proteolysis, indicating that this O-linked disaccharide appears to play an important role in protecting the IIM protein from degradation (Fig. 4). Unlike vertebrate mucins, insect PM proteins are embedded in a chitin fibril network. The inability to extract the IIM from PMs with various detergents and extreme conditions in the absence of a reducing agent suggest that IIM is tightly associated with the chitin-rich PM matrix and that disulfide bonding is seemingly important for this association.
Mucins from mammals and other vertebrates have been extensively studied (2, 3, 29–35). In contrast, knowledge on invertebrate mucins is very limited. There have been only a few mucin-like proteins described in invertebrates. Among them are the glue proteins from Drosophila (36), the mucin-like proteins from protozoans (37, 38), a secretory mucin from the nidamental gland of squid (39), a mucin-like protein from Drosophila cell culture (40), and a membrane associated mucin-like protein from hemocytes of Drosophila melanogaster (41). IIM is a new type among the invertebrate mucins and represents the first intestinal mucin identified from an invertebrate.
It has been reported that in insects PMs shield the midgut epithelial surfaces and can provide some level of protection from microbial invasion and infection (7, 8). The present study extends previous work on the degradation of PM proteins by a baculovirus enhancin (16, 17) by demonstrating that an intestinal mucin (IIM), the major PM protein in T. ni larvae, is the target substrate for the enhancin. The degradation of mucin leads to the disruption of this intestinal barrier and supports the proposed mode of action for enhancins (17). The presence of an IIM protein and its degradation by enhancin is not restricted to the species, T. ni. Another mucin, similar to the IIM from T. ni PMs, was also isolated from Pseudaletia unipuncta PMs and biochemically characterized (unpublished data). This mucin is also degraded by the TnGV enhancin and degradation was correlated with enhanced baculovirus infections in P. unipuncta larvae.
T. ni PMs are present in all larval instars and at all stages between molts (unpublished data). Therefore, IIM may play a protective role throughout the entire larval period. No mucin-degrading protease has been previously reported to be associated with a virus to assist the penetration of a pathogen through a mucinous protective barrier; therefore, this study represents a novel concept in animal virus pathogenesis. Further studies on the specific recognition sites and cleavage of mucins by baculovirus enhancins, and the biological properties of IIM and enhancins, will be necessary for a comprehensive understanding of the role of enhancins in the pathogenesis of virus infections.
Acknowledgments
We thank R. St. Leger for discussions and suggestions for the research, and H. A. Wood and K. A. McKenna for their critical review of the manuscript. This research was supported in part by grants from Biotechnology Research and Development Corp. (Peoria, IL) and the Cooperative State Research Service, U.S. Department of Agriculture, under Agreement No. 95-37302-1889.
ABBREVIATIONS
- PM
peritrophic membrane
- IIM
insect intestinal mucin
- TnGV
Trichoplusia ni granulosis virus
- AcMNPV
Autographa californica multiple nuclear polyhedrosis virus
References
- 1.Forstner J F, Forstner G G. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. Vol. 2. New York: Raven; 1994. pp. 1255–1283. [Google Scholar]
- 2.Strous G J, Dekker J. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 3.Forstner J F, Oliver M G, Sylvester F A. In: Infections of the Gastrointestinal Tract. Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. New York: Raven; 1995. pp. 71–88. [Google Scholar]
- 4.Drumm B, Roberton A M, Sherman P M. Infect Immun. 1988;56:2437–2442. doi: 10.1128/iai.56.9.2437-2442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantle M, Basaraba L, Peacock S C, Gall D G. Infect Immun. 1989;57:3292–3299. doi: 10.1128/iai.57.11.3292-3299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C C, Baylor M, Bass D M. Gastroenterology. 1993;105:84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 7.Spence K D. In: Physiology of the Insect Epidermis. Binnington K, Retnakaran A, editors. East Melbourne, Victoria, Australia: CSIRO Publications; 1991. pp. 77–93. [Google Scholar]
- 8.Peters W. Zoophysiology. Vol. 30. Berlin: Springer; 1992. [Google Scholar]
- 9.Huber M, Cabib E, Miller L H. Proc Natl Acad Sci USA. 1991;88:2807–2810. doi: 10.1073/pnas.88.7.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono M, Kato S. Bull Sericul Exp Stn. 1968;23:1–8. [Google Scholar]
- 11.Zimmermann U, Mehlan D, Peters W. Comp Biochem Physiol B. 1975;51:181–186. doi: 10.1016/0305-0491(73)90206-x. [DOI] [PubMed] [Google Scholar]
- 12.Adang M J, Spence K D. Comp Biochem Physiol A. 1982;75:645–649. [Google Scholar]
- 13.Stamm B, D’Haese J, Peter W. J Insect Physiol. 1978;24:1–8. [Google Scholar]
- 14.Rupp R A, Spence K D. Insect Biochem. 1985;15:147–154. [Google Scholar]
- 15.Elvin C M, Vuocolo T, Pearson R D, East I J, Riding G A, Eisemann C H, Tellam R L. J Biol Chem. 1996;271:8925–8935. doi: 10.1074/jbc.271.15.8925. [DOI] [PubMed] [Google Scholar]
- 16.Derksen A C G, Granados R R. Virology. 1988;167:242–250. doi: 10.1016/0042-6822(88)90074-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Hammer D A, Granados R R. J Gen Virol. 1994;66:541–550. doi: 10.1099/0022-1317-75-8-1961. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Corsaro B G, Granados R R. J Gen Virol. 1991;72:2645–2651. doi: 10.1099/0022-1317-72-11-2645. [DOI] [PubMed] [Google Scholar]
- 19.Lepore L S, Roelvink P R, Granados R R. J Invertebr Pathol. 1996;68:131–140. doi: 10.1006/jipa.1996.0070. [DOI] [PubMed] [Google Scholar]
- 20.Gallo L G, Corsaro B G, Hughes P R, Granados R R. J Invertebr Pathol. 1991;58:203–210. [Google Scholar]
- 21.Wood H A. Virology. 1980;102:21–27. doi: 10.1016/0042-6822(80)90066-5. [DOI] [PubMed] [Google Scholar]
- 22.Weber K, Osborn M. J Biol Chem. 1969;244:4406. [PubMed] [Google Scholar]
- 23.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lee C, Levin A, Branton D. Anal Biochem. 1987;166:308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- 25.Ozols J. Methods Enzymol. 1990;182:587–601. doi: 10.1016/0076-6879(90)82046-5. [DOI] [PubMed] [Google Scholar]
- 26.Calza R E, Irwin D C, Wilson D B. Biochemistry. 1985;24:7797–7804. [Google Scholar]
- 27.Adang M J, Spence K D. Cell Tissue Res. 1981;218:141–147. doi: 10.1007/BF00210100. [DOI] [PubMed] [Google Scholar]
- 28.Devine P L, McKenzie I F C. BioEssays. 1992;14:619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- 29.Gendler S J, Spicer A P. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 30.Carlstedt I, Sheehan J K, Corfield A P, Gallagher J T. Essays Biochem. 1985;20:40–139. [PubMed] [Google Scholar]
- 31.Verma M, Davidson E A. Glycoconjugate J. 1994;11:172–179. doi: 10.1007/BF00731215. [DOI] [PubMed] [Google Scholar]
- 32.Forstner G. Annu Rev Physiol. 1995;57:585–605. doi: 10.1146/annurev.ph.57.030195.003101. [DOI] [PubMed] [Google Scholar]
- 33.Bansil R, Stanley E, LaMont J T. Annu Rev Physiol. 1995;57:635–657. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 34.Tabak L A. Annu Rev Physiol. 1995;57:547–564. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 35.Lichtenberger L M. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 36.Garfinkel M D, Pruitt R E, Meyerowitz E M. J Mol Biol. 1983;168:765–789. doi: 10.1016/s0022-2836(83)80074-6. [DOI] [PubMed] [Google Scholar]
- 37.Murray P J, Spithill T W. J Biol Chem. 1991;266:24477–24484. [PubMed] [Google Scholar]
- 38.Di Noia J M, Sanchez D O, Frasch A C C. J Biol Chem. 1995;270:24146–24149. doi: 10.1074/jbc.270.41.24146. [DOI] [PubMed] [Google Scholar]
- 39.Kimura S, Sugiura Y, Mizuno H, Kato N, Hanaoka Y. Fish Sci. 1994;60:193–197. [Google Scholar]
- 40.Kramerov A A, Arbatsky N P, Rozovsky Y M, Mikhaleva E A, Polesskaya O O, Gvozdev V A, Shibaev V N. FEBS Lett. 1996;378:213–218. doi: 10.1016/0014-5793(95)01444-6. [DOI] [PubMed] [Google Scholar]
- 41.Theopold U, Samakovlis C, Erdjument-Bromage H, Dillon N, Axelsson B, Schmidt O, Tempst P, Hultmark D. J Biol Chem. 1996;271:12708–12715. doi: 10.1074/jbc.271.22.12708. [DOI] [PubMed] [Google Scholar]