Abstract
The membrane-distal headpiece of integrins has evolved to specifically bind large extracellular protein ligands, but the molecular architecture of the resulting complexes has not been determined. We used molecular electron microscopy to determine the three-dimensional structure of the ligand-binding headpiece of integrin α5β1 complexed with fragments of its physiological ligand fibronectin. The density map for the unliganded α5β1 headpiece shows a ‘closed’ conformation similar to that seen in the αVβ3 crystal structure. By contrast, binding to fibronectin induces an ‘open’ conformation with a dramatic, ∼80° change in the angle of the hybrid domain of the β subunit relative to its I-like domain. The fibronectin fragment binds to the interface between the β-propeller and I-like domains in the integrin headpiece through the RGD-containing module 10, but direct contact of the synergy-region-containing module 9 to integrin is not evident. This finding is corroborated by kinetic analysis of real-time binding data, which shows that the synergy site greatly enhances kon but has little effect on the stability or koff of the complex.
Keywords: conformational change/electron microscopy/fibronectin/integrin/single-particle analysis
Introduction
The integrin family of cell adhesion receptors comprises 24 distinct αβ heterodimers that recognize glycoprotein ligands in the extracellular matrix or on cell surfaces (Hynes, 2002). Integrins and their ligands play fundamental roles in all events that involve cell adhesion, detachment and migration, the hallmarks of multicellular organisms. Strict and dynamic control of the integrin’s affinity for ligand by cellular mechanisms is of crucial importance, since ligand binding results in integrin signaling with the potential of changing the cell’s fate. Aberrant interactions of integrins with their ligands have been implicated in many pathophysiological states. Therefore elucidation of the mechanisms for ligand recognition and signaling induced by ligand binding is key to the development of effective drugs against numerous disease processes including cancer metastasis, thrombosis and autoimmunity.
Many members of the integrin family, including α5β1, α8β1, αIIbβ3, αVβ3, αVβ5, αVβ6 and αVβ8, recognize an Arg–Gly–Asp (RGD) motif within their ligands. These ligands include fibronectin (Fn), fibrinogen, vitronectin, von Willebrand factor and many other large glycoproteins. Peptides containing this motif can efficiently block these integrin–ligand interactions. However, it is the residues outside the RGD motif that provide specificity as well as high affinity for each integrin–ligand pair. α5β1 integrin and Fn form a prototypic integrin–ligand pair (Hynes and Yamada, 1982; Pierschbacher and Ruoslahti, 1984). This receptor–ligand pair is functionally very important because it mediates fibronectin fibril formation and governs extracellular matrix assembly, which is vital to cell function in vivo (Cukierman et al., 2001). The interaction between α5β1 and Fn is fundamental for vertebrate development, since lack of α5β1 or Fn results in early embryonic lethality (George et al., 1993; Yang et al., 1993; Goh et al., 1997). In addition to the RGD sequence present in Fn type III module 10, a set of residues present in Fn type III module 9 (synergy site) contribute to high-affinity recognition by α5β1 (Obara et al., 1988; Aota et al., 1994; Mould et al., 1997, 2000; Redick et al., 2000; Altroff et al., 2003).
The crystal structure of the extracellular domain of αVβ3 integrin (Xiong et al., 2001) has established a basis to think about integrin function on the atomic level. The subsequent structure of αVβ3 in complex with a ligand-mimetic peptide (Xiong et al., 2002) provided a first glimpse as to how integrins recognize the RGD tripeptide motif, where Arg and Asp side chains bridge the integrin α and β subunits at the center of the ligand binding pocket. Recently, we have shown that binding to a small ligand-mimetic peptide converted the αVβ3 integrin extracellular domain from the compact ‘bent’ conformation seen in the crystal structure into a tall extended conformation, and that this conversion is essential for integrin activation (Takagi et al., 2002b). These studies utilized small peptides containing RGD; however, structural information using a protein ligand is needed in order to understand the contribution of residues outside the RGD–integrin interface to the specificity and stability of the physiological integrin–ligand complex, and to integrin conformation. Electron microscope images of integrins in complex with macromolecule ligands have previously been reported (Weisel et al., 1992; Du et al., 1993) but provide limited information due to both the flexible nature of the complex and the limitation of the rotary shadowing approach that prevents the determination of a three-dimensional (3D) reconstruction. Using a recombinant α5β1 headpiece fragment and Fn fragments, we present here for the first time 3D information on how an integrin globular head domain composed of both subunits binds its physiological protein ligand. Together with binding kinetics measurements, new insights emerge on the contribution of the synergy site in Fn to the binding to α5β1 integrin.
Results
Preparation and ligand binding activity of the α5β1 head fragment
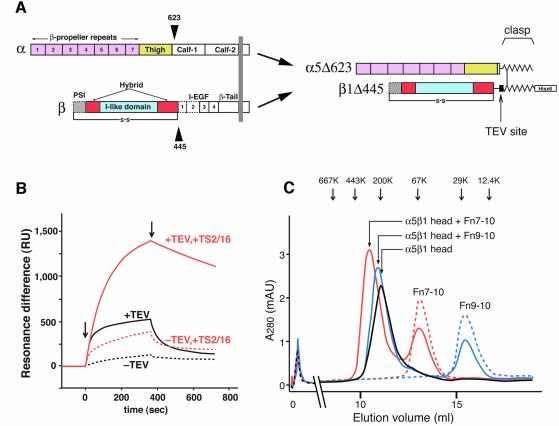
Our previous electron microscopic studies on αVβ3 (Takagi et al., 2002b) showed that the integrin stalk regions are highly flexible. Lack of clear electron density in the midregion of the β subunit in the crystal structure also indicated structural flexibility in this region (Xiong et al., 2001). In order to reduce the resulting structural variability of the integrin extracellular segment, we truncated both α5 and β1 subunits to obtain an integrin fragment consisting exclusively of the ligand-binding headpiece (Figure 1A).
Fig. 1. The truncated α5β1 headpiece retains its functional integrity. (A) The domain organization within the primary structure of integrin α5β1 is shown on the left, and the design of the recombinant soluble α5β1 headpiece is shown on the right. Disulfide-bonded α-helical coiled-coil domains were attached to the C-termini of the truncated subunits to act as a clasp (Takagi et al., 2001). Domains included in the headpiece fragment are color coded as follows: α5 β-propeller repeat domain in pink, α5 thigh domain in yellow, β1 PSI domain in gray, β1 I-like domain in cyan and β1 hybrid domain in red. Domains not resolved in the crystal structure are depicted by dotted lines, and the position of the long-range disulfide bond present in the β subunit is shown below. (B) Binding analysis of Fn9–10 to the α5β1 head fragment by surface plasmon resonance. Clasped (–TEV, dotted lines) or unclasped (+TEV, solid lines) α5β1 headpieces were preincubated with (red lines) or without (black lines) a 3-fold molar excess of TS2/16 Fab fragment for more than 10 min and infused at a concentration of 50 nM onto the sensor surface coated with 650 RU of Fn9–10. Arrows indicate start- and end-points of the injections. (C) Gel filtration chromatography of the α5β1 headpiece with bound ligands. The TEV-cleaved α5β1 headpiece (∼70 pmol) was incubated with 150 pmol of Fn9–10 (blue), Fn7–10 (red) or without ligands (black) in the presence of 1 mM Mn2+ for 1 h and separated on a Superdex 200 column. Chromatograms for 150 pmol Fn7–10 (red dotted) or Fn9–10 (blue dotted) alone are also shown. The elution positions of standard proteins are indicated by arrows (667 kDa, thyroglobulin; 443 kDa, apoferritin; 200 kDa, β-amylase; 67 kDa, serum albumin; 29 kDa, carbonic anhydrase; 12.4 kDa, cytochrome c).
The α5 subunit was truncated after the thigh domain and β1 after the hybrid domain, thus retaining Cys444 predicted to form a long-range disulfide bridge with Cys7 (Figure 1A). Acidic/basic α-helical coiled-coil peptides containing disulfide-bridge forming Cys residues were attached to the C-terminus of the subunits to stabilize the heterodimer with a clasp (Figure 1A) (Takagi et al., 2001). Furthermore, a hexahistidine tag was fused to the C-terminus of the basic peptide to facilitate purification, and a TEV cleavage site was introduced before the coiled-coil domain to enable release of the C-terminal clasp. Cotransfection of these truncated subunits into CHO lec cells resulted in production and secretion of the clasped α5β1 headpiece. A version without the clasp did not express well in transfected cells (data not shown). A similarly truncated α5β1 protein (α5Δ613/β1Δ455) has been reported (Coe et al., 2001), but a different C-terminal clasp was utilized in that construct.
In a buffer containing 1 mM Mn2+ the clasped α5β1 headpiece bound very weakly to a fibronectin fragment containing Fn type III modules 9 and 10 (Fn9–10) (Figure 1B, black dotted sensorgram). The binding increased marginally when the α5β1 headpiece was preincubated with a Fab fragment derived from the activating anti-β1 mAb TS2/16 (red dotted sensorgram). In contrast, ligand binding activity increased significantly when the C-terminal clasp was released by TEV protease cleavage (black solid line), and binding became maximal in the presence of the activating TS2/16 Fab fragment (red solid line). The dissociation constant of Fn9–10 from this maximally activated α5β1 headpiece was calculated from the sensorgrams as 1.68 ± 0.57 nM (n = 5). This value is comparable to that of recombinant soluble full-length α5β1 (1.54 ± 0.27 nM, n = 6), indicating that the α5β1 headpiece retained full ligand binding potential despite the absence of the tailpiece segments.
To form receptor–ligand complexes, unclasped α5β1 headpiece at 7 µM was mixed with 15 µM Fn7–10 or Fn9–10 fragments in a buffer containing 1 mM Mn2+. The complexes were sufficiently stable to be separated from free integrins and Fn fragments by gel filtration chromatography (Figure 1C). Binding between α5β1 headpiece and Fn fragments had 1:1 stoichiometry because the use of a 2:1 molar excess of Fn fragments resulted in nearly 50% depletion of the free Fn7–10 and Fn9–10 peaks (Figure 1C). The complex peaks showed no shoulders corresponding to free integrin, and their positions were consistent with the expected molecular size increases by the binding of Fn9–10 (20 kDa) and Fn7–10 (40 kDa), respectively. Therefore it is concluded that under this condition all the integrin molecules are saturated with bound ligand.
Closed α5β1 headpiece in the absence of ligand
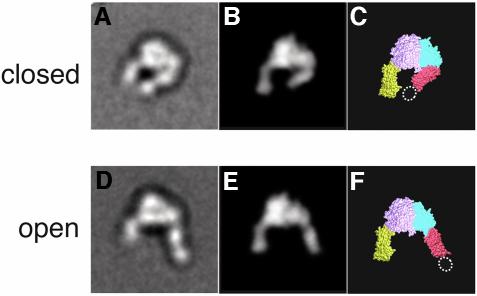
We first imaged the unliganded unclasped α5β1 headpiece by negative stain electron microscopy (EM). The majority of the projection averages (>90%) showed a very uniform conformation of the headpiece, both in Ca2+-containing buffer (Figure 2A) and Mn2+-containing buffer (not shown). The projection average of the α5β1 headpiece was remarkably similar to the crystal structure of the extracellular domain of αVβ3 (Xiong et al., 2001). Therefore we calculated a molecular envelope of the αVβ3 headpiece at 25 Å resolution from the atomic coordinates and calculated regularly spaced projections at 2° intervals. Cross-correlation of these αVβ3 projections with the α5β1 projection averages determined by negative stain EM indeed identified a very similar projection (Figure 3B) with a cross-correlation coefficient (CCC) of 1070. The average CCC for all projections was 870 ± 104 with the worst CCC being 726, indicating that the projection with the CCC of 1070 is indeed by far the best match with the EM projection average. The excellent correlation of the EM projection average with the X-ray structure projection enabled us to assign the densities in the EM map (Figure 3A) to individual integrin domains (Figure 3C). The globular head is asymmetric with a large density corresponding to the large β-propeller domain of the α subunit (Figure 3C, pink) and a smaller density corresponding to the I-like domain of the β subunit (Figure 3C, cyan). The α5 thigh domain (Figure 3C, yellow) and the β1 hybrid domain (Figure 3C, red) protrude from the longer axis of the globular head at an obtuse and an acute angle, respectively. Therefore the unliganded α5β1 headpiece assumes a conformation we refer to as ‘closed’, which was previously visualized by EM of αVβ3 containing the complete extracellular domain (Takagi et al., 2002b). The position of the β1 PSI domain, which is present in the α5β1 headpiece construct but is lacking in the αVβ3 crystal structure (Figure 3C, dotted oval), can be clearly identified as an extra density present on the tip of the β tail. The very high percentage of particles in the closed conformation (>90%) confirmed that this is the most stable conformation of the unliganded α5β1 headpiece, even in the absence of the tailpiece.
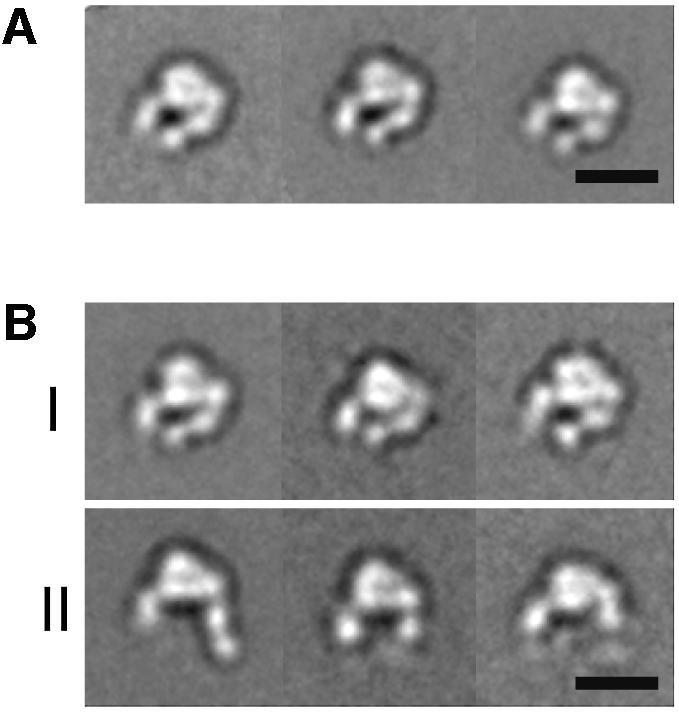
Fig. 2. Projection averages of the α5β1 headpiece obtained by negative stain EM. The unclasped α5β1 headpiece was incubated (A) without or (B) with 1 mM RGD peptide and imaged in the EM. In the +RGD condition, particles with closed (group I) and open (group II) conformations were observed. The three most typical averages for each group are shown, each containing 200–600 particles. Scale bars, 100 Å.
Fig. 3. Two different integrin headpiece conformations. A representative projection average from (A) unliganded and (D) liganded α5β1 headpiece was used to identify the best-correlating projections calculated from a 25 Å density map created from αVβ3 headpiece models (B and E). The model for the open αVβ3 headpiece was prepared as described in the text and Materials and methods section. The models are shown in CPK representation (C and F) using the same color code as in Figure 1A.
RGD binding opens the α5β1 headpiece
The same preparation of the unclasped α5β1 headpiece was incubated with 1 mM GRGDNP peptide in 1 mM Ca2+ and also imaged by negative stain EM. Although the majority of the particles still showed a closed conformation identical to that in the absence of ligand (Figure 2B, group I), about a quarter of the particles changed their shape (Figure 2B, group II). In these particles, the β hybrid domain moved away from the α5 subunit, so that the two tails were more widely separated than in the closed conformation. In these ‘open’ conformations, the PSI domain was visualized in some of the averages but disappeared in others, suggesting that the hybrid–PSI connection is mobile. Conversion to the open conformation in the RGD-treated integrin headpiece is consistent with the idea that the ‘hybrid domain swing’ is conformationally linked to ligand binding (Takagi et al., 2002b). We created a model for the αVβ3 headpiece in the open conformation (see Materials and methods). When we cross-correlated the projections from this model with the EM average (Figure 3D), we again identified a very similar projection view (Figure 3E) with a CCC of 1077, whereas the worst correlating projection yielded a CCC of 538 (with an average of 800 ± 130). It is not clear why only about a quarter of the molecules changed their shape upon RGD binding, since this peptide efficiently blocked Fn binding by full-length α5β1 in BIAcore assays (data not shown). Complete inhibition was achieved at 1 µM (IC50 ≈ 300 nM) so that 1 mM RGD should have saturated all the binding sites. Therefore the association of linear RGDNP peptide to α5β1 may not be sufficient to completely shift the conformational equilibrium of the headpiece fragment toward the open conformation.
Projection averages of Fn fragment and its complex with α5β1
Despite its very small size (∼40 kDa), projection averages of the negatively stained four-domain fibronectin fragment Fn7–10 showed a distinct shape with individual FnIII domains being clearly resolved (Figure 4A). The majority of the Fn7–10 fragments adopted a loosely extended conformation, but the interdomain angles seemed to be very flexible as illustrated by the presence of ‘folded’ particles (Figure 4A). Flexibility is consistent with the intermodule flexibility suggested by NMR studies (Copie et al., 1998) and the small interfaces between individual modules in the crystal structure, which were suggested to give rise to intermodule mobility, especially at the interface between modules 9 and 10 (Leahy et al., 1996). Next, we imaged a complex of the α5β1 headpiece and the Fn7–10 fragment formed in the presence of 1 mM Mn2+. About 80% of the particles had no ligand bound and showed the closed conformation identical to the one in the absence of ligands (data not shown). This is expected considering the fact that we purified the complex from the free Fn fragments and that the complex has a relatively fast dissociation rate in the absence of activating mAb (Figure 1B, black solid tracing). On the other hand, the remaining 20% had a three-legged appearance, with the C-termini of α and β chains and the Fn7–10 fragment each forming one of the three legs (Figure 4B).
Fig. 4. α5β1 head in complex with Fn fragment. Projection averages are shown for (A) Fn7–10 alone, (B) α5β1 complex with Fn7–10 and (C) α5β1 complex with Fn9–10. The projection averages of the α5β1/Fn7–10 complexes are subgrouped (groups I, II and III) based on the orientation of the bound ligand, and several representative raw images are shown below (raw). As in Figure 2, the three most populated averages for each group are shown. The assignment of the FnIII modules (black ovals) in the complex is shown in the schematic drawing to the right. The modules missing in the averages due to linker flexibility are depicted by gray ovals. Scale bars, 100 Å.
The projection structures of the α5β1 headpieces complexed with Fn7–10 provide several important insights into the ligand-recognition mechanism of α5β1 integrin. Firstly, Fn fragment bound to the globular head at its ‘top’ face, which is composed of blades 2 and 3 of the α-subunit β-propeller domain and the face of the β-subunit I-like domain containing the metal-ion-dependent adhesion site (MIDAS) and a ‘specificity-determining loop’ (Takagi et al., 2002a). Critical involvement of these segments has been suggested by many mutational and antibody mapping studies (for review see Humphries, 2000), and is now directly confirmed by our structure. Secondly, all of the α5β1 headpieces with a bound Fn fragment assumed the open conformation, with a nearly 80° swing-out of the β tail compared with the closed conformation. This unambiguously proves that there is a direct link between ligand binding and the opening of the angle between the I-like domain and the hybrid domain. It also explains why the presence of a C-terminal clasp, which cross-links the tips of the α and β tails and thus hinders the opening of the tails, has to be removed for the α5β1 headpiece fragment to gain full activity (Figure 1B). Thirdly, the α5 β-propeller and the β1 I-like domain, which together form the ligand-binding globular head, stay associated together upon ligand binding. This finding argues against the hypothesis that integrin activation/ligand binding is accompanied by a substantial separation of the headpieces of the two subunits (Hantgan et al., 1999; Liddington and Ginsberg, 2002). Lastly and most importantly, the binding of the Fn7–10 fragment to the integrin headpiece was restricted to a single contact point at the end of the Fn7–10 fragment. The remaining segments of the molecule showed no defined orientation with respect to the integrin headpiece and radiated out from it in many different directions (Figure 4B, compare groups I, II and III). Because of flexibility between adjacent FnIII modules, the most distal FnIII module tended to be averaged out in our projection averages. However, this module was present in the raw images (Figure 4B, raw), indicating that the Fn module in contact with the integrin was the 10th. This allowed us to assign the positions of the four modules in the averages using the size of a single Fn module as a guide (Figure 4A and B, right). The contact site of Fn module 10 with the integrin headpiece is located at the α/β interface, slightly shifted toward the I-like domain of the β subunit. The adjacent FnIII module 9 is positioned very close to the α5 subunit, but in most averages the two protein domains do not seem to make contact. Group I in Figure 4B, representing ∼28% of the α5β1-Fn7–10 complexes, appears to have a relatively large contact area, which may include interactions of FnIII module 9 with the integrin headpiece. However, in groups II and III, representing 58% and 14% of the complexes, respectively, only FnIII module 10 appears to interact with the integrin headpiece.
When a two-domain fragment of fibronectin (Fn9–10) was used to form a complex with the α5β1 headpiece, we could only see density corresponding to FnIII module 10 in the resulting averages (Figure 4C; compare with Figure 2B, group II). The adjacent module 9 was averaged out most likely due to a combined effect of its small size (compared with the remainder of the molecule) and the flexibility between modules 9 and 10. The disappearance of module 9 in the projection averages corroborates the lack of a strong interaction between the synergy site in module 9 and the integrin headpiece. All the particles that had the extra density corresponding to the bound ligand assumed the open conformation, indicating that the two-domain Fn9–10 fragment had the same effect on the integrin conformation as the four-domain Fn7–10 fragment.
Three-dimensional reconstruction
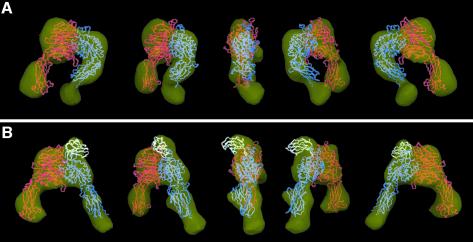
We used the random conical tilt approach (Radermacher et al., 1987) to calculate a 3D reconstruction of the negatively stained α5β1 headpiece and its complex with Fn9–10 (Figure 5A and B). With a size of 170 kDa, the integrin headpiece is among the smallest molecules so far for which a structure has been determined by single-particle EM.
Fig. 5. Surface-rendered density maps of the α5β1 headpiece in (A) the unliganded closed and (B) the ligand-bound open conformation. The unmodified headpiece segments of the αVβ3 crystal structure or the open αVβ3 model were manually fitted into the 3D density map of the unliganded and ligand-bound α5β1 headpiece, respectively. Cα worm tracings for αV, β3 and Fn10 segments are colored in red, blue and white, respectively. Views are successive 45° rotations about the vertical figure axis. The figure was generated using DINO (Philippsen, 2002).
The resulting density map of the unliganded headpiece revealed all the expected integrin domains and allowed for a good fit with the segment of the αVβ3 crystal structure that corresponds to the headpiece (Figure 5A). However, the molecular envelope determined by EM for the unliganded α5β1 headpiece appears to have a slightly wider opening angle between the α5 thigh domain and the β1 hybrid domain than in the αVβ3 crystal structure. This might be due to structural differences between the two integrins or it may reflect a slightly more relaxed conformation of the closed integrin headpiece conformation in the absence of crystal packing constraints. The extra density at the tip of the β tail in our 3D map corresponds well to the expected location of the PSI domain, which is present in our α5β1 headpiece but is absent in the αVβ3 crystal structure.
In the 3D map of the α5β1 headpiece in complex with Fn9–10, the orientation of the β subunit hybrid and PSI domains was very different compared with the unliganded headpiece (Figure 5B). To adjust the atomic model of the closed αVβ3 headpiece to our EM map of the liganded headpiece, the hybrid domain had to be rotated as a rigid body away from the remainder of the molecule by an angle of about 80°. The resulting model for the open conformation of the αVβ3 headpiece fits very well into the 3D map (Figure 5B), with the exception of an extra density at the top of the α5β1 headpiece that represented the bound ligand. The size of this extra density was sufficient to accommodate an FnIII module, consistent with our fit of the Fn10 module to this position (Figure 5B, white Cα tracing). The oblong Fn10 module was docked into the extra density present in the α5β1-Fn9–10 complex using the C- to N-terminal orientation derived from the images of the bound Fn7–10 fragment. In this docking model the expected ligand-coordinating metal in the β subunit’s MIDAS motif is about 10 Å away from the Asp residue in the RGD loop. Given the ∼25 Å resolution of our EM map and the flexibility of the RGD loop seen in NMR structures, revealing a pairwise RMS deviation of 7 Å for the side-chain Oδ atoms of the Asp (Copie et al., 1998), our fit is compatible with direct coordination of the oxygen in the Asp side chain to the metal ion in the MIDAS motif.
The synergy site in FnIII module 9 has been hypothesized to be a secondary interaction site for α5β1, directly binding to the β-propeller domain of the α5 subunit (Mould et al., 1997; Redick et al., 2000). This hypothesis was based on the observations that a mutant Fn fragment lacking an intact synergy site showed greatly reduced affinity to α5β1 compared with the wild-type fragment. However, a great reduction of total affinity does not necessarily imply a direct involvement of the mutated residue in the binding interface. The lack of an obvious interaction between the FnIII module 9 and the α5β1 headpiece in our projection and 3D maps led us to reconsider the two-site hypothesis.
Kinetic analysis of α5β1 binding to both wild-type and mutant Fn7–10
To determine the effect of the synergy site mutation on the kinetic behavior of Fn binding to α5β1, we performed real-time surface plasmon resonance binding assays. Wild-type and two different synergy site mutant Fn7–10 fragments were immobilized at the same density on a sensorchip via C-terminally tagged biotin (Takagi et al., 2001). One mutant (R1379A) has a single Ala mutation in the synergy site and shows mildly reduced cell adhesion activity, whereas the other mutant (R1374A/P1376A/R1379A, abbreviated as RPR/AAA) has two more Ala mutations that abolished the synergy effect of the ninth repeat on the adhesion activity (Redick et al., 2000). Binding of injected soluble α5β1 was monitored in the presence of 1 mM Mn2+. Comparison of the concentration-dependent binding of wild-type and mutant ligands revealed an 11- and 45-fold decrease in the affinity of the R1379A and RPR/AAA mutants, respectively (Figure 6 and Table I). However, when we calculated the kinetic parameters for the binding, it became clear that the mutation primarily affected the association phase (5- and 26-fold decrease of kon for R1379A and RPR/AAA, respectively), whereas the dissociation phase was only slightly affected (∼2-fold increase in koff for both mutants). In general, mutations in residues that are part of protein–protein interfaces affect mostly the dissociation rate with minimal effects on the association rate (Cunningham and Wells, 1993; Clackson et al., 1998; Wu et al., 2002). The reason for this is that the association phase in most protein–protein interactions is diffusion controlled (Northrup and Erickson, 1992) and, analogous to the protein-folding process (Fersht, 1997), there are many pathways that lead to an initial encounter between two components. The loss of Arg1379 cost only 0.4 kcal/mol for the stability of the complex while decreasing the rate of complex formation by a factor of 5 (0.96 kcal/mol). Moreover, the additional loss of Pro1376 and Arg1374 had no effect on the stability of the complex but resulted in an additional 5-fold reduction in the association rate. This strongly suggests that the synergy site exerts its positive effect on α5β1 binding by facilitating the initial encounter, rather than by contributing to the protein–protein interaction surface.
Fig. 6. Comparison of the kinetics of α5β1 binding to recombinant Fn7–10 fragment. Either wild-type, R1379A or RPR/AAA synergy mutant Fn7–10 fragments were immobilized on the sensor chip at the same density (500 RU), and full-length α5β1 was injected at 20 µl/min. Traces show increasing concentrations (7.5, 15, 30 and 60 nM) of α5β1 analyte.
Table I. Kinetic parameters for α5β1 binding to various Fn7–10 fragments.
| Fn7–10 ligand | kon (× 105 M–1 s–1) | koff (× 10–3 s–1) | KD (nM) |
|---|---|---|---|
| Wild type | 14.9 ± 4.9 | 6.5 ± 0.1 | 4.4 |
| R1379A | 2.8 ± 0.8 | 14.0 ± 1.4 | 50 |
| RPR/AAA | 0.58 ± 0.16 | 11.3 ± 2.0 | 195 |
Surface plasmon resonance binding was performed as described in Materials and methods and the kinetic parameters were derived from the sensorgrams shown in Figure 6. Values are mean ± SE obtained from four different concentrations.
Discussion
Here, a 3D reconstruction by single-particle EM yields the first 3D picture of a heterodimeric integrin headpiece in complex with a physiological protein ligand. Visualization of the protein ligand bound to the ‘top’ face of the integrin headpiece confirms the location of the ligand-binding site suggested by many structural, mutational and antibody mapping studies. The large conformational change associated with ligand binding unambiguously establishes the central role of the hybrid/I-like domain angle change in affinity regulation. The magnitude of the interdomain conformational change seen here is extraordinary; the hybrid domain swings out by about 80° and moves the tip of the PSI domain some 70 Å away from the α subunit.
A resting integrin on the cell surface assumes a bent conformation unfavorable for the recognition and binding of large macromolecular ligands (Takagi et al., 2002b). When integrins are activated by either cellular activation mechanisms or artificial extracellular reagents, a straightening of the receptor molecule is observed (Takagi and Springer, 2002). However, the global unbending of the receptor per se is not likely to be responsible for the increased affinity, since the release of a C-terminal clasp (and hence the acquisition of freedom of the hybrid domain to swing) in the headpiece fragment used here increases ligand affinity even in the complete absence of a tailpiece. Therefore the swing of the hybrid domain must directly affect the conformation of the ligand-binding site on the top of the molecule. However, in the context of a full-length integrin, the same movement would also induce the straightening of the bent receptor via disruption of headpiece–tailpiece interactions. Thus the hybrid domain acts as a mechanical device that couples the conformational state of the ligand-binding site to the overall conformation of the receptor. For cell surface integrins, acquisition of high affinity by a local conformational change and better ligand accessibility by receptor extension may both be important to ensure efficient cell adhesion.
Our EM observations and kinetic measurements of the α5β1–Fn interactions do not support the two-site binding model, which proposes that the RGD loop and the synergy site latch simultaneously onto widely separated binding pockets on the β1 and α5 subunits, respectively. Instead, our findings suggest that FnIII module 9 supports integrin binding in an indirect fashion, although its contribution to the overall affinity is substantial. Recent studies showed that the synergistic activity of FnIII module 9 correlates with its effect on the stability of the Fn9–10 fragment (Grant et al., 1997; Altroff et al., 2001, 2003). In one study it was shown that the mutation of a residue located on the opposite face from the synergy site in Fn9, and hence not able to contribute directly to integrin binding, canceled the deleterious effect of a synergy site mutation (Altroff et al., 2003). Since the Fn9–Fn10 intermodule linkage and the RGD loop are highly flexible in solution (Baron et al., 1992; Copie et al., 1998), the role of the synergy region in module 9 may therefore lie in orienting the RGD loop in a way that leads to optimal exposure of the RGD sequence for binding to an integrin receptor.
Another possible explanation for the synergy effect of module 9 is long-range electrostatic steering. A model of the α5β1 integrin headpiece created on the basis of the crystal structure of αVβ3 revealed that there is an extensive stretch of acidic surface on the top of the α/β interface, where the ligand-binding site is located. Aside from Asp1495, which is part of the RGD sequence, the Fn9–10 segment contains several conserved acidic residues in the vicinity of the RGD loop. The association rate of wild-type Fn7–10 to α5β1 falls in the range that is typical for diffusion-limited interactions between proteins (0.5–5 × 106 M–1s–1) (Northrup and Erickson, 1992), whereas those of the synergy site mutants are below this range. Taken together, these observations suggest that the association of mutant Fn7–10 to α5β1 is hampered by electrostatic repulsion, which is neutralized by basic residues contributed by the synergy site in wild-type Fn7–10. The additive effect of R1374A and R1379A mutations on the koff of Fn7–10 supports this idea. Further support for this hypothesis is provided by the finding that mutating basic residues outside the classical synergy region also severely perturbed integrin binding (Redick et al., 2000). Structural and mutational studies on protein recognition have shown that residues outside the interaction site can make an important contribution to affinity by affecting the on-rate (Clackson and Wells, 1995). Our studies suggest that the synergy site may contribute in an analogous manner, although we cannot completely rule out a direct interaction. It is likely that the synergy site mediates transient and weak long-range interactions between Fn and the α5 subunit that do not result in a tight association that can persist during the adsorption/staining procedure of EM sample preparation. Determination of a high-resolution crystal structure of an α5β1–Fn complex would help to resolve the remaining ambiguities concerning the contribution of individual domains/residues in complex formation.
A series of studies have shown that residues in the α5 subunit contribute directly to the binding of Fn (Mould et al., 1997; Humphries et al., 2000). Our EM structures are consistent with these studies, because module 10 interacts with the interface between the α and the β subunit. Some of the residues recognized by anti-α5 blocking mAb seem to be located outside the α/β interface (Burrows et al., 1999), but the binding of a large IgG molecule may occlude Fn approach, especially if module 10 has an N-terminal extension (i.e. module 9).
The present study unequivocally establishes that hybrid domain swing-out is directly linked with ligand binding. Since the I-like domain is connected through both its N- and C-termini to the hybrid domain, widening of the angle between the I-like and hybrid domains is predicted to be tightly linked to a downward displacement of the C-terminal α-helix of the I-like domain, based on the overall topology of the interdomain connections (Takagi and Springer, 2002). Ligand docking and the subsequent direct coordination of an acidic residue in the ligand (e.g. Asp1495 of the RGD motif of fibronectin) to the MIDAS metal ion would induce this helix movement via a rearrangement of MIDAS coordinating residues, analogous to the shape-shifting pathway in α I domains (Shimaoka et al., 2003). Hybrid domain swing-out at the headpiece induced by ligand binding would in turn trigger a global conformational change and ultimately change the conformation of the cytoplasmic tails that link integrins to the cytoskeleton, leading to cellular signaling. Therefore in our study we have captured the first step of the integrin outside-in signaling pathway.
Materials and methods
Production of soluble recombinant β5β1 headpiece
Soluble truncated α5 (α5Δ623-AHCys) and β1 (β1Δ445-tev-BHCys) constructs were prepared from wild-type human α5 and β1 cDNAs by overlap extension PCR using the same design as for soluble clasped α5β1 (Takagi et al., 2001), except that a hexahistidine tag was fused to the C-terminus of β1. The soluble integrin headpiece was purified from culture supernatant of stably transfected CHO lec 3.2.8.1 cells as described previously, and stored at 1–2 mg/ml in TBS (50 mM Tris–HCl pH 7.5, 150 mM NaCl) containing 1 mM CaCl2 and 1 mM MgCl2 at 4°C. Release of the C-terminal clasp was achieved by incubation with 250 U/ml TEV protease (Invitrogen) at 25°C for 16 h. Complete cleavage was confirmed by the conversion of the single ∼150 kDa band (corresponding to the covalent dimer) to an 85 and a 55 kDa band (corresponding to truncated α5 and β1 monomer, respectively) on non-reducing SDS–PAGE. Unclasped α5β1, or its complex with ligand, was purified on a Superdex 200 HR column and the peak fraction was kept on ice for less than 30 min before the preparation of EM samples.
Preparation of Fn fragments
Recombinant FnIII fragments were produced using a bacterial expression system as described previously (Takagi et al., 2001). Wild-type, R1379A or R1374A/P1376A/R1379A (RPR/AAA) versions of the Fn7–10 construct (gifts from Harold P.Erickson) (Redick et al., 2000) were modified to contain a C-terminal cysteine, which was used for the biotinylation and subsequent immobilization onto streptavidin-sensor chips (Takagi et al., 2001). Fn7–10 and Fn9–10 with unmodified C-termini were prepared as described (Leahy et al., 1994).
Electron microscopy and image processing
Samples were adsorbed to glow-discharged carbon-coated copper grids, washed with two drops of deionized water and stained with two drops of freshly prepared 0.75% uranyl formate. Grids were inspected with a Philips Tecnai 12 electron microscope operated at 120 kV, and images were recorded at a nominal magnification of 52 000× using low-dose procedures. Images were digitized with a Zeiss SCAI scanner using a step size of 7 µm and 3 × 3 pixels were averaged to yield a final pixel size of 4 Å at the specimen level. The SPIDER image processing package (Frank et al., 1996) was used for particle selection and for all image processing steps. For the α5β1 headpiece in Ca2+ 9179 particles were selected from 26 micrographs, and for the headpiece in complex with the RGD peptide 8872 particles were selected from 42 micrographs. For the headpiece in complex with the Fn7–10 fragment we only selected particles which clearly had a ligand bound (4292 particles from 69 images). In all cases particles were classified and grouped into 50 classes using the multireference alignment procedure, and the particles in each class were averaged to produce class averages. The model for the open αVβ3 headpiece was created using the corresponding segment of the liganded αVβ3 (1LG5) (Xiong et al., 2002) by rotating the hybrid domain as a rigid body away from the remainder of the molecule by an angle of about 80°. For comparison with the crystal structures of αVβ3, the models were resolution filtered to 25 Å and projections calculated at an angular interval of 2° were cross-correlated with selected class averages. The reprojections with the highest correlation coefficient are shown in Figure 3B and E. The 3D reconstructions of the unliganded and the Fn9–10-liganded headpiece were calculated using the random conical tilt approach. Then, 9555 particle pairs were selected from 25 pairs of 60° and 0° tilted images. The particles selected from the untilted images were classified into 10 groups, and the largest classes representing the unliganded headpiece (2672 particles) and the Fn9–10-liganded headpiece (1005 particles) were used to calculate 3D reconstructions. The corresponding particles from the tilted images were used to calculate an initial 3D map using weighted backprojection (Radermacher, 1992). The model was refined by iterative projection matching of the original images with reprojections from the 3D map at increasingly finer angular intervals. Finally, 500 untilted images for the unliganded headpiece and 1300 images for the Fn9–10-liganded headpiece were included in the dataset. Fourier shell correlation (FSC) using the FSC > 0.5 criterion indicated a resolution of 20 Å for the unliganded headpiece and 24 Å for the Fn9–10-liganded headpiece. The density maps shown in Figure 3 were both resolution filtered to 24 Å.
Surface plasmon resonance
Experiments were done using the BIAcore 3000 (Biacore AB). Dilutions of α5β1 headpiece fragment (clasped or unclasped) in TBS containing 1 mM MnCl2 were injected at 20 µl/min into flow cells containing 500–800 RU of Fn fragment. All measurements were baseline corrected by subtracting the sensorgram obtained with control streptavidin surface and kinetic parameters were determined by fitting the data to a 1:1 Langmuir binding model using BIAevaluation software ver3.0.
Acknowledgments
Acknowledgements
We thank H.P.Erickson for providing the mutant Fn constructs. This work was supported by NIH grant HL48675 (to T.A.S. and J.T.). The molecular EM facility at Harvard Medical School was established by a generous donation from the Giovanni Armenise Harvard Center for Structural Biology and is maintained by funds from NIH grant GM62580.
References
- Altroff H., van Der Walle,C.F., Asselin,J., Fairless,R., Campbell,I.D. and Mardon,H.J. (2001) The eighth FIII domain of human fibronectin promotes integrin α5β1 binding via stabilization of the ninth FIII domain. J. Biol. Chem., 276, 38885–38892. [DOI] [PubMed] [Google Scholar]
- Altroff H., Choulier,L. and Mardon,H.J. (2003) Synergistic activity of the ninth and tenth FIII domains of human fibronectin depends upon structural stability. J. Biol. Chem., 278, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aota S., Nomizu,M. and Yamada,K.M. (1994) The short amino acid sequence Pro–His–Ser–Arg–Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem., 269, 24756–24761. [PubMed] [Google Scholar]
- Baron M., Main,A.L., Driscoll,P.C., Mardon,H.J., Boyd,J. and Campbell,I.D. (1992) 1H NMR assignment and secondary structure of the cell adhesion type III module of fibronectin. Biochemistry, 31, 2068–2073. [DOI] [PubMed] [Google Scholar]
- Burrows L., Clark,K., Mould,A.P. and Humphries,M.J. (1999) Fine mapping of inhibitory anti-α 5 monoclonal antibody epitopes that differentially affect integrin–ligand binding. Biochem. J., 344, 527–533. [PMC free article] [PubMed] [Google Scholar]
- Clackson T. and Wells,J.A. (1995) A hot spot of binding energy in a hormone-receptor interface Science, 267, 383–386. [DOI] [PubMed] [Google Scholar]
- Clackson T., Ultsch,M.H., Wells,J.A. and de Vos,A.M. (1998) Structural and functional analysis of the 1:1 growth hormone:receptor complex reveals the molecular basis for receptor affinity. J. Mol. Biol., 277, 1111–1128. [DOI] [PubMed] [Google Scholar]
- Coe A.P., Askari,J.A., Kline,A.D., Robinson,M.K., Kirby,H., Stephens,P.E. and Humphries,M.J. (2001) Generation of a minimal α5β1 integrin-Fc fragment. J. Biol. Chem., 276, 35854–35866. [DOI] [PubMed] [Google Scholar]
- Copie V., Tomita,Y., Akiyama,S.K., Aota,S., Yamada,K.M., Venable,R.M., Pastor,R.W., Krueger,S. and Torchia,D.A. (1998) Solution structure and dynamics of linked cell attachment modules of mouse fibronectin containing the RGD and synergy regions: comparison with the human fibronectin crystal structure. J. Mol. Biol., 277, 663–682. [DOI] [PubMed] [Google Scholar]
- Cukierman E., Pankov,R., Stevens,D.R. and Yamada,K.M. (2001) Taking cell-matrix adhesions to the third dimension. Science, 294, 1708–1712. [DOI] [PubMed] [Google Scholar]
- Cunningham B.C. and Wells,J.A. (1993) Comparison of a structural and a functional epitope. J. Mol. Biol., 234, 554–563. [DOI] [PubMed] [Google Scholar]
- Du X., Gu,M., Weisel,J.W., Nagaswami,C., Bennett,J.S., Bowditch,R. and Ginsberg,M.H. (1993) Long range propagation of conformational changes in integrin αIIbβ3. J. Biol. Chem., 268, 23087–23092. [PubMed] [Google Scholar]
- Fersht A.R. (1997) Nucleation mechanisms in protein folding. Curr. Opin. Struct. Biol., 7, 3–9. [DOI] [PubMed] [Google Scholar]
- Frank J., Radermacher,M., Penczek,P., Zhu,J., Li,Y., Ladjadj,M. and Leith,A. (1996) SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol., 116, 190–199. [DOI] [PubMed] [Google Scholar]
- George E.L., Georges-Labouesse,E.N., Patel-King,R.S., Rayburn,H. and Hynes,R.O. (1993) Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development, 119, 1079–1091. [DOI] [PubMed] [Google Scholar]
- Goh K.L., Yang,J.T. and Hynes,R.O. (1997) Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development, 124, 4309–4319. [DOI] [PubMed] [Google Scholar]
- Grant R.P., Spitzfaden,C., Altroff,H., Campbell,I.D. and Mardon,H.J. (1997) Structural requirements for biological activity of the ninth and tenth FIII domains of human fibronectin. J. Biol. Chem., 272, 6159–6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan R.R., Paumi,C., Rocco,M. and Weisel,J.W. (1999) Effects of ligand-mimetic peptides Arg–Gly–Asp–X (X = Phe, Trp, Ser) on alphaIIbbeta3 integrin conformation and oligomerization. Biochemistry, 38, 14461–14474. [DOI] [PubMed] [Google Scholar]
- Humphries J.D., Askari,J.A., Zhang,X.P., Takada,Y., Humphries,M.J. and Mould,A.P. (2000) Molecular basis of ligand recognition by integrin α5β1. II. Specificity of Arg–Gly–Asp binding is determined by Trp157 of the alpha subunit. J. Biol. Chem., 275, 20337–20345. [DOI] [PubMed] [Google Scholar]
- Humphries M.J. (2000) Integrin structure. Biochem. Soc. Trans, 28, 311–339. [PubMed] [Google Scholar]
- Hynes R.O. (2002) Integrins: bi-directional, allosteric, signalling machines. Cell, 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. and Yamada,K.M. (1982) Fibronectins:multifunctional modular glycoproteins. J. Cell Biol., 95, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy D.J., Erickson,H.P., Aukhil,I., Joshi,P. and Hendrickson,W.A. (1994) Crystallization of a fragment of human fibronectin: introduction of methionine by site-directed mutagenesis to allow phasing via selenomethionine. Proteins, 19, 48–54. [DOI] [PubMed] [Google Scholar]
- Leahy D.J., Aukhil,I. and Erickson,H.P. (1996) 2.0 angstrom crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell, 84, 155–164. [DOI] [PubMed] [Google Scholar]
- Liddington R.C. and Ginsberg,M.H. (2002) Integrin activation takes shape. J. Cell Biol., 158, 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A.P., Askari,J.A., Aota,S., Yamada,K.M., Irie,A., Takada,Y., Mardon,H.J. and Humphries,M.J. (1997) Defining the topology of integrin α5β1–fibronectin interactions using inhibitory anti-α5 and anti-β1 monoclonal antibodies. J. Biol. Chem., 272, 17283–17292. [DOI] [PubMed] [Google Scholar]
- Mould A.P., Askari,J.A. and Humphries,M.J. (2000) Molecular basis of ligand recognition by integrin α5β1: specificity of ligand binding is determined by amino acid sequences in the second and third NH2-terminal repeats of the α subunit. J. Biol. Chem., 275, 20324–20336. [DOI] [PubMed] [Google Scholar]
- Northrup S.H. and Erickson,H.P. (1992) Kinetics of protein–protein association explained by Brownian dynamics computer simulation. Proc. Natl Acad. Sci. USA, 89, 3338–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara M., Kang,M.S. and Yamada,K.M. (1988) Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell, 53, 649–657. [DOI] [PubMed] [Google Scholar]
- Philippsen A. (2002) DINO: Visualizing structural biology. http://www.dino3d.org. [Google Scholar]
- Pierschbacher M.D. and Ruoslahti,E. (1984) Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature, 309, 30–33. [DOI] [PubMed] [Google Scholar]
- Radermacher M. (1992) Weighted backprojection methods in electron tomography. In Frank,J. (ed.), Electron Tomography. Plenum Press, New York, pp. 91–116. [Google Scholar]
- Radermacher M., Wagenknecht,T., Verschoor,A. and Frank,J. (1987) Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J. Microsc., 146, 113–136. [DOI] [PubMed] [Google Scholar]
- Redick S.D., Settles,D.L., Briscoe,G. and Erickson,H.P. (2000) Defining fibronectin’s cell adhesion synergy site by site-directed mutagenesis. J. Cell Biol., 149, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka M., et al. (2003) Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell, 112, 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J. and Springer,T.A. (2002) Integrin activation and structural rearrangement. Immunol. Rev., 186, 141–163. [DOI] [PubMed] [Google Scholar]
- Takagi J., Erickson,H.P. and Springer,T.A. (2001) C-terminal opening mimics ‘inside-out’ activation of integrin α5β1. Nat. Struct. Biol., 8, 412–416. [DOI] [PubMed] [Google Scholar]
- Takagi J., Debottis,D.P., Erickson,H.P. and Springer,T.A. (2002a) The role of specificity-determining loop of the integrin β-subunit I-like domain in folding, association with the α subunit and ligand binding. Biochemistry, 41, 4339–4347. [DOI] [PubMed] [Google Scholar]
- Takagi J., Petre,B.M., Walz,T. and Springer,T.A. (2002b) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell, 110, 599–611. [DOI] [PubMed] [Google Scholar]
- Weisel J.W., Nagaswami,C., Vilaire,G. and Bennett,J.S. (1992) Examination of the platelet membrane glycoprotein IIb–IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J. Biol. Chem., 267, 16637–16643. [PubMed] [Google Scholar]
- Wu L.C., Tuot,D.S., Lyons,D.S., Garcia,K.C. and Davis,M.M. (2002) Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature, 418, 552–556. [DOI] [PubMed] [Google Scholar]
- Xiong J.P., Stehle,T., Diefenbach,B., Zhang,R., Dunker,R., Scott,D.L., Joachimiak,A., Goodman,S.L. and Arnaout,M.A. (2001) Crystal structure of the extracellular segment of integrin αVβ3. Science, 294, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J.P., Stehle,T., Zhang,R., Joachimiak,A., Frech,M., Goodman,S.L. and Arnaout,M.A. (2002) Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg–Gly–Asp ligand. Science, 296, 151–155. [DOI] [PubMed] [Google Scholar]
- Yang J.T., Rayburn,H. and Hynes,R.O. (1993) Embryonic mesodermal defects in α5 integrin-deficient mice. Development, 119, 1093–1105. [DOI] [PubMed] [Google Scholar]