Abstract
The protective effect of onion against oxidative stress in streptozotosin-induced diabetic rats was investigated in comparison with that of quercetin aglycone. We measured oxidative stress biomarkers involving the susceptibility of the plasma against copper ion-induced lipid peroxidation, which was estimated by the amounts of thiobarbituric acid-reactive substances (TBARS) and cholesteryl ester hydroperoxides, and urine TBARS and 8-hydroxydeoxyguanosine contents. After the 12-week feeding period, plasma glucose levels and these biomarkers increased in diabetic rats compared to normal rats. In diabetic rats fed a 6.0% onion diet (quercetin equivalent: 0.023%), quercetin metabolites accumulated in the plasma at concentrations of approximately 35 µM. Onion intake decreased plasma glucose levels and lowered the oxidative stress biomarkers. On the other hand, quercetin metabolites in the plasma of rats fed a diet with 0.023% quercetin aglycone were found at lower concentrations (14.2 µM) than the rats fed the onion diet. Furthermore, oxidative stress biomarkers were higher in the quercetin diet group compared to the onion diet group. These results strongly suggest that onion intake suppresses diabetes-induced oxidative stress more effectively than the intake of the same amount of quercetin aglycone alone.
Keywords: onion, quercetin, oxidative stress, diabetes, antioxidant
Introduction
Food plants contain a variety of polyphenolic compounds including flavonoids. In recent years, flavonoids have attracted much attention for their physiological functions. Dietary flavonoids are expected to have a protective effect against atherosclerosis which can lead to coronary heart disease by acting, at least in part, as an antioxidant on the oxidation of low-density lipoproteins and other oxidative events [1–3]. Epidemiological studies have shown an inverse relationship between the intake of flavonoids and the risk of coronary heart disease [4–6]. Quercetin, a typical flavonol-type flavonoid, is ubiquitously present in vegetables, fruits and tea, mostly as its glycoside form. It composes the largest part of flavonoid intake from daily foods and is known to possess high antioxidant activity. Thus, dietary quercetin is thought to be important in the light of the prevention of oxidative stress-related diseases.
Absorption and metabolic conversion of quercetin have been investigated by a large number of researchers and it is now clarified that the main pathway for quercetin glycosides is conversion to conjugate metabolites by deglycosylation and following glucuronide/sulfate conjugation [7–9]. Although quercetin glycosides are subject to deglycosidation by enterobacteria for absorption in large intestine, the presence of a glucoside-hydrolyzing activity in small intestinal cells [10] and their glucose transport system also participate in the glucoside absorption [11, 12]. Therefore, the small intestine acts as an effective absorption site for glucose-bound glycosides.
Onion (Allium cepa L.) is known as a quercetin-rich vegetable [13] and has a high content of quercetin glucosides (mainly quercetin 4'-glucoside and quercetin 3,4'-diglucoside) [14, 15]. Previous studies have reported the absorption of quercetin from dietary onion and its consequences [16–26]. We have previously shown that co-ingestion of lipids or emulsifiers enhanced the accumulation of quercetin metabolites in rat blood plasma after the short-term intake of onion from the diet [16], as well as the absorption efficiency of orally administered quercetin in rats [17]. Onion intake was found to improve the diabetic status, including protection of DNA against oxidative damage, lowering of peroxidized lipids in the circulation and urine, and hypoglycemic and hypocholesterolemic effects [20, 22, 23]. Quercetin has already been shown to reduce the oxidative stress in streptozotocin (STZ)-induced diabetic rats [24, 25]. However, the significance of onion intake as a dietary source of quercetin for preventing diabetes-related oxidative stress is still unclear.
This study aimed to clarify the in vivo antioxidative effect of onion intake by assessing oxidative stress biomarkers in STZ-induced diabetic rats and comparing them with those of the rats fed a diet containing the same amount of quercetin aglycone as onion.
Materials and Methods
Materials
One variety of yellow-type onion (Allium cepa L.), ‘Doctor quercy’, was cultivated in Hokkaido and was supplied by Takii Seed Co. (Kyoto, Japan). This onion sample was freeze-dried and pulverized in a sample mill (WB-1 Wonder Blender, Osaka Chemical Co., Osaka, Japan). Cornstarch, sucrose, casein, cellulose powder, AIN-76 mineral mix and AIN-76 vitamin mix supplemented with choline hydrogen tartrate were purchased from Oriental Yeast Co. (Tokyo, Japan) to prepare semipurified diets. STZ, quercetin dehydrate, soybean oil and glutathione were purchased from Wako Pure Chemical Ind. (Osaka, Japan). Sulfatase type H-5 (from Helix pomatia), 2-thiobarbituric acid, glutathione (GSH) reductase, NADPH, and tert-butylhydroperoxide were purchased from Sigma Chemical Co. (St. Louis, MO). Quercetin 3-glucoside (Q3G) and isorhamnetin were obtained from Extrasynthese (Geney, France). Quercetin 4'-α-d-glucoside (Q4'G) and quercetin 3,4'-α-d-diglucoside (Q3,4'G) were supplied by Dr. T. Tsushida of the National Food Research Institute (Tsukuba, Japan). Cholesteryl ester hydroperoxides (CEOOH) for a standard compound were prepared by the method described by Arai et al. [27]. All other chemicals were of analytical or HPLC grade.
Measurement of quercetin glucosides in onion
Quercetin glucosides were extracted by suspending 200 mg of onion powder in 4 ml of 70% methanol for 24 h in darkness at room temperature with occasional vigorous shaking. After centrifuging at 3000 × g for 5 min, the extraction was repeated. The supernatants were combined and 70% methanol was added to a volume of 10 ml. The extract was passed through a 0.20-µm filter and analyzed by HPLC under the following conditions: column, TSK gel ODS-80Ts (5 µm, 4.6 × 150 mm; Tosoh, Tokyo, Japan); mobile phase, water-methanol-acetic acid (30:68:2, v/v/v); flow rate, 0.9 ml/min; column temperature, 35°C. The quercetin glucosides eluted from the column were monitored at 360 nm. They were identified from their retention times against those of respective standard compounds, and their concentrations were calculated using standard curves for each quercetin glucoside.
Animals and diets
Nine-week-old male Wistar rats weighing 190–200 g were purchased from Japan SLC (Hamamatsu, Japan) and fed a commercial diet (MF, Oriental Yeast Co., Tokyo, Japan) for 5 days before being used in the experiment. The animals were kept in an environmentally controlled animal facility operated on a 12 h dark/light cycle at 23 ± 1°C and 55% humidity. Diabetes was induced by a single intraperitoneal injection of STZ (25 mg/kg of body weight in 0.9% NaCl). Three days after this injection, fasting blood glucose levels were monitored using a commercial kit (glucose CII-test Wako; Wako Pure Chemical Ind., Osaka, Japan). Rats with blood glucose levels above 230 mg/dl were divided into three diabetic groups (diabetic control (DC), diabetic-onion (DO), and diabetic-quercetin (DQ)) of 6 rats each. One group of 6 untreated rats was included in this study as normal control (NC) group. For the following 12 weeks, each group of rats was fed the semipurified basal diet (NC and DC groups), onion diet containing 6.0% onion powder (DO group) or quercetin diet containing 0.026% quercetin dihydrate (DQ group) as shown in Table 1 ad libitum. Both onion diet and quercetin diet contained equivalent amounts of quercetin (0.023% quercetin aglycone). Body weights of the rats were monitored at weekly intervals, and plasma quercetin metabolite concentrations were determined at 3-week intervals. At the end of the experimental period, 24 h urine samples were collected. After an overnight fast, the blood was collected from the hearts under ether anesthesia and plasma was immediately separated by centrifugation at 1000 × g for 15 min at 4°C. The liver, kidney and heart were taken and weighed after perfused with a PBS (10 mM phosphate-buffered saline, pH 7.2) containing 0.16 mg/ml heparin to remove any red blood cells and clots. Liver and kidney samples were immediately frozen in liquid nitrogen. Plasma, urine and organ samples were stored at –80°C until analysis. These animal experiments were performed under the guidelines for animal experiments according to Notification No. 6 of the Japanese government.
Table 1.
Composition of the diets (%)
| Ingredient | Basal diet | Onion diet | Quercetin diet |
|---|---|---|---|
| Cornstarch | 48.2 | 45.3 | 48.1 |
| Sucrose | 9.9 | 9.3 | 9.9 |
| Casein | 24.7 | 23.2 | 24.7 |
| Cellulose powder | 7.9 | 7.4 | 7.9 |
| Mineral mix1 | 3.4 | 3.2 | 3.4 |
| Vitamin mix2 | 1.0 | 0.9 | 1.0 |
| Soybean oil | 4.9 | 4.7 | 4.9 |
| Onion powder | 0.0 | 6.0 | 0.0 |
| Quercetin dihydrate | 0.0 | 0.0 | 0.026 |
AIN-76 mineral mix (Oriental Yeast Co., Japan)
AIN-76 vitamin mix supplemented with choline hydrogen tartrate (Oriental Yeast Co.)
Determination of quercetin metabolites in rat plasma
The quercetin metabolites in rat plasma were quantitatively determined by HPLC according to the method previously described [17]. Plasma (50 µl) was mixed with 50 µl of a sulfatase type H-5 (25 units of sulfatase and 500 units of β-glucuronidase) solution in a 0.1 mM sodium acetate buffer at pH 5.0. The mixture was incubated at 37°C for 50 min. The released compounds were extracted by adding 900 µl of methanol-acetic acid (100:5, v/v) to the reaction mixture, vortexing for 30 s, sonicating for 30 s, again vortexing for 30 s, and finally centrifuging for 5 min at 4°C and 5000 × g. The supernatant was diluted with water (1:1, v/v), and 20 µl was injected into a CAPCELL PAK C18 MG HPLC column (5 µm, 4.6 × 150 mm; Shiseido, Tokyo, Japan). Isocratic elution was carried out with a mobile phase composed of water-methanol-acetic acid (53:45:2, v/v/v) and 50 mM lithium acetate at a flow rate of 0.9 ml/min. The eluate was monitored with a coulometric electrochemical detector (Coulochem II with a model 5010 analytical cell; ESA, Chelmsford, MA). The potential of electrodes 1 and 2 was +100 mV and +800 mV, respectively, vs the Pd reference. Quercetin and isorhamnetin were determined by an external standard method. The detection limits for quercetin and isorhamnetin were 5 nM and 10 nM, respectively, with a linear detector response up to 20 µM.
Determination of cholesterol and triacylglycerol in plasma
Total cholesterol, high-density lipoprotein cholesterol, free cholesterol and triacylglycerol in plasma were determined with respective enzymatic assay kits (Wako Pure Chemical Ind., Japan). The content of cholesteryl ester was calculated by subtracting free cholesterol from total cholesterol.
Measurement of thiobarbituric acid-reactive substances (TBARS) and CEOOH
The plasma TBARS concentration was determined by the fluorometric assay of Yagi [28]. The urea TBARS concentration was measured by the method of Kosugi et al. [29]. The liver TBARS levels were determined by the method of Uchiyama and Mihara [30] using the homogenate, which was prepared by homogenizing the liver with 9 volumes of 20 mM phosphate buffer (pH 7.4). For the measurement of CEOOH in plasma, 100 µl of plasma which was 4 times diluted with PBS (pH 7.4) was added to 3 ml of methanol containing 2.5 mM butylhydroxytoluene (BHT) and 3 ml of hexane. After vigorous mixing, the hexane layer was obtained and evaporated in vacuo. The residue was mixed with the solution of methanol/chloroform (95:5, v/v) and subjected to HPLC analysis for the determination of CEOOH [31]. HPLC was performed with a column of TSK-gel Octyl-80Ts (5 µm, 4.6 × 150 mm, Tosoh) and a mobile phase of 97% methanol at a flow rate of 1.0 ml/min. CEOOH was detected at 235 nm absorption.
Copper ion-induced lipid peroxidation of plasma and measurement of lipid peroxidation level
CuSO4 was added to plasma which was 4 times diluted with PBS (pH 7.4) at the final concentration of 0.5 mM and incubated at 37°C with continuous shaking. The peroxidation level after 24 h incubation was monitored with TBARS assay and CEOOH concentrations as described above.
Measurement of plasma antioxidative activity
The antioxidative activity was measured with an ELISA kit “Potential Anti Oxidant” for measuring a copper ion-reducing activity (Nikken SEIL Co. LTD., Japan Institute of the Control of Aging, Fukuroi, Japan).
Determination of 8-hydroxydeoxyguanosine (8-OHdG) and creatinine in urine
8-OHdG and creatinine in urine were determined with an ELISA kit “New 8-OHdG Check” (Nikken SEIL Co. LTD., Japan Institute of the Control of Aging) and a creatinine assay kit (Wako Pure Chemical Ind., Japan), respectively.
Measurement of liver glutathione peroxidase (GPx) activity
Livers (0.5–1.0 g) were exactly weighed, added with 9 volumes of PBS (10 mM, pH 7.2), and homogenized with Polytron homogenizer (KINEMATICA, AG, Co., Littau-Lucerne, Switzerland). After centrifugation for 5 min at 4°C and 10,000 × g, the resulting supernatants were diluted 100 times and used as liver samples for measuring GPx activity. All procedures were performed in an ice bath and the liver samples were stored at –80°C until assay. GPx activity was determined according to the method described by Flohe and Gunzler [32]. For the assay, 0.1 ml of Tris-HCl buffer (1 M, pH 8.0) added with 5 mM EDTA, 0.5 ml of distilled water, 0.1 ml of GSH (20 mM), 0.1 ml of GSH reductase (10 U/ml), 0.1 ml of NADPH (2 mM), and 0.1 ml of liver sample were mixed in the cuvette and prewarmed for 2 min at 37°C. Then 0.1 ml of tert-butylhydroperoxide (7 mM) was added and the consumption of NADPH at 37°C was monitored at 340 nm for 5 min.
Data analysis
Each reported value is presented as the mean ± SD. A statistical analysis was conducted by Dunnett’s post hoc multiple-comparison test to identify significantly different means, using StatView for Windows Ver. 5.0 (SAS Institute, Cary, NC). The level of significance was set at p<0.05.
Results
Content of quercetin glucosides in onion
The content of quercetin glucosides in the freeze-dried onion used here were determined by HPLC analysis (Table 2). Q3,4'G and Q4'G were present as major quercetin glucosides and Q3G as a minor glucoside. The total content of quercetin glucosides in this onion was 3.84 ± 0.31 mg of quercetin aglycone equivalent per gram of dry weight.
Table 2.
Quercetin glucoside content of onion ‘Doctor quercy’
| Compound | Content (mg of quercetin aglycone equivalent/g dry weight) |
|---|---|
| Quercetin 3,4'-diglucoside | 1.49 ± 0.12 |
| Quercetin 3-glucoside | 0.07 ± 0.01 |
| Quercetin 4'-glucoside | 2.28 ± 0.18 |
| Total | 3.84 ± 0.31 |
Values are means ± SD (n = 3).
Effect of onion on growth, food intake and organ weight
Table 3 shows the average food intake during the feeding period, the body weight gain and the organ weights after the feeding period. The average food intake was significantly higher in the diabetic rat groups (DC, DO and DQ), compared to normal rat group (NC). Average quercetin intakes in DO and DQ groups were 6.91 ± 0.53 and 6.69 ± 0.44 mg as quercetin aglycone equivalent per kilogram of initial body weight, respectively, and the difference of the average quercetin intakes between the two groups was insignificant. Despite more food intake, the body weight of diabetic groups (DC, DO and DQ) was reduced after the trial, in contrast to the NC group. Of organ weights, the liver and kidney weights were significantly higher in the diabetic rat groups (DC, DO and DQ) compared to the NC group, although no significant differences were observed among the three diabetic rat groups.
Table 3.
Average food intake, body weight gain and organ weights in rats after the feeding period
| Group1 | NC | DC | DO | DQ |
|---|---|---|---|---|
| Average food intake (g/day) | 16.8 ± 1.1a | 29.4 ± 2.1b | 30.0 ± 2.3b | 29.1 ± 1.9b |
| Body weight gain (g) | 72 ± 9a | –39 ± 16b | –22 ± 19b | –44 ± 9b |
| Organ weight (% of body weight) | ||||
| Heart | 0.28 ± 0.02 | 0.35 ± 0.06 | 0.31 ± 0.02 | 0.32 ± 0.03 |
| Liver | 2.53 ± 0.17a | 3.39 ± 0.30b | 3.26 ± 0.38b | 3.32 ± 0.34b |
| Kidney | 0.67 ± 0.06a | 1.27 ± 0.13b | 1.18 ± 0.09b | 1.21 ± 0.17b |
NC, normal rats fed basal diet; DC, diabetic rats fed basal diet; DO, diabetic rats fed onion diet; DQ, diabetic rats fed quercetin diet. Rats were kept on the diets ad libitum for 12 weeks. Onion diet and quercetin diet contained equal amounts of quercetin (0.023% quercetin aglycone). Values are means ± SD (n = 6). Values with different superscripts are significantly different (p<0.05).
Accumulation of quercetin metabolites in plasma
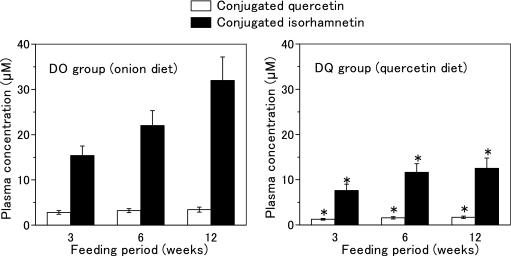
In either NC or DC group, no prominent peak corresponding to quercetin aglycone appeared in the chromatogram of the extract from rat plasma treated with sulfatase H-5, which possesses both sulfatase activity and glucuronidase activity. On the other hand, two distinct peaks corresponding to quercetin aglycone and isorhamnetin (3'-methoxyquercetin), as identified in our previous papers [16, 17], emerged in the chromatograms of the DO and DQ groups. However, no peak appeared in the chromatograms without sulfatase H-5 treatment. These results indicate that conjugated quercetin and conjugated isorhamnetin accumulated in the plasma, which liberated their aglycone by hydrolysis with sulfatase H-5. Fig. 1 shows the plasma concentrations of conjugated quercetin and conjugated isorhamnetin of the DO and DQ groups during the feeding period. The total quercetin metabolite concentrations, which were calculated as the sum of conjugated quercetin and conjugated isorhamnetin concentrations, were significantly higher in the DO group compared to the DQ group.
Fig. 1.
Concentrations of quercetin metabolites in the plasma of diabetic rats fed onion diet or quercetin diet. Onion diet and quercetin diet containing equal amounts of quercetin (0.023% quercetin aglycone) were fed diabetic rats ad libitum for 12 weeks. Values are means ± SD (n = 6).
*Significantly different from the rat group fed onion diet (p<0.05).
Effect of onion intake and quercetin intake on glucose, cholesterol and triacylglycerol levels in blood plasma
Table 4 shows the concentrations of glucose, cholesterol and triacylglycerol in blood plasma after the feeding period. Plasma glucose levels in the DC group were remarkably higher than that of the NC group. Increased glucose levels in diabetic rats were lowered by onion intake (DO group) but not by quercetin intake (DQ group). Plasma triacylglycerol levels were lower in the DO and DQ groups compared to the DC group. On the other hand, no significant effects of both onion intake and quercetin intake on cholesterol levels were observed.
Table 4.
Concentrations of glucose, cholesterol and triacylglycerol in rat plasma after the feeding period.
| Group1 | NC | DC | DO | DQ |
|---|---|---|---|---|
| Glucose (mg/dl) | 126 ± 18a | 515 ± 47b | 437 ± 32c | 509 ± 41b |
| Total cholesterol | 68 ± 9 | 83 ± 10 | 77 ± 15 | 85 ± 14 |
| HDL-cholesterol | 57 ± 6 | 61 ± 8 | 66 ± 12 | 71 ± 13 |
| Free cholesterol | 19 ± 3 | 24 ± 4 | 22 ± 4 | 25 ± 5 |
| Esterified cholesterol | 49 ± 6 | 59 ± 6 | 55 ± 11 | 60 ± 9 |
| Triacylglycerol | 73 ± 12a | 116 ± 17b | 69 ± 14a | 84 ± 15a |
NC, normal rats fed basal diet; DC, diabetic rats fed basal diet; DO, diabetic rats fed onion diet; DQ, diabetic rats fed quercetin diet. Rats were kept on the diets ad libitum for 12 weeks. Onion diet and quercetin diet contained equal amounts of quercetin (0.023% quercetin aglycone). Values are means ± SD (n = 6). Values with different superscripts are significantly different (p<0.05).
Effect of onion intake and quercetin intake on TBARS and CEOOH levels in plasma and liver
The DC group showed higher plasma TBARS and CEOOH levels than the NC group (Table 5). These lipid peroxidation biomarkers tended to be lower in the DO and DQ groups than in the DC group, although no significant difference was observed among the three groups. Liver TBARS levels were not significantly different between the DC and NC groups. No significant differences were observed among the DC, DO and DQ groups.
Table 5.
TBARS and CEOOH levels in plasma and liver of rats after the feeding period.
| Group1 | NC | DC | DO | DQ |
|---|---|---|---|---|
| Plasma | ||||
| TBARS (µM) | 6.4 ± 1.1a | 10.0 ± 1.9b | 8.1 ± 1.5ab | 8.4 ± 1.6ab |
| CEOOH (nM) | 2.7 ± 0.5a | 7.4 ± 1.5b | 6.2 ± 1.2b | 6.5 ± 1.3b |
| Liver | ||||
| TBARS (nmol/g of liver) | 128 ± 11 | 136 ± 16 | 130 ± 12 | 132 ± 10 |
NC, normal rats fed basal diet; DC, diabetic rats fed basal diet; DO, diabetic rats fed onion diet; DQ, diabetic rats fed quercetin diet. Rats were kept on the diets ad libitum for 12 weeks. Onion diet and quercetin diet contained equal amounts of quercetin (0.023% quercetin aglycone). Values are means ± SD (n = 6). Values with different superscripts are significantly different (p<0.05).
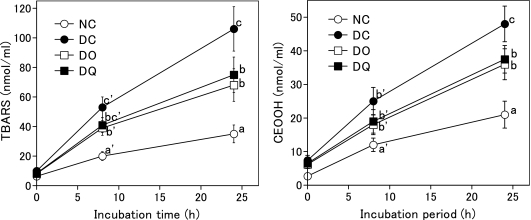
Susceptibility of plasma to lipid peroxidation
Fig. 2 shows TBARS and CEOOH levels in rat plasma after incubation with copper ion. Both levels significantly increased in the order of NC group<DO group and DQ group<DC group after 24 h incubation. This result indicates that the susceptibility of plasma to copper ion-induced lipid peroxidation of diabetic rats was higher than that of normal rats and that the intake of onion or quercetin suppressed diabetes-induced enhancement of the susceptibility to plasma lipid peroxidation.
Fig. 2.
Copper ion-induced lipid peroxidation in rat plasma after the feeding period. Rats were kept on basal diet, onion diet, or quercetin diet ad libitum for 12 weeks. Onion diet and quercetin diet contained equal amounts of quercetin (0.023% quercetin aglycone). NC, normal rats fed basal diet; DC, diabetic rats fed basal diet; DO, diabetic rats fed onion diet; DQ, diabetic rats fed quercetin diet. Values are means ± SD (n = 6). Values with different superscripts are significantly different (p<0.05).
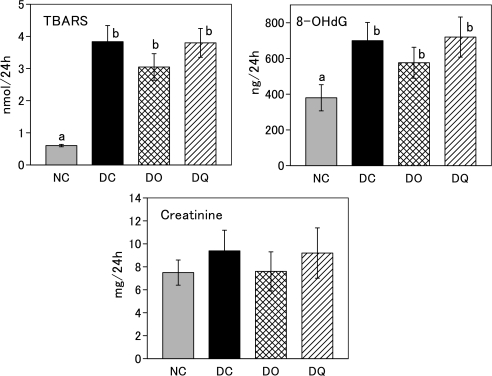
Effect of onion intake or quercetin intake on TBARS, 8-OHdG and creatinine levels in urine
TBARS and 8-OHdG in urine were markedly increased in DC group compared to NC group (Fig. 3). Although no significant differences were observed in TBARS and 8-OHdG among the three diabetic rat groups, the DO group tended to exhibit lower values than the DC and DQ groups. Creatinine level did not differ significantly among the four groups.
Fig. 3.
TBARS, 8-OHdG and creatinine levels in rat urine after the feeding period. Rats were kept on basal diet, onion diet, or quercetin diet ad libitum for 12 weeks. Onion diet and quercetin diet contained equal amounts of quercetin (0.023% quercetin aglycone). NC, normal rats fed basal diet; DC, diabetic rats fed basal diet; DO, diabetic rats fed onion diet; DQ, diabetic rats fed quercetin diet. Values are means ± SD (n = 6). Values with different superscripts are significantly different (p<0.05).
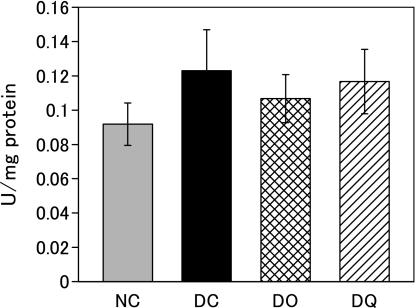
Effect of onion intake and quercetin intake on hepatic GPx activity
Fig. 4 shows hepatic GPx activity after the feeding period. Diabetic rats (DC group) tended to exhibit higher activity compared to normal rats (NC group). No significant difference was observed in the activity among the DC, DO and DQ groups, indicating that the neither onion intake nor quercetin intake affected hepatic GPx activity under diabetic conditions.
Fig. 4.
Hepatic glutathione peroxidase activity after the feeding period. Rats were kept on basal diet, onion diet, or quercetin diet ad libitum for 12 weeks. Onion diet and quercetin diet contained equal amounts of quercetin (0.023% quercetin aglycone). NC, normal rats fed basal diet; DC, diabetic rats fed basal diet; DO, diabetic rats fed onion diet; DQ, diabetic rats fed quercetin diet. Values are means ± SD (n = 6).
Discussion
The composition of quercetin glucosides in the onion of this study agreed with those reported previously [14, 15]. From the total content of 3.84 ± 0.31 mg quercetin aglycone equivalent per gram of dry weight and the water content of 10.1% of this onion, the total quercetin glucoside content was calculated as 38.0 mg of quercetin aglycone equivalent per 100 g fresh weight. Since most yellow onion varieties cultivated in Japan and USA contain less than 30 mg of quercetin aglycone equivalent per 100 g fresh weight [14, 15], the onion variety used in this study is one of the richest in quercetin glucosides among the yellow onion varieties.
Conjugated quercetin and conjugated isorhamnetin accumulated as quercetin metabolites in the blood plasma of rats fed the onion diet or the quercetin diet. The plasma concentrations of conjugated isorhamnetin were higher than those of conjugated quercetin in both rat groups. Isorhamnetin, that is 3'-methoxyquercetin, has been reported to have in vitro antioxidative activity, although the activity is lower than quercetin [33]. Therefore, the conjugates of isorhamnetin are expected to act as antioxidants in vivo.
Approximately twice higher concentrations of quercetin metabolites were accumulated in the plasma of the onion diet group compared to the quercetin diet group, despite of the intake of the same amount of quercetin as its aglycone equivalent (Fig. 1). It is therefore likely that quercetin glucosides in onion (mainly Q4'G and Q3,4'G) are absorbed more efficiently than quercetin aglycone. Superior absorption of quercetin glucoside to its aglycone may be explained by quercetin glucoside-specific cellular uptake through an intestinal sodium-dependent glucose transporter or effective hydrolysis of quercetin glucoside by β-glucosidase activity of intestinal epithelial cells [11, 12]. Alternatively, some coexisting components in onion may enhance its intestinal absorption.
It is known that STZ-induced diabetic rats exhibit increases in serum glucose concentrations and the weight ratio of kidney to body weight and the decrease of the body weight gain [34, 35]. The present study also showed the same effects in STZ administration to the rats. Neither onion diet nor quercetin diet affected the weight ratio of kidney to body weight and the body weight gain in the diabetic rats. However, plasma glucose levels were significantly decreased by onion diet, as reported previously by Babu et al. [22]. On the other hand, quercetin diet showed no significant effect on plasma glucose level. This result indicates that onion intake, but not quercetin intake, is effective for suppressing the development of diabetes.
Both onion diet and quercetin diet lowered the plasma triacylglycerol level, which is known to increase in STZ-induced diabetic rats [22]. It has been reported that lipid peroxidation products including TBARS in plasma [22, 25] and tissues [36] were increased in STZ-induced diabetes and that plasma TBARS was lowered by onion diet or quercetin diets [22, 25]. In the present study, we found that both onion diet and quercetin diet showed a tendency to lower both plasma TBARS and CEOOH.
Susceptibility of the plasma to lipid peroxidation, as indicated by the increase of TBARS and CEOOH after the addition of copper ion, was significantly attenuated by both onion diet and quercetin diet, indicating that both diets enhance the antioxidative activity of blood plasma. Sanders et al. [24] showed that the oral administration of quercetin in diabetic rats brought about significant decrease in hepatic GPx activity, which is known to be increased in diabetic animals [37, 38]. In our study, hepatic GPx activity tended to be lowered by both onion intake and quercetin intake. Three oxidative stress biomarkers in urine, that is, 8-OHdG, TBARS and creatinine, tended to be reduced by onion diet but not by quercetin diet, indicating that the attenuating effect of onion intake against oxidative stress in diabetes is superior to that of quercetin intake.
There are some previous papers on the hypoglycemic effects [25, 39] and insulin secretion-enhancing effects [40, 41] of quercetin. The intraperitoneal injection of quercetin (10 and 15 mg/kg body weight) for 10 days [39] and oral administration of quercetin (50 and 80 mg/kg body weight) for 45 days [25] were shown to decrease the plasma glucose level of STZ-induced diabetic rats. From these findings, the hypoglycemic effect of onion intake observed in the present study may be attributed to quercetin in onion. The result that quercetin intake had no significant hypoglycemic effect in this experiment is likely to be caused by lower absorption efficiency and bioavailability of quercetin aglycone compared to quercetin glucosides in onion.
The superiority of onion intake to quercetin diet in lowering oxidative stress biomarkers in urine also may be at least in part caused by higher absorption efficiency and bioavailability of quercetin glucosides compared to its aglycone. Onion contains sulfur-containing compounds such as dialkyl disulfides and their oxidized thiols, which can trap electrons from other systems [42]. Onion oil containing these compounds has been reported to have an antioxidative effect against the oxidative damage caused by nicotine in experimental animals [43, 44]. Thus, these constituents also may contribute to the protective effects of onion against oxidative stress in STZ-induced diabetic rats.
In conclusion, quercetin glucosides from onion accumulated in the plasma as their conjugated metabolites at higher concentration compared to the ingestion of equal amounts of quercetin aglycone, and dietary onion lowered plasma glucose and oxidative stress biomarkers either more effectively or equally to quercetin diet. Overall, the protective effects of quercetin-rich onion intake seem to be higher or at least equal to the intake of quercetin aglycone in diabetes-induced oxidative stress. Further studies on the antioxidative effect of quercetin glucosides and other components including sulfur-containing compounds in diabetes are required to assess the significance of dietary onion in the attenuation of diabetes-related oxidative stress.
Acknowledgments
We thank Dr. T. Tsushida, National Food Research Institute, for kindly presenting quercetin glucosides, and Takii Seed Co. for providing the onion. This study was partly supported by the Ministry of Agriculture, Forestry and Fishery Food Project, Japan.
References
- 1.de Whalley C.V., Rankin S.M., Hoult J.R., Jessup W., Leake D. Flavonoids inhibit the oxidative modification of low density lipoproteins by macropharges. Biochem. Pharmacol. 1990;39:1743–1750. doi: 10.1016/0006-2952(90)90120-a. [DOI] [PubMed] [Google Scholar]
- 2.Miura S., Watanabe J., Sano M., Tomita T., Osawa T., Hara Y., Tomita I. Effects of various natural antioxidants on the Cu2+-mediated oxidative modification of low-density lipoprotein. Biol. Pharm. Bull. 1995;18:1–4. doi: 10.1248/bpb.18.1. [DOI] [PubMed] [Google Scholar]
- 3.da Silva E.L., Tsushida T., Terao J. Inhibition of mammalian 15-lipoxygenase-dependent lipid peroxidation in low-density lipoprotein by quercetin and quercetin monoglucosides. Arch. Biochem. Biophys. 1998;349:313–320. doi: 10.1006/abbi.1997.0455. [DOI] [PubMed] [Google Scholar]
- 4.Hertog M.G.L., Kromhout D., Aravanis C., Blackburn H., Buzina R., Fidanza F., Giampaoli S., Jansen A., Menotti A., Nedelijkovic S., Pekkarinen M., Simic B.S., Toshoma H., Feskens E.J.M., Hollman P.C.H., Katan M. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 5.Knekt P., Jarvinen R., Reunanen A., Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. Br. Med. J. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yochum L., Kushi L., Meyer K., Folsom A. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 7.Terao J. Dietary flavonoids as antioxidants in vivo: conjugated metabolites of (–)-epicatechin and quercetin participate in antioxidative defense in blood plasma. J. Med. Investigation. 1999;46:159–168. [PubMed] [Google Scholar]
- 8.Murota K., Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 9.Terao J. Quercetin glycosides: their absorption, metabolism and antioxidant activity. Vitamins. 2005;79:3–11. [Google Scholar]
- 10.Day A.J., Canada F.J., Diaz J.C., Kroon P.A., Mclauchlan R., Faulds C.B., Plumb G.W., Morgan M.R., Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolyzed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 11.Walgren R.A., Lin J.T., Kinne R.K., Walle T. Cellular uptake of dietary flavonoid quercetin 4'-beta-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000;294:837–843. [PubMed] [Google Scholar]
- 12.Gee J.M., DuPont M.S., Day A.J., Plumb G.W., Williamson G., Johnson I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- 13.Azuma K., Nakayama M., Koshioka M., Ippoushi K., Yamaguchi Y., Kohata K., Yamauchi Y., Ito H., Higashio H. Phenolic antioxidants from the leaves of Corchorus olitorius L. J. Agric. Food Chem. 1999;47:3963–3966. doi: 10.1021/jf990347p. [DOI] [PubMed] [Google Scholar]
- 14.Tsushida T., Suzuki M. Content of flavonol glucosides and some properties of enzymes metabolizing the glucosides in onion. Nippon Shokuhin Kagaku Kogaku Kaishi. 1996;43:642–649. [Google Scholar]
- 15.Price K.R., Bacon J.R., Rhodes J.C. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa L.) J. Agric. Food Chem. 1997;45:938–942. [Google Scholar]
- 16.Azuma K., Ippoushi K., Ito H., Horie H., Terao J. Enhancing effect of lipids and emulsifiers on the accumulation of quercetin metabolites in blood plasma after the short-term ingestion of onion by rats. Biosci. Biotechnol. Biochem. 2003;67:2548–2555. doi: 10.1271/bbb.67.2548. [DOI] [PubMed] [Google Scholar]
- 17.Azuma K., Ippoushi K., Ito H., Higashio H., Terao J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J. Agric. Food Chem. 2002;50:1706–1712. doi: 10.1021/jf0112421. [DOI] [PubMed] [Google Scholar]
- 18.McAnlis G.T., McEneny J., Pearce J., Young I.S. Absorption and antioxidant effects of quercetin from onions, in man. Eur. J. Clin. Nutr. 1999;53:92–96. doi: 10.1038/sj.ejcn.1600682. [DOI] [PubMed] [Google Scholar]
- 19.Moon J.-H., Nakata R., Oshima S., Inafuku T., Terao J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regulatory Integrative Comp. Phys. 2000;279:R461–R467. doi: 10.1152/ajpregu.2000.279.2.R461. [DOI] [PubMed] [Google Scholar]
- 20.Lean M.E.J., Noroozi M., Kelly I., Burns J., Talwar D., Sattar N., Crozier N. Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes. 1999;48:176–181. doi: 10.2337/diabetes.48.1.176. [DOI] [PubMed] [Google Scholar]
- 21.Boyle S.P., Dobson V.L., Duthie S.J., Kyle J.A.M., Collins A.R. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000;39:213–223. doi: 10.1007/s003940070014. [DOI] [PubMed] [Google Scholar]
- 22.Babu P.S., Srinivasan K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol. Cell. Biochem. 1997;175:49–57. doi: 10.1023/a:1006881027166. [DOI] [PubMed] [Google Scholar]
- 23.Babu P.S., Srinivasan K. Renal lesions in streptozotocin-induced diabetic rats maintained on onion and capsaicin containing diets. J. Nutr. Biochem. 1999;10:477–483. doi: 10.1016/s0955-2863(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 24.Sanders R.A., Rauscher F.M., Watkins J.B. Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxic. 2001;15:143–149. doi: 10.1002/jbt.11. [DOI] [PubMed] [Google Scholar]
- 25.Mahesh T., Menon V.P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004;18:123–127. doi: 10.1002/ptr.1374. [DOI] [PubMed] [Google Scholar]
- 26.Femia A.P., Caderni G., Ianni M., Salvadori M., Schijlen E., Collins G., Bovy A., Dolara P. Effect of diets fortified with tomatoes and onions with variable quercetin-glycoside content on azoxymethane-induced aberrant crypt foci in the colon of rats. Eur. J. Nutr. 2003;42:346–352. doi: 10.1007/s00394-003-0431-5. [DOI] [PubMed] [Google Scholar]
- 27.Arai H., Terao J., Abdalla D.S.P., Suzuki T., Takama K. Coulometric detection in high-performance liquid chromatographic analysis of cholesteryl ester hydroperoxides. Free Radic. Biol. Med. 1996;20:365–371. doi: 10.1016/0891-5849(96)02062-x. [DOI] [PubMed] [Google Scholar]
- 28.Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–331. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 29.Kosugi H., Kojima T., Kikugawa K. Characteristics of the thiobarbituric acid reactivity of oxidized fats and oils. J. Am. Oil Chem. Soc. 1991;68:51–55. [Google Scholar]
- 30.Uchiyama M., Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 31.da Silva E.L., Piskula M.K., Yamamoto N., Moon J.-H., Terao J. Quercetin metabolites inhibit copper ion-induced lipid peroxidation in rat plasma. FEBS Lett. 1998;430:405–408. doi: 10.1016/s0014-5793(98)00709-1. [DOI] [PubMed] [Google Scholar]
- 32.Flohe L., Gunzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 33.Letan A. The relation of structure to antioxidant activity of quercetin and some of its derivatives. I Primary activity. J. Food Sci. 1966;31:518–523. [Google Scholar]
- 34.Ree S.J., Choe W.K., Ha T.U. The effect of vitamin E on the antioxidative defense mechanism in streptozotocin-induced diabetic rats. Nippon Shokuhin Eiyou Gakkaishi. 1995;48:451–457. [Google Scholar]
- 35.Sugimoto E., Igarashi K., Kubo K., Molyneux J., Kubomura K. Protective effects of boysenberry anthocyanins on oxidative stress in diabetic rats. Food Sci. Technol. Res. 2003;9:345–349. [Google Scholar]
- 36.Sun F., Iwaguchi K., Shudo R., Nagaki Y., Tanaka K., Ikeda K., Tokumaru S., Kojo S. Change in tissue concentrations of lipid hydroperoxides, vitamin C and vitamin E in rats with strepotozotocin-induced diabetes. Clin. Sci. 1999;96:185–190. [PubMed] [Google Scholar]
- 37.Kakker R., Kalra J., Mantha S.V., Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 38.Rauscher F.M., Sanders R.A., Watkins J.B. Effects of piperine on antioxidant pathways in tissues from normal and streptozotocin-induced diabetic rats. J. Mol. Biochem. Toxicol. 2000;14:329–334. doi: 10.1002/1099-0461(2000)14:6<329::AID-JBT5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.Vessal M., Hemmati M., Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2003;135C:357–364. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 40.Hii C.S., Howell S.L. Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. J. Endocrinol. 1985;107:1–8. doi: 10.1677/joe.0.1070001. [DOI] [PubMed] [Google Scholar]
- 41.Best L., Trebilcock R., Tomlinson S. Acute stimulation of pancreatic islets by inhibitors of lactic acid transport. Biochem. Pharmacol. 1991;41:405–409. doi: 10.1016/0006-2952(91)90537-f. [DOI] [PubMed] [Google Scholar]
- 42.Klanns-Dieter A. In: Sulfur contend free radicals. in Radioprotectors and anticarcinogens. Nygaard O.F., Simic M.G., editors. Academic press; New York: 1983. pp. 23–42. [Google Scholar]
- 43.Helen A., Rajasree C.R., Krishnakumar K., Augusti K.T., Vijayammal P.L. Antioxidant role of oils isolated from garlic (Allium sativum Linn) and onion (Allium cepa Linn) on nicotine-induced lipid peroxidation. Vet. Human Toxicol. 1999;41:316–319. [PubMed] [Google Scholar]
- 44.Helen A., Krishnakumar K., Vijayammal P.L., Augusti K.T. Antioxidant effect of onion (Allium cepa Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicol. Lett. 2000;116:61–68. doi: 10.1016/s0378-4274(00)00208-3. [DOI] [PubMed] [Google Scholar]