Abstract
Blue light regulates many physiological and developmental processes in fungi. Most of the blue light responses in the ascomycete Neurospora crassa are dependent on the two blue light regulatory proteins White Collar (WC)-1 and -2. WC-1 has recently been shown to be the first fungal blue light photoreceptor. In the present study, we characterize the Neurospora protein VIVID. VIVID shows a partial sequence similarity with plant blue light photoreceptors. In addition, we found that VIVID non-covalently binds a flavin chromophore. Upon illumination with blue light, VIVID undergoes a photocycle indicative of the formation of a flavin-cysteinyl adduct. VVD is localized in the cytoplasm and is only present after light induction. A loss-of-function vvd mutant was insensitive to increases in light intensities. Furthermore, mutational analysis of the photoactive cysteine indicated that the formation of a flavin-cysteinyl adduct is essential for VIVID functions in vivo. Our results show that VIVID is a second fungal blue light photoreceptor which enables Neurospora to perceive and respond to daily changes in light intensity.
Keywords: blue light/flavin/Neurospora crassa/photoreceptor/VIVID
Introduction
Light is a very important environmental signal that regulates development and metabolism in most organisms. In plants, light is crucial for the photosynthetic conversion of solar energy into chemical energy. It is therefore not surprising that plants have developed a complex light perception and signal transduction machinery that facilitates the adjustment to ambient light conditions and also helps to avoid the deleterious effects of sunlight (Neff et al., 2000). Plants are capable of sensing the quality, intensity and direction of light. The perception of light is carried out by photoreceptor molecules consisting of a protein with either one or several chromophore moieties attached to it. The light signal perceived by the chromophore results in conformational changes of the photoreceptor that initialize the transduction of the light signaling cascade, eventually leading to the light response of the organism. Higher plants possess a variety of photoreceptors including the red/far-red light absorbing phytochromes and the blue light-absorbing cryptochromes and phototropins (Batschauer, 1998; Christie and Briggs, 2001).
The capacity of sensing and responding to light is also widespread in non-photosynthetic organisms such as bacteria and fungi. In fungi, several developmental and physiological processes have been reported to be influenced by light (for a review see Lauter, 1996; Linden et al., 1997a). The most prominent light response in the ascomycete Neurospora crassa is the light-regulated biosynthesis of the photo-protective carotenoids (Schrott, 1980, 1981; Harding and Turner, 1981). Only traces of carotenoids can be detected in dark-grown mycelia, resulting in an almost white phenotype. Upon illumination with blue light, all carotenoid biosynthesis genes are up-regulated on a transcriptional level, leading to a fast accumulation of orange-colored carotenoids (Linden, 2002). Other Neurospora responses to light include the light entrainment of the circadian clock, the formation of spores and phototropism (Harding and Melles, 1983; Lauter et al., 1997; Dunlap, 1999). All Neurospora light responses described so far are only triggered by blue light. The blue light perception and signaling have received much attention during the last decades, culminating in the recent characterization of the first fungal blue light photoreceptor (Froehlich et al., 2002; He et al., 2002). The photoreceptor White Collar (WC)-1 is a flavin-type photoreceptor with FAD as a chromophore. In addition to the photoreceptor function, WC-1 also acts as a transcription factor with a zinc-finger DNA binding domain, a nuclear localization signal and protein–protein interaction domain (Ballario et al., 1996). Another protein involved in blue light signaling in Neurospora is WC-2, which also shows features of a transcription factor and which was found to form a complex with WC-1 (Linden and Macino, 1997; Talora et al., 1999). Since the two White Collar proteins are the only essential components for light perception and blue light signaling identified today, the signaling cascade seems to be very short in Neurospora. Thus, the WC-1/WC-2 complex is localized in the nucleus and directly targets the light signal to the promoters of blue light-regulated genes.
Nevertheless, other mutants and proteins have been described that interfere with blue light signaling in Neurospora (Carattoli et al., 1995; Linden et al., 1997b). Among these, the vivid (vvd) mutant shows particularly interesting features. The mutant shows an increased accumulation of carotenoids under constant illumination (leading to the name vivid coloration), which is due to a sustained expression of carotenoid genes in the light (Perkins et al., 1997; Schwerdtfeger and Linden, 2001). In contrast, most blue light-regulated genes are down-regulated in constant light in the wild type after 2 h, a phenomenon called photoadaptation (Baima et al., 1991). Furthermore, VVD was found to be controlled by the circadian clock and to modulate the light input to the circadian pacemaker (Heintzen et al., 2001).
In the present study, we show that VVD is a fungal blue light photoreceptor. VVD is capable of binding a flavin-type chromophore. Moreover, the purified protein undergoes a blue light-induced photocycle that indicates the formation of a cysteinyl-flavin adduct. This photocycle matches the photocycle of the flavin-binding (LOV) domains of the plant blue light photoreceptor phototropin. Mutational analysis of the photoactive cysteine suggests that the formation of a flavin-cysteinyl adduct is essential also for the in vivo functions of VVD. Although previous results suggest VVD as a negative factor of light signal transduction, we show that a vvd loss-of-function mutant has defects in a subset of the Neurospora blue light responses. Our results show that VVD represents a new type of photoreceptor that enables Neurospora to detect and to adapt to daily changes in light intensities.
Results
VVD has a putative chromophore binding motif
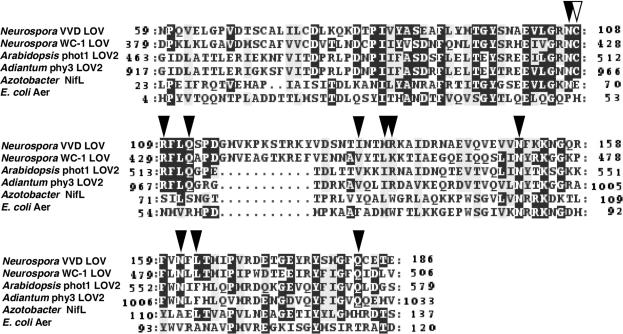
VVD is a small protein of 186 amino acids that contains only one functional domain (Heintzen et al., 2001). A sequence alignment of VVD with other proteins showed considerable sequence similarities with the chromophore binding domains of various blue light photoreceptors and other sensor proteins (Figure 1). VVD showed highest sequence similarities with the so-called light, oxygen and voltage (LOV) domains of the fungal blue light photoreceptor WC-1 and the plant blue light photoreceptors phototropin and phy3 (72, 61 and 54% similarity, respectively). LOV domains constitute a subset of the PER, ARNT and SIM (PAS)-domain protein superfamily mediating both ligand binding and protein–protein interactions (Taylor and Zhulin, 1999). A lower sequence similarity was found with other FAD-binding motifs of the bacterial sensor proteins NifL and Aer. LOV domains were shown to bind either flavin-adenine dinucleotide (FAD) or flavin mononucleotide (FMN), and several amino acids have been identified which seem to be involved in the interaction with the chromophore moiety (Crosson and Moffat, 2002). Interestingly, all 11 flavin-interacting amino acid residues including the photoactive Cys are also conserved in the VVD LOV domain (Figure 1). The similarity to FAD and FMN-binding domains of sensor proteins suggested that VVD may also bind a flavin-type chromophore.
Fig. 1. Sequence alignment of VVD with the WC-1 LOV domain and FAD and FMN-binding domains of plant photoreceptors and redox sensing proteins. Identical residues are shown in black, similar residues are shaded in gray. Flavin-interacting residues as well as the photoactive cysteine of phy3 are marked by black and open arrowheads, respectively (DDBJ/EMBL/GenBank accession Nos: VVD, AAK08514; WC-1, Q01371; Arabidopsis phot1, AAC01753; Adiantum phy3, T30891; Azotobacter NifL, P30663, E.coli Aer, P50466).
VVD is capable of binding FAD and FMN
To investigate this hypothesis we expressed the entire open reading frame of VVD as a His-tagged fusion protein in Escherichia coli (Figure 2, lane 2). When growth was carried out at room temperature, ∼50% of the expressed VVD fusion protein was soluble. The soluble protein was purified to near homogeneity by affinity chromatography with Ni-NTA agarose under native conditions (Figure 2, lane 3). Upon elution, the fusion protein was found to co-purify with a yellow pigment. This result indicated the presence of a chromophore associated with VVD.
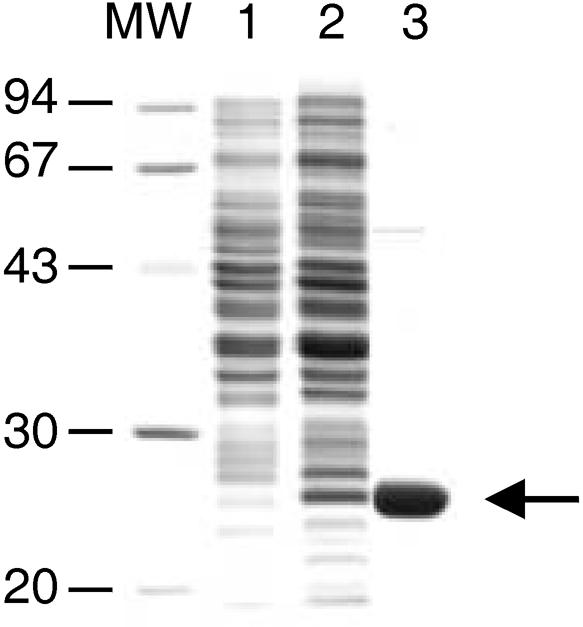
Fig. 2. Expression in E.coli and purification of VVD. Proteins were separated on a 15% SDS–PAGE. Non-induced (lane 1) and induced E.coli cells expressing VVD as His-tagged fusion protein (lane 2), VVD protein after purification by affinity chromatography. The arrow on the right shows the expressed and purified VVD protein.
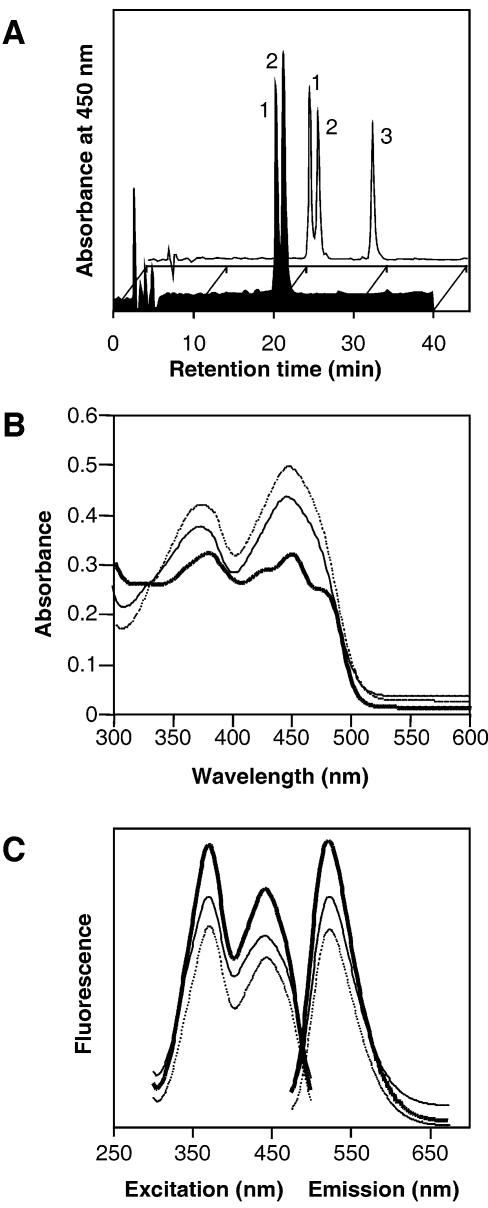
We further investigated the nature of this chromophore. The purified protein was extracted several times with chloroform to remove proteins and lipids and the yellow pigment was found in the aqueous solution suggesting a non-covalent attachment between VVD and the chromophore. The aqueous chromophore solution was subjected to high-performance liquid chromatography (HPLC) analysis using a C18 reverse phase column. Upon separation two peaks were observed indicating the presence of two different chromophores (Figure 3A, black profile, peak 1 and 2). When compared with a HPLC separation of a mixture of FAD, FMN and riboflavin, the VVD chromophores showed the same retention times as FAD and FMN standards (Figure 3A, transparent profile, peak 1 and 2). The ratio of FAD to FMN varied from 0.5 to 1.0 depending on the protein preparation. The purified VVD protein showed absorbance maxima at 380 and 450 nm, which were very similar to the absorption spectra of other flavin-binding LOV domains (Figure 3B, solid line) (Swartz et al., 2001). When an SDS denaturation of VVD protein fraction was carried out, a typical flavin spectrum was observed, indicating the release of the chromophore from the protein (Figure 3B, dashed line). The released VVD chromophores were further analyzed by fluorescence spectroscopy. The chromophores were fluorescent with excitation and emission maxima identical to those of FMN and FAD (Figure 3C). A quantification of the chromophores and the VVD fusion protein was carried out and a molar chromophore to protein ratio of 0.61 ± 0.07 was observed, which was comparable to the chromophore/protein ratio previously described for the LOV domains of phototropin (0.6–1.0; Christie et al., 1999). Thus, the VVD fusion protein seems to be associated with one flavin chromophore, which is either FAD or FMN.
Fig. 3. VVD non-covalently binds FAD and FMN. (A) HPLC analysis of the VVD chromophores (black profile) and an aqueous mixtures (transparent profile) of FAD (peak 1), FMN (peak 2) and riboflavin (peak 3). (B) Absorbance spectra of the purified VVD protein before (solid line) and after SDS denaturation (dashed line) and of an aqueous FAD solution (dotted line). (C) Fluorescence excitation (left, 525 nm emission) and emission spectra (right, 370 nm excitation) of the VVD chromophores (solid line), FAD (dashed line) and FMN (dotted line).
The blue light-induced photocycle of VVD
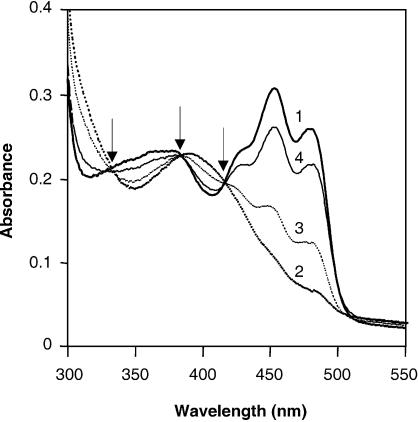
The plant blue light photoreceptor phototropin exhibits characteristic light-induced absorbance changes upon transfer from the dark to the light (Briggs and Christie, 2002; Crosson and Moffat, 2002). To investigate whether VVD also shows a photocycle, absorbance spectra of purified VVD were recorded following incubation in the dark, after a short light pulse and after subsequent dark incubations (Figure 4). The VVD absorbance spectrum in the dark showed a major peak at 450 nm and two minor peaks at 428 and 478 nm (Figure 4, spectrum 1). Upon light incubation for 30 s, an almost complete loss of absorption in the blue light range was observed combined with the appearance of a new peak near 390 nm (Figure 4, spectrum 2). When the protein was subsequently incubated in the dark, the light-induced absorbance change was reversible with complete recovery after ∼5 h (Figure 4, spectra 3, 4 and 1). The dark recovery rate of VVD was very long in comparison to the dark recovery rates of seconds to a few minutes reported for higher plant LOV domains (Briggs and Christie, 2002). However, a similarly long dark recovery rate of 120 min has been described for a prokaryotic protein containing a LOV domain (Losi et al., 2002). The authors proposed that the long dark recovery rate may be due to the fact that the entire protein was applied in their study, whereas the shorter recovery rates have been determined expressing only the respective LOV domains. The same reason may also be responsible for the long recovery rates detected in the present study. Alternatively, the long recovery rate of VVD may be due to the fact that the experiment was carried out at 4°C. Therefore, the same experiment was repeated at room temperature. However, the VVD protein was not soluble under this condition, making it impossible to test this hypothesis.
Fig. 4. The VVD protein exhibits a reversible light-induced absorbance change. Absorbance spectra were recorded from purified VVD protein after incubation in the dark (spectrum 1), after a 30 s light induction (100 µmol photons/m2/s for 30 s, provided by universal white lamps Osram L65W/25S; spectrum 2) and after subsequent incubations in the dark for 10 min (spectrum 3), 2 h (spectrum 4) and 5 h (spectrum 1) at 4°C. The three isosbestic points are indicated by arrows.
Only blue light lead to the observed light-induced absorbance changes, whereas light with a wavelength >500 nm was completely ineffective (data not shown). We also identified the three isosbestic points which are characteristic for the formation of a flavin-cysteinyl adduct (Figure 4) (Briggs and Christie, 2002). Thus, following the transfer from dark to light, VVD undergoes a photocycle that matches the photocycle of the flavin-binding (LOV) domains of phototropin.
The VVD protein is expressed only in the light and is localized in the cytoplasm
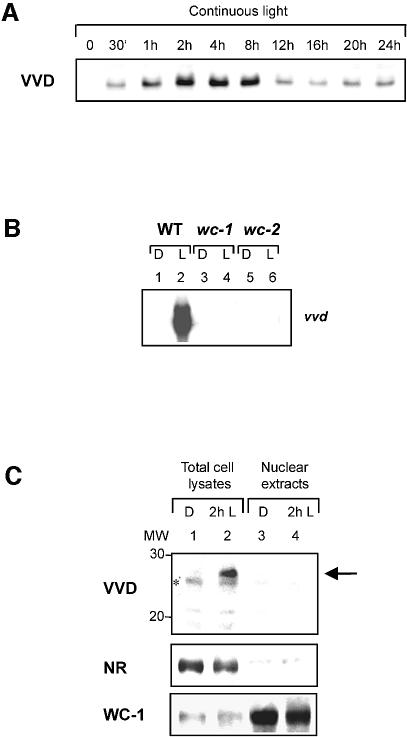
The regulation and localization of the VVD protein in N.crassa was investigated. A VVD antiserum was produced that specifically detected VVD in Neurospora total cell extracts (Figure 5). The VVD protein was observed only as a consequence of a light induction and was not detected in dark-grown mycelia (Figure 5A). Under continuous light conditions, VVD showed a sustained expression with a maximum after 2 h of light. To obtain additional information on the light-induced expression of VVD, the gene expression of vvd was analyzed in wc-1 and wc-2 mutants (Figure 5B). Light-regulated expression was only observed in the wild type, but was found to be abolished in both wc mutants. This result indicated the dependency of vvd expression on the photoreceptor WC-1 and on a functional WC-1/WC-2 complex.
Fig. 5. VVD is expressed only in the light and localized in the cytoplasm. (A) Expression of the VVD protein in N.crassa mycelia after growth in the dark and after different times of continuous light induction. (B) Northern blot analysis of the vvd gene expression in the Neurospora wild type and in wc-1 and wc-2 mutant background. Cultures were harvested either after growth in the dark (D) or after a low light induction for 30 min (L). (C) Western blot analysis of VVD, nitrate reductase (NR) and WC-1 in nuclear extracts and total cell lysates of Neurospora wild type. Total cell lysates (lanes 1 and 2) and nuclear extracts (lanes 3 and 4) were prepared after cultivation in the dark and after an additional incubation for 2 h in the light. The arrow on the right shows the VVD protein. A non-specific cross reaction of the VVD antiserum is indicated by an asterisk.
All the previously isolated Neurospora blue light regulatory proteins, including the photoreceptor WC-1, are nuclear proteins (Schwerdtfeger and Linden, 2000; Denault et al., 2001). These findings suggest that blue light perception and signaling are entirely limited to the nuclear compartment. The finding that VVD affects WC-1 phosphorylation and signal transduction furthermore pointed at a possible direct interaction of VVD with the WC-1/WC-2 complex in the nuclear compartment (Heintzen et al., 2001; Schwerdtfeger and Linden, 2001). To investigate this hypothesis, a biochemical fractionation technique was applied to study the subcellular localization of VVD in the wild-type Neurospora (Figure 5C). For the confirmation of the purity of the nuclear fraction, two control proteins were used as markers. WC-1 was found to be enriched in the nuclear fraction, whereas the cytoplasmic protein nitrate reductase was only found in the total cell lysates but not in the nuclear fraction (Figure 5C, lower and middle panels). Consequently, the nuclear fractions were essentially free of cytoplasmic contaminations. In immunoblot analysis of VVD using the same fractions, VVD was detected in the total cell lysates of light-induced Neurospora mycelia, but not in the nuclear fractions (Figure 5C, upper panel). We concluded that the VVD protein is synthesized as a consequence of a light induction and localized in the cytoplasm.
The light responses of the photoreceptor VVD
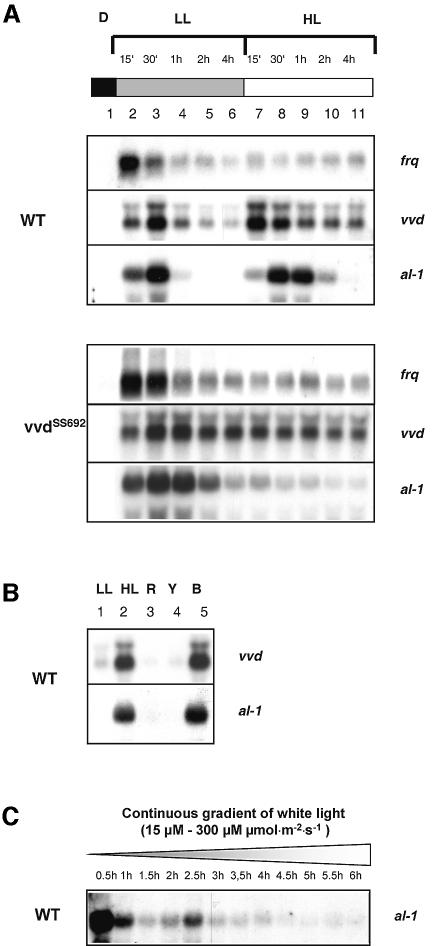
Previous results showed that one function of VVD is the down-regulation of light responses after a light induction (Heintzen et al., 2001; Schwerdtfeger and Linden, 2001; Shrode et al., 2001). We looked for blue light responses that are abolished in the VVD mutant background. To this end, a Neurospora wild type and a loss-of-function vvd mutant were cultivated in complete darkness and successive light inductions were carried out using different light intensities (Figure 6A). Despite its null phenotype, a vvd transcript can still be detected in the vvdSS692 mutant allele (Figure 6A, lower panel) (Heintzen et al., 2001). This enabled us to also investigate the vvd gene regulation in a vvd mutant background. Light induction of the carotenoid biosynthesis gene al-1, vvd and the circadian clock gene frq was not affected in the vvd mutant, confirming earlier results that VVD is not involved in initial light perception (Figure 6A, lower panel, lanes 1 and 2). The defect in the down-regulation of light-induced genes can clearly be seen in the vvd mutant background. In comparison with the wild type, the expression of all genes was more persistent in the vvd mutant after a first light induction of 1, 2 and 4 h (Figure 6A, lower and upper panels, lanes 4–6). A second light response was observed after a subsequent high light induction merely in the wild type (Figure 6A, upper panel, lanes 7–9). The high light-induced increase in gene expression was only detected for al-1 and vvd transcripts, whereas no significant up-regulation was found for frq (Figure 6A, upper panel, lanes 7–9). In contrast, the vvd mutant was insensitive to the increase in light intensity and none of the genes examined showed any changes in transcription levels in response to high light (Figure 4A, lower panel, lanes 7–9; Schwerdtfeger and Linden, 2001).
Fig. 6. The vvd mutant is insensitive to high light during growth under continuous illumination. (A) Cultures of Neurospora wild type and mutant vvdSS692 were grown in the dark and illuminated with low light (LL) for 4 h and subsequently with high light (HL) for an additional 4 h. Mycelia were harvested at the indicated time points. (B) Dark-grown wild-type mycelia were subjected to a 4 h low light induction (LL) and subsequently illuminated with either white (HL), red (R), yellow (Y) or blue light (B) for 1 h. (C) Cultures of Neurospora wild type were grown in the dark and illuminated using a continuous light gradient of white light. An initial light intensity of 15 µmol/m2/s was applied and light was gradually increased up to 300 µmol/m2/s after 6 h. Mycelia were harvested at the indicated time points. For northern blot analysis the carotenoid biosynthesis gene al-1, the circadian clock gene frq and vvd were used as specific probes.
If the flavoprotein VVD is directly involved in perception of increasing light intensities, we would expect this response to also be dependent on blue light. Indeed, when low light-treated wild-type cultures were illuminated with red and yellow light, no increase in al-1 and vvd mRNA levels was detected (Figure 6B, lanes 3 and 4). Only blue light led to an increase in transcript levels comparable to a high light induction with white light (Figure 6B, lanes 2 and 5).
Our results indicate that VVD functions in the activation of light-regulated genes in response to increased illumination. However, the sudden changes in light intensities applied for these experiments hardly reflect the environmental growth conditions of N.crassa under natural light conditions. It was therefore interesting to obtain information on the capability of this fungus to perceive gradually increasing light intensities (Figure 6C). Under these conditions, the expression of the carotenoid biosynthesis gene al-1 showed a sustained expression that is in contrast to the complete down-regulation of al-1 mRNA observed under constant light conditions after 2 h (Figure 6A, upper panel, lane 5). Thus, the Neurospora wild type is able to detect and to respond to small changes in light conditions and over a wide range of light intensities.
The photoactive cysteine is essential for the function of VVD in vivo
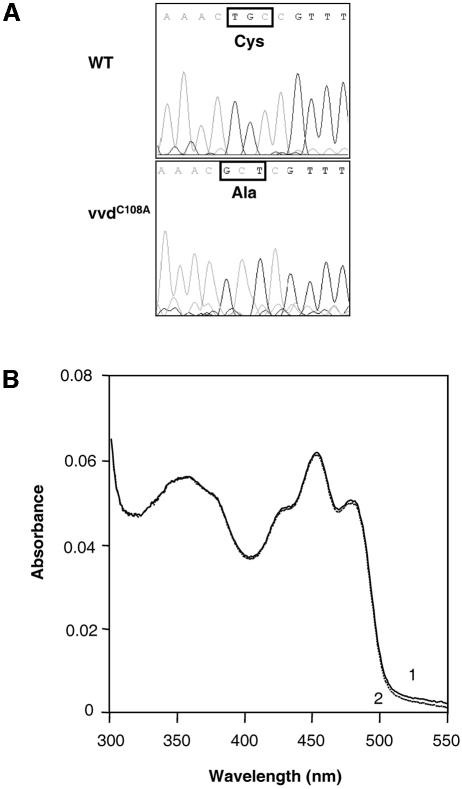
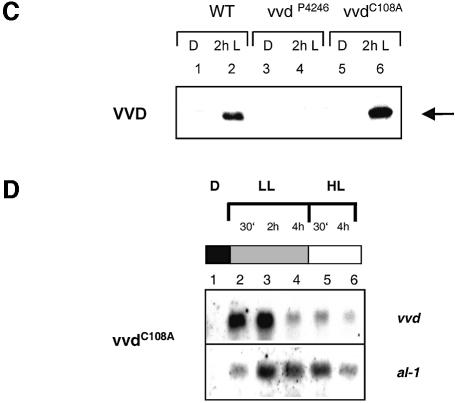
The VVD protein seems to play a dual role in light regulation in Neurospora, namely the down-regulation of light responses after a light induction and the detection of increasing light intensities under constant light conditions. It was interesting to determine whether the photoreceptor function of VVD is involved in both processes. We modified the photoactive Cys108 by site-directed mutagenesis. Introduction of the mutations led to an exchange of cysteine for alanine in the VVD protein at this position (Cys108→Ala; Figure 7A). Subsequently, the entire open reading frame of vvdC108A was expressed as a His6-tagged fusion protein in E.coli and VVDC108A was purified to near homogeneity as described above. The VVDC108A polypeptide was unaffected in its capacity to bind the flavin chromophore, although the absorption spectrum showed some minor changes in comparison to the spectrum of the wild-type protein (Figures 4 and 7B). However, in contrast to the wild-type protein, VVDC108A did not exhibit a light-induced absorbance change (Figure 7B, spectra 1 and 2). The complete loss of photochemical reactions was also observed in other LOV domains in which the photoactive cysteine had been exchanged, which seems to be due to the lack of a thiol group for cysteinyl adduct formation (Salomon et al., 2000; Christie et al., 2002; Holzer et al., 2002). Furthermore, the mutation of the photoactive cysteine also resulted in the inactivation of the plant LOV domains in vivo. To examine the effect of the Cys108→Ala mutation on the function of VVD in vivo, VVD was expressed as His-tagged fusion protein in a vvd null mutant under control of its own promoter. The vvdP4246mutant applied for these experiments represents another null mutant in which accumulation of the vvd transcript was not observed (Heintzen et al., 2001). In a control experiment, we first expressed wild-type VVD as His-tagged fusion protein. In these transformants wild-type phenotype was recovered and down-regulation of light-regulated genes as well as low light–high light responses were comparable to the wild type (Figure 6; data not shown). Next, the VVDC108A protein was expressed as His-tagged fusion in the mutant vvdP4246. Using the VVD antibody, the VVD protein was detected in the wild type and in the vvdC108A transformants, but not in the vvdP4249 mutant (Figure 7C, lanes 2, 4 and 6). However, despite the expression of VVDC108A, the transformants showed a typical vvd phenotype with increased carotenoid accumulation in the light (data not shown). In addition, down-regulation of light-regulated genes, as well as the higher gene expression in response to increased light intensities, was not observed in these transformants (Figure 7D). In contrast to the wild type, the vvdC108A transformants showed a sustained expression of al-1 after 4 h of low light (Figures 6A and 7D, lane 4). Furthermore, a second light induction with higher light intensities did not lead to the up-regulation of vvd and al-1 transcripts (Figure 7D, lane 5). Several transformants were analyzed and exhibited similar light induction patterns, as shown in Figure 7D. We concluded that light perception as well as the formation of the cysteinyl adduct are essential for the in vivo functions of VVD.
Fig. 7. Cysteine108 is essential for the light-induced absorbance change in vitro and for the in vivo functions of VVD in photoadaptation. (A) Sequencing results of the vvd wild-type gene and of the vvdC108A gene (genomic DNA construct) following site-directed mutagenesis. (B) Absorbance spectra from purified VVD protein expressed in E.coli after incubation in the dark (spectrum 1) and after a 30 s light induction (100 µmol photons/m2/s, provided by universal white lamps Osram L65W/25S; spectrum 2). (C) Western blot analysis of VVD in Neurospora wild-type, vvdP4246 mutant and in a vvdp4246 mutant after transformation with the vvdC108A gene. Total cell lysates were prepared after cultivation in the dark (lanes 1, 3 and 5) and after an additional incubation for 2 h in the light (lanes 2, 4 and 6). The arrow on the right shows the VVD protein. (D) The vvdC108A mutant is defective in both photoadaptation and in the up-regulation of vvd and al-1 in response to higher light. Cultures of the vvdC108A strain were grown in the dark and illuminated with low light (LL) for 4 h and subsequently with high light (HL) for an additional 4 h. Mycelia were harvested at the indicated time points. For northern blot analysis the carotenoid biosynthesis gene al-1 and vvd were used as specific probes.
Discussion
Here, we show that the Neurospora protein VVD is a blue light photoreceptor. Following the heterologous expression in E.coli, VVD was found to be associated with a flavin-type chromophore (Figure 3). Upon illumination, the native VVD protein underwent a blue light-induced absorbance change that was fully reversible in the dark (Figure 4). This photocycle was also reported for the LOV domains of the plant blue light photoreceptor phototropin and indicates the formation of a reversible covalent bond between the conserved cysteine in the VVD LOV domain and the flavin chromophore (Briggs and Christie, 2002). The formation of the cysteinyl-flavin adduct results in subtle structural changes of the flavin-binding pocket (Crosson and Moffat, 2002). The latter changes are thought to represent the initial event in light signal transduction and seem to lead to the activation of the intra-molecular kinase in phototropins. However, VVD is different in this respect since it only contains the LOV motif and additional signaling domains have not been identified so far. It is therefore plausible that blue light signaling of VVD is carried out via the interaction with other proteins and illumination with blue light results in the promotion or disruption of these protein–protein interactions. VVD is capable of binding both FAD and FMN. The determination of the molar chromophore/protein ratio indicated that VVD is associated with only one flavin chromophore, which is either FMN or FAD. The entire VVD protein fraction was found to be photochemically active furthermore emphasizing that both FAD- and FMN-bound VVD proteins are capable of undergoing this photocycle (Figure 4). Similarly, it was shown that the LOV domains of the higher plant photoreceptor phototropin are also capable of binding both FMN and FAD following the heterologous expression in E.coli (M.Salomon, personal communication). However, it is important to note that this versatility in chromophore binding may be due to the expression in E.coli. The nature of the VVD chromophore in vivo has yet to be determined.
If VVD plays a role in blue light perception in N.crassa, what are the light responses regulated by this photoreceptor? Our results confirm previous findings that VVD is not required for initial light perception (Figure 6). However, VVD plays a crucial role in the light response of Neurospora to increasing light intensities following a first light induction (Figure 6). The transcriptional gene activation in response to increasing light intensities was only observed under blue light, further supporting our hypotheses that VVD functions as blue light photoreceptor for this response (Figure 6). The involvement of a second photoreceptor in the Neurospora light perception mechanism has previously been proposed and explains many earlier data. For example, Neurospora wild type showed a biphasic fluence response curve for the biosynthesis of carotenoids, the phase-shifting of the circadian rhythm of conidiation and for the expression of genes involved in spore formation (Sargent and Briggs, 1967; Schrott, 1980; Corrochano et al., 1995). After a first light induction the fluence response curve showed a saturation plateau that was independent of the light intensity applied. A second phase of the responses was only observed after a distinct period of illumination (15–16 min) suggesting the de novo biosynthesis of components involved in light perception. This is in accordance with the fact that VVD can be detected after a light induction of 15 min (unpublished results). In addition, it has previously been shown that dark-grown Neurospora wild type is not capable of differentiating between two different light intensities (above saturating light intensities) (Schwerdtfeger and Linden, 2001). The capacity of responding to increasing light intensities is only acquired after a first light induction. Thus, VVD is synthesized in response to a first light induction (Figures 5 and 6). The transcriptional up-regulation of the VVD mRNA is brought about by the photoreceptor WC-1 and the WC-1/WC-2 complex (Figure 5; Dragovic et al., 2002). Subsequently, VVD carries out two different functions. First, the initial light perception system is down-regulated leading to the transient expression of light-regulated genes. The down-regulation is also indicated by the transient phosphorylation pattern of WC-1, which is dependent on VVD (Schwerdtfeger and Linden, 2000, 2001; Heintzen et al., 2001). Secondly, VVD is required for the perception of light intensity changes resulting in the up-regulation of a subset of light-regulated genes in response to higher light. Interestingly, both functions are dependent on the formation of a cysteinyl adduct (Figure 7). Thus, not only the perception of light intensity changes but also the repressor function of VVD require a functional LOV domain. Our findings that the formation of the cysteinyl adduct is essential for VVD functions in vivo provide additional evidence that VVD indeed represents a photoreceptor. In domain swapping experiments it has recently been shown that the VVD LOV domain is capable of partially replacing the WC-1 LOV domain in light perception further supporting our results (Cheng et al., 2003).
What are the other components of the VVD light signaling system? The presence of a PAS/LOV domain in VVD as well as the dependency of the phosphorylation status of WC-1 on VVD led to the hypothesis that VVD may directly interact with the WC-1/WC-2 complex (Heintzen et al., 2001). However, VVD was strictly localized in the cytoplasm and was not detected in the nuclei of N.crassa (Figure 5). In view of the fact that both WC-1 and WC-2 are nuclear proteins, signaling of VVD via a direct interaction with the WC-1/WC-2 complex is rather unlikely. As a consequence, other signaling components must participate in the signal transduction process which remain elusive today. Despite this uncertainty, our data suggest that quantity of VVD present in the cell is an important factor participating in the response to different light intensities. Thus, in comparison, the low light induction pattern of the vvd transcript, a higher and sustained expression was observed during the subsequent high light induction, which was also reflected by higher VVD protein quantities (Figure 6; unpublished results). The higher expression of VVD under these conditions is due to autoregulation as indicated by the lack of this response in the VVD null mutant strain (Figure 6).
Besides al-1, VVD was shown to regulate the expression of additional carotenoid biosynthesis genes and of other light-regulated genes of unknown functions (Schwerdtfeger and Linden, 2001; unpublished results). Furthermore, several blue light-regulated genes of spore formation such as con-6 and con-10 are down-regulated by VVD following a light induction (Shrode et al., 2001). VVD also interferes with its own expression and seems to modulate the light input to the circadian pacemaker (Figure 6; Heintzen et al., 2001). These findings suggest that VVD is a general regulatory protein of most light responses in N.crassa.
In conclusion, our results show that Neurospora has a dual light perception system with at least two photoreceptors, namely WC-1 and VVD. Initial light perception is responsible for dark to light transitions with WC-1 and WC-2 as signal transduction proteins. In contrast, the blue light photoreceptor VVD is an essential component of a second light signaling system that enables Neurospora to detect and to adapt to daily changes in light intensities. We show that even subtle and gradual light intensity changes are perceived leading to the fine-tuning of the light responses to the prevailing environmental light conditions (Figure 6). The evolutionary advantage of this dual light perception system for Neurospora is evident. The repeated production of carotenoids in response to increasing light intensities provides protection against photodamage and anticipates the destruction of carotenoids by high light. In contrast, for the light entrainment of the circadian clock, which is manifested by the up-regulation of the frq gene, the onset of light is more relevant than light intensity. There is a surprising analogy to the higher plant light sensing system, where individual photoreceptors of the phytochrome and cryptochrome families are responsible for dark to light transitions and for growth under daily light changes.
Materials and methods
Neurospora strains and growth conditions
Neurospora wild-type strain 74OR23-1A (FGSC 987) and the mutants vvdSS692 (FGSC 7852) and vvdP4246 (FGSC 7854) were obtained from the Fungal Genetic Stock Center (Kansas City, KS). Production of the wc-1 and wc-2 null mutants applied in this study have been described previously (Talora et al., 1999; Collett et al., 2002) Growth of Neurospora was performed in Vogel’s minimal medium supplemented with 1.5% sucrose in the dark (Davis and deSerres, 1970). Mycelia were collected by filtration either directly under red safety light or after various illumination times (15 µmol photons/m2/s for low light, 55 µmol/m2/s for high light, red, yellow and blue light). Philips 36W TL 14 lamps (red, light emission 600–720 nm, maximum at 660 nm), Osram L 36W/63 lamps (yellow, light emission 520–680 nm, maximum at 580 nm) and Osram L 36W/67 lamps (blue, light emission 400–530 nm, maximum at 450 nm) were used for light quality experiments.
Expression and purification of the native VVD protein
Plasmid pQECS105 containing the entire ORF of VVD ligated into the pQE expression vector (Qiagen) was transformed into E.coli BLR21 (DE3) cells. Expression was carried out at 21.5°C for 12 h in the light and VVD production was induced by addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside at an OD600 of 0.050. The pelleted cells were resuspended in H50 buffer (50 mM HEPES pH 7.5, 225 mM NaCl, 2 mM MgCl) and lyzed by sonication. The cell debris was removed by ultracentrifugation at 180 000 g for 90 min. After centrifugation, 2.5 M saccharose (10% v/v) was added to the supernatant. The native VVD protein was subsequently purified by affinity chromatography on Ni-NTA agarose according to the Qiagen protocol.
Spectroscopic analysis
Purified VVD protein was extracted several times with chloroform and the organic phase was discarded. The aqueous phase was separated on a C18 reverse phase column (Machery-Nagel) starting with methanol:10 mM sodium phosphate buffer pH 6.0 (10:90 v/v) as eluent. Subsequently, a linear gradient to 40% methanol was applied for 35 min. HPLC profiles and absorbance spectra were recorded using either a Kontron Diode Array Detector 440 or a Hitachi U-2000 Spectrophotometer. Fluorescence excitation and emission spectra were obtained with a Hitachi F-2000 Fluorescence Spectrophotometer. For the detection of the VVD photocycle the purified protein was illuminated with 100 µM photons/m2/s (provided by universal white lamps Osram L65W/25S) for 30 s. To estimate the quantity of flavins in a mixture of FAD and FMN a mean extinction coefficient of 1.18 × 104/M/cm was applied, which was derived from the respective extinction coefficients of FAD and FMN (Faeder and Siegel, 1973).
VVD antibody
For the production of the VVD antibody, a DNA fragment coding for a partial VVD polypeptide (amino acids 55–186) was amplified by PCR (Oligonucleotides: vvd-BamHI CCCGGATCCAGATTATCAACAGG CCAAACCC and vvd-SacI CAAGAGCGCCATGCCCAATCGC) and ligated into the pQE32 vector (Qiagen). Escherichia coli host strain SG13009 (pREP4) was used for transformation. Expression of the recombinant proteins was carried out according to the QIA express protocol (Qiagen). The expressed His-VVD fusion proteins were purified by ion exchange chromatography (DE52; Whatman). After a further purification step by SDS preparative gel electrophoreses and electro-elution, the purified polypeptides were used for the immunization of rabbits according to standard protocols (Harlow and Lane, 1988). The IgG antibodies were isolated using protein A–Sepharose (Pharmacia) and further purified by affinity chromatography using recombinant VVD polypeptides coupled to CNBr–Sepharose (Pharmacia) as described previously (Schwerdtfeger and Linden, 2000).
Production of a vvdC108A mutant strain
The different VVD polypeptides were expressed as His-tagged fusion protein in E.coli and in Neurospora. The genomic DNA construct used for complementation experiments in E.coli contained a His6-tag and was constructed by overlapping PCR (oligonucleotides: vvdI-C-Ter-His, GTGATGGTGATGGTGTTCCGTTTCGCACTGG and vvdII-C-Ter-His, CACCATCACCATTGAAAGCGGCGAG). The PCR product was amplified again using the following oligonucleotides: vvd-05-ApaI CAAGTGTCGTAGGGCCCGTGG and vvd-04 TTCATTGCAGTGT CCCACTCG. The resulting 3.7 kb fragment was subsequently ligated into the vector pCB1004 (plasmid pCBCS108) and applied for site-directed mutagenesis. The vvd cDNA (plasmid pQECS105) as well as the genomic DNA fragment (plasmid pCBCS108) were modified by site-directed mutagenesis using the quick mutagenesis kit (Stratagene). The following oligonucleotides were used for the introduction of the C108A mutation: VVD-C>A-1, GGGGAGAAACGCTCGTTTTCTTCAGTCA CCC; VVD-C>A-2, GGGTGACTGAAGAAAACGAGCGTTTCTCC CC. The introduction of the mutation in both constructs was verified by DNA sequence analysis, respectively. The transformation of Neurospora was carried out by electroporation according to Garceau et al. (1997). Several transformants were isolated by at least three rounds of purifications and used for further analysis.
Preparation of Neurospora nuclei and western blot analysis
Total cell lysates and nuclei were prepared according to a method described previously (Baum and Giles, 1985) with minor modifications (Schwerdtfeger and Linden, 2000). The purity of the nuclear fraction was checked by fluorescence microscopy using a Zeiss fluorescence microscope and ethidium bromide (100 µg/ml) as fluorescence dye.
Western blot analysis was carried out as described previously (Schwerdtfeger and Linden, 2000). For SDS–PAGE equal quantities of total cell lysates (∼120 µg protein) and nuclear fractions (∼70 µg protein), respectively, were applied. In addition to protein quantifications, total cells lysates and nuclear fractions were separated on SDS gels and stained with Coomassie Blue prior to western blot analysis in order to allow adjustment of protein concentrations. For the detection of VVD, proteins were separated on 15% SDS gels, whereas 7.5% SDS gels were used for WC-1 and nitrate reductase.
Northern blot analysis
RNA was isolated according to the RNA extraction procedure described by Sokolowsky et al. (1990). For northern blot analysis, total RNA (10µg) was denatured in formaldehyde, electrophoresed on a 1.5% agarose gel containing 10% formaldehyde and transferred to positively charged nylon membranes (Roche). Hybridization and detection was carried out according to the DIG system user’s guide for filter hybridization (Roche). The al-1, vvd and frq probes were labeled by PCR using specific oligonucleotides and the DIG DNA labeling mixture (Roche). Typical results of at least three independent experiments are shown.
Acknowledgments
Acknowledgements
We are grateful to S.Ghisla, University of Konstanz, Germany for help with the flavin analysis. C.S. is grateful to J.C.Dunlap and J.F.Loros, Dartmouth Medical School, Hanover, USA: part of the work was carried out in their laboratory and was supported by a grant to J.C.Dunlap (National Institutes of Health, GM 34985). The authors thank S.Kuhn and A.Feyel for excellent technical assistance and G.A.Marzluf, Ohio State University, for providing the nitrate reductase antiserum. Furthermore, we would like to thank M.Salomon, Botanisches Institut München, Germany, for sharing unpublished data. We are grateful to T.Krude for suggestions and to E.O’Halloran for her assistance in the preparation of the manuscript. The work was supported by the Deutsche Forschungsgemeinschaft (LI 819/2-1) and was only possible due to the generous support of P.Böger, Konstanz, Germany.
References
- Baima S., Macino,G. and Morelli,G. (1991) Photoregulation of the albino-3 gene in Neurospora crassa. J. Photochem. Photobiol. B, 11, 107–115. [DOI] [PubMed] [Google Scholar]
- Ballario P., Vittorioso,P., Magrelli,A., Talora,C., Cabibbo,A. and Macino,G. (1996) White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J., 15, 1650–1657. [PMC free article] [PubMed] [Google Scholar]
- Batschauer A. (1998) Photoreceptors of higher plants. Planta, 206, 479–492. [DOI] [PubMed] [Google Scholar]
- Baum J.A. and Giles,N.H. (1985) Genetic control of chromatin structure 5′ to the qa-x and qa-2 genes of Neurospora. J. Mol. Biol., 182, 79–89. [DOI] [PubMed] [Google Scholar]
- Briggs W.R. and Christie,J.M. (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci., 7, 204–210. [DOI] [PubMed] [Google Scholar]
- Carattoli A., Kato,E., Rodriguez-Franco,M., Stuart,W.D. and Macino,G. (1995) A chimeric light-regulated amino acid transport system allows the isolation of blue light regulator (blr) mutants of Neurospora crassa. Proc. Natl Acad. Sci. USA, 92, 6612–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., He,Q., Yang,Y., Wang,L. and Liu,Y. (2003) Functional conservation of light, oxygen, or voltage domains in light sensing. Proc. Natl Acad. Sci. USA, 100, 5938–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M. and Briggs,W.R. (2001) Blue light sensing in higher plants. J. Biol. Chem., 276, 11457–11460. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Reymond,P., Powell,G.K., Bernasconi,P., Raibekas,A.A., Liscum,E. and Briggs,W.R. (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science, 282, 1698–1701. [DOI] [PubMed] [Google Scholar]
- Christie J.M., Salomon,M., Nozue,K., Wada,M. and Briggs,W.R. (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl Acad. Sci. USA, 96, 8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M., Swartz,T.E., Bogomolni,R.A. and Briggs,W.R. (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J., 32, 205–219. [DOI] [PubMed] [Google Scholar]
- Collett M.A., Garceau,N., Dunlap,J.C. and Loros,J.J. (2002) Light and clock expression of the neurospora clock gene frequency is differentially driven by but dependent on WHITE COLLAR-2. Genetics, 160, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano L.M., Lauter,F.R., Ebbole,D.J. and Yanofsky,C. (1995) Light and developmental regulation of the gene con-10 of Neurospora crassa. Dev. Biol., 167, 190–200. [DOI] [PubMed] [Google Scholar]
- Crosson S. and Moffat,K. (2002) Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell, 14, 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.,H. and deSerres,F. (1970) Genetic and microbial research techniques for Neurospora crassa. Methods Enzymol., 17A, 79–143. [Google Scholar]
- Denault D.L., Loros,J.J. and Dunlap,J.C. (2001) WC-2 mediates WC-1-FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J., 20, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic Z., Tan,Y., Gorl,M., Roenneberg,T. and Merrow,M. (2002) Light reception and circadian behaviour in ‘blind’ and ‘clock-less’ mutants of Neurospora crassa. EMBO J., 21, 3643–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap J.C. (1999) Molecular bases for circadian clocks. Cell, 96, 271–290. [DOI] [PubMed] [Google Scholar]
- Faeder E.J. and Siegel,L.M. (1973) A rapid micromethod for the determination of FMN and FAD in mixtures. Anal. Biochem., 53, 332–336. [DOI] [PubMed] [Google Scholar]
- Froehlich A.C., Liu,Y., Loros,J.J. and Dunlap,J.C. (2002) White collar-1, a circadian blue light photoreceptor, binds to the frequency promoter. Science, 297, 815–819. [DOI] [PubMed] [Google Scholar]
- Garceau N.Y., Liu,Y., Loros,J.J. and Dunlap,J.C. (1997) Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell, 89, 469–476. [DOI] [PubMed] [Google Scholar]
- Harding R.W. and Turner,R.V. (1981) Photoregulation of the carotenoid biosynthetic pathway in albino and white collar mutants of Neurospora crassa. Plant Physiol., 68, 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R.W. and Melles,S. (1983) Genetic analysis of phototropism of Neurospora crassa perithecial beaks using white collar and albino mutants. Plant Physiol., 72, 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- He Q., Cheng,P., Yang,Y., Wang,L., Gardner,K.H. and Liu,Y. (2002) White collar-1, a DNA binding transcription factor and a light sensor. Science, 297, 840–843. [DOI] [PubMed] [Google Scholar]
- Heintzen C., Loros,J.J. and Dunlap,J.C. (2001) The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating and regulates clock resetting. Cell, 104, 453–464. [DOI] [PubMed] [Google Scholar]
- Holzer W., Penzkofer,A., Fuhrmann,M. and Hegemann,P. (2002) Spectroscopic characterization of flavin mononucleotide bound to the LOV1 domain of Phot1 from Chlamydomonas reinhardtii. Photochem. Photobiol., 75, 479–487. [DOI] [PubMed] [Google Scholar]
- Lauter F.R. (1996) Molecular genetics of fungal photobiology. J. Genet., 75, 375–386. [Google Scholar]
- Lauter F.R., Yamashiro,C.T. and Yanofsky,C. (1997) Light stimulation of conidiation in Neurospora crassa: studies with the wild-type strain and mutants wc-1, wc-2 and acon-2. J. Photochem. Photobiol. B, 37, 203–211. [Google Scholar]
- Linden H. (2002) Blue light perception and signal transduction in Neurospora crassa. In Osiewacz,H.D. (ed.), Molecular Biology of Fungal Development. Marcel Dekker, New York, NY, pp. 165–185. [Google Scholar]
- Linden H. and Macino,G. (1997) White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J., 16, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H., Ballario,P. and Macino,G. (1997a) Blue light regulation in Neurospora crassa. Fungal Genet. Biol., 22, 141–150. [DOI] [PubMed] [Google Scholar]
- Linden H., Rodriguez-Franco,M. and Macino,G. (1997b) Mutants of Neurospora crassa defective in regulation of blue light perception. Mol. Gen. Genet., 254, 111–118. [DOI] [PubMed] [Google Scholar]
- Losi A., Polverini,E., Quest,B. and Gärtner,W. (2002) First evidence for phototropin-related blue-light receptors in procaryotes. Biophys. J., 82, 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M.M., Fankhauser,C. and Chory,J. (2000) Light: an indicator of time and place. Genes Dev., 14, 257–271. [PubMed] [Google Scholar]
- Perkins D.D., Margolin,B.S., Selker,E.U. and Haedo,S.D. (1997) Occurence of repeat induced point mutation in long segmental duplications of Neurospora. Genetics, 147, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M., Christie,J.M., Knieb,E., Lempert,U. and Briggs,W.R. (2000) Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry, 39, 9401–9410. [DOI] [PubMed] [Google Scholar]
- Sargent M.L. and Briggs,W.R. (1967) The effects of light on a circadian rhythm of conidiation in Neurospora. Plant Physiol., 42, 1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott E.L. (1980) Fluence response relationship of carotenogenesis in Neurospora crassa. Planta, 150, 174–179. [DOI] [PubMed] [Google Scholar]
- Schrott E. (1981) The biphasic fluence response of carotenogenesis in Neurospora crassa: temporal insensitivity of the photoreceptor system. Planta, 151, 371–374. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger C. and Linden,H. (2000) Localization and light-dependent phosphorylation of white collar 1 and 2, the two central components of blue light signaling in Neurospora crassa. Eur. J. Biochem., 267, 414–422. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger C. and Linden,H. (2001) Blue light adaptation and desensitization of light signal transduction in Neurospora crassa. Mol. Microbiol., 39, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Shrode L.B., Lewis,Z.A., White,L.D., Bell-Pedersen,D. and Ebbole,D.J. (2001) vvd is required for light adaptation of conidiation-specific genes of Neurospora crassa, but not circadian conidiation. Fungal Genet. Biol., 32, 169–181. [DOI] [PubMed] [Google Scholar]
- Sokolowsky V., Kaldenhoff,R., Ricci,M. and Russo,V.E.A. (1990) Fast and reliable mini-prep RNA extraction from Neurospora crassa. Fungal Genet. Newsl., 37, 41–43. [Google Scholar]
- Swartz T.E., Corchnoy,S.B., Christie,J.M., Lewis,J.W., Szundi,I., Briggs,W.R. and Bogomolni,R.A. (2001) The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem., 276, 36493–36500. [DOI] [PubMed] [Google Scholar]
- Talora C., Franchi,L., Linden,H., Ballario,P. and Macino,G. (1999) Role of a white collar-1–white collar-2 complex in blue-light signal transduction. EMBO J., 18, 4961–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B.L. and Zhulin,I.B. (1999) PAS domains: internal sensors of oxygen, redox potential and light. Microbiol. Mol. Biol. Rev., 63, 479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]