Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) have gastrointestinal side effects such as dyspepsia, peptic ulcer, hemorrhage, and perforation. Misoprostol and PPIs have been used to prevent NSAID-induced gastroduodenal injury. Rebamipide increases gastric mucus and stimulates the production of endogenous prostaglandins. The prophylactic effect of rebamipide on NSAID-induced gastrointestinal complications is unknown. The aim of this study was to compare NSAID-induced gastrointestinal complications in rebamipide- and misoprostol-treated groups. Patients were randomized to two groups and took a conventional NSAID plus rebamipide or misoprostol for 12 weeks. Gastric mucosal damage was evaluated by endoscopy at screening and the end of the study. The prevalences of active gastric ulcer were 7/176 (3.9%) in the rebamipide group and 3/156 (1.9%) in the misoprostol group. The prevalences of peptic ulcer were 8/176 (4.5%) in the rebamipide group and 7/156 (4.4%) in the misoprostol group. The cumulative incidences of peptic ulcer in the high-risk subgroup were 6/151 (4.0%) for rebamipide and 6/154 (3.9%) for misoprostol. In conclusion, rebamipide prevented NSAID-induced peptic ulcer as effectively as misoprostol in patients on long-term NSAID therapy. Rebamipide may be a useful therapeutic option for the prevention of NSAID-induced gastrointestinal ulcer because of its therapeutic effect and safety.
Keywords: rebamipide, misoprostol, NSAID, gastrointestinal tract, clinical trial
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) contribute to the management of arthritis and other inflammatory conditions. Upper gastrointestinal (GI) adverse events, ranging from dyspepsia to life-threatening complications such as perforation, are the primary side effects associated with NSAIDs. Gastroprotective agents are therefore often prescribed concomitantly with NSAIDs. Ulcers can be documented endoscopically in up to 40% of chronic NSAID users; however, it is estimated that as many as 85% of these ulcers never become clinically apparent [1]. Misoprostol and omeprazole, are only prescribed drugs in decreasing the NSAID-associated GI mucosal injury. Prophylaxis by gastroprotective agents decreases ulcer complications associated with long-term NSAID use.
Misoprostol is a synthetic prostaglandin (PG) E1 analogue that has been shown to be gastroprotective by augmenting depleted mucosal defense factors and inhibiting gastric acid secretion. Numerous studies have been performed on the use of misoprostol to prevent NSAID-induced gastrointestinal complications. A dose–response study showed that 600 ug and 800 ug doses of misoprostol yielded similar protective effects [2]. Misoprostol reduces the risk of endoscopic ulcers, even at doses of 400, 600, and 800 µg/day [2–4]. However, a high incidence of diarrhea has been reported and documented in many patients on continuous misoprostol treatment.
Rebamipide (2-(4-chlorobenzoylamino)-3-[2-(1H)-quinolinon-4-yl]-propionic acid) (Otsuka Pharmaceutical Co., Tokyo) is a cytoprotective antiulcer drug that enhances defense mechanisms in the gastric mucosa by increasing gastric mucus and stimulating the production of endogenous prostaglandins, and has been reported to reduce gastric mucosal injury [5, 6]. The anti-inflammatory effects of rebamipide are due to its inhibitory effect on the production of superoxides [7]. Rebamipide significantly accelerated ulcer healing, in association with the expression of epidermal growth factor and epidermal growth factor receptor [8]. The efficacy of rebamipide in preventing NSAID-induced gastric injury has been reported in healthy volunteers on indomethacin treatment [9]. However, the efficacy of rebamipide in preventing NSAID-induced gastrointestinal complications has not been determined. The purpose of this study was to evaluate the cumulative incidence of NSAID-induced gastrointestinal complications such as ulcers in rebamipide- and misoprostol-treated groups in a randomized, multicenter, controlled trial.
Methods
Study design
A randomized, multicenter, controlled, open-label trial was performed in Korea, China, and Thailand. The subjects were recruited for treatment sequences in random fashion according to a randomization schedule for the treatment period from departments of rheumatology and internal medicine outpatient clinics. Individuals who were at least 18 years of age with rheumatoid arthritis, osteoarthritis, or ankylosing spondylitis or any other condition that required continuous NSAID therapy for more than 12 weeks were included in this study. Exclusion Criteria were as follows: individuals with a history of a serious medical condition (chronic liver disease or chronic renal disease); individuals with any other clinically significant gastrointestinal diseases confirmed by endoscopy, such as GERD, esophageal varix, peptic ulcer, or malignancy; those with a history of any documented gastric surgery or any malignant disease; those reporting recent use (within 4 weeks prior to the study) of sucralfate, H2-RA, misoprostol, PPIs, prokinetics, or any other medications that could affect acid secretion or gastrointestinal motility; and those reporting recent use (within 4 weeks prior to the study) of any NSAIDs, corticosteroids, anticholinergics, antineoplastics, or anticoagulants.
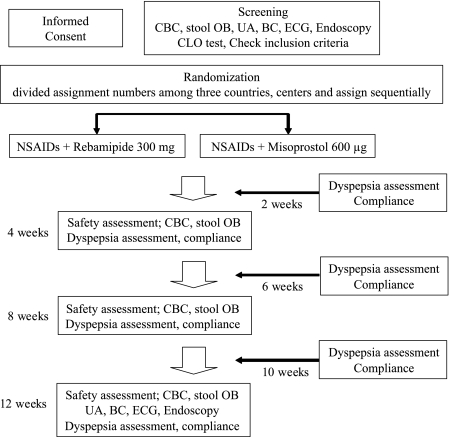
Patients were randomly assigned to receive either 100 mg rebamipide or 200 µg misoprostol t.i.d. for 12 weeks. Assessment of dyspepsia using a diary card and compliance with the trial drug and NSAIDs was performed every 2 weeks until 12 weeks (Fig. 1). Concomitant use of NSAIDs during this study was limited to aceclofenac, diclofenac, fenoprofen, ibuprofen, naproxen, and sulindac. Patients with a rate of compliance of less than 85% with study drugs and minimum daily dosage of NSAIDs were excluded from the study. The severity of dyspeptic symptoms was scored on a four-point scale. The study protocol was approved by the Ethics Committees of each of the 12 participating hospitals, and written informed consent was obtained from each of the patients.
Fig. 1.
Flow diagram of subjects progress through the study
Randomization
Subjects were recruited for the treatment sequences in random fashion according to a randomization schedule for the treatment period. A randomization number that was associated with a specific treatment with either rebamipide or misoprostol was assigned to each patient included in the study. The treatment was randomly assigned using an allocation ratio of 1:1 for the two treatment groups. Patients were allocated to the next available randomization number at each center. Allocation of randomized numbers was performed using the SAS program.
Endoscopic evaluation
Two endoscopists reviewed still images to establish standardized reporting criteria for ulcers and other lesions. At endoscopy, video or photographic images were recorded separately for the esophagus, gastric body, gastric antrum, duodenal bulb, and second part of the duodenum. An ulcer was defined as an excavated mucosal break 3 mm or more in diameter, measured with biopsy forceps or a custom-made device. Erosions were defined as superficial mucosal breaks, and intramucosal hemorrhages as hemorrhagic lesions without overlying mucosal breaks. Endoscopic mucosal damage was evaluated using the modified Lanza score, of 0 to 5, during the screening period and at the end of the study. Endoscopic diagnoses of subjects were reviewed after the study by four different endoscopists not participating in this study. Helicobacter pylori status was determined by CLO test (Kimberly-Clark Corporation, Irving, TX) during screening endoscopy.
Unscheduled endoscopy
Subjects who had intractable symptoms of dyspepsia, who were positive for occult blood in stool, had anemia (more than 1 g/dL decrease from baseline value of hemoglobin), hematemesis or hematochezia, or required rescue antacid consumption for more than 7 days underwent unscheduled endoscopy to ensure patient safety.
Evaluation of adverse events
Adverse events were of the following categories: (1) Symptoms of dyspepsia: the following gastrointestinal symptoms were to be recorded in the symptom diary-fullness, early satiety, bloating, nausea, vomiting, heartburn, and acid regurgitation. (2) Gastrointestinal complications, defined as intractable abdominal pain, intractable diarrhea, and intractable symptoms of dyspepsia. The criterion for intractable abdominal pain was moderate pain on the four-point scale lasting more than 3 days per week. The criterion for intractable diarrhea was more than 4 per day for more than 3 days per week. The criterion for intractable symptoms of dyspepsia was moderate pain on the four-point scale lasting more than 7 days between visits.
End points
The primary endpoint was the cumulative incidence of gastric or duodenal ulceration at 12 weeks. The secondary endpoint was the cumulative incidence of adverse events.
Statistical analysis
We referred to the results of a previous clinical trial in determining the trial size [10]. Equivalence of rebamipide to misoprostol at the primary endpoint was considered demonstrated when the 95% two-sided confidence intervals of the difference between the rebamipide and misoprostol groups was not less than the pre-specified limit of equivalence of –0.07 and not more than the pre-specified limit of equivalence of 0.07. A categorical model underlying the confidence interval with the center and treatment as fixed factors was used. Laboratory tests, age, sex, H. pylori infection status, and body weight measurements from pretreatment to posttreatment were analyzed both within and between the treatment groups using Student’s unpaired t test or the Wilcoxon rank-sum test. A shift table with chi-square analysis of the change in normal range from baseline was last values over the course of the study. All statistical tests were two-sided, with a 5% level of significance. In addition, 95% two-sided confidence intervals were used. The SAS program (Ver. 8.1) was used for statistical analyses.
Results
Demographic characteristics of patients
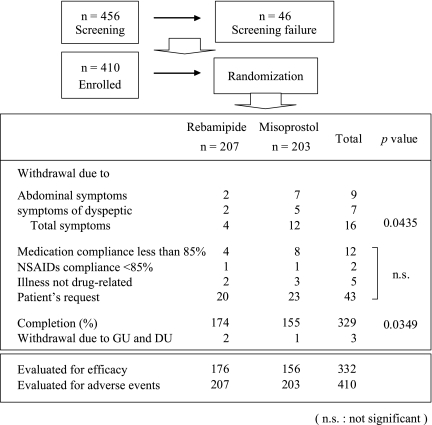
A total of 456 patients were scheduled to undergo endoscopic screening for enrollment in this trial. Forty-six patients were excluded during the screening period, and 410 patients were enrolled and evaluated (Fig. 2). The numbers of enrolled patients from China, Korea, and Thailand were 174, 153, and 83, respectively. The treatment groups were well-balanced with respect to treatment with NSAIDs, sex, age, and frequency of H. pylori infection (Table 1). The most commonly used NSAIDs were diclofenac (36.8%), aceclofenac (34.9%), and naproxen (7.8%) (Table 2).
Fig. 2.
Enrollment and randomization of the studied population
Symptoms; Two patients had intractable pain, and 2 were vomiting in the rebamipide group. One had stomach pain, 4 had abdominal distension, 2 had lower abdominal pain, and 5 had intractable diarrhea in the misoprostol group.
Table 1.
Demographic characteristics of enrolled patients
| Characteristics | Rebamipide | Misoprostol | p value | |
|---|---|---|---|---|
| Number | 207 | 203 | ||
| Sex (M:F) | 54:153 | 67:136 | 0.1244 | |
| Age (mean ± SE) | 48 ± 11 | 46 ± 12 | 0.3754 | |
| H. pylori-positive | 48.5% | 41.7% | 0.2342 | |
| Pre MLS | 0 | 128 | 137 | 0.2633 |
| 1 | 38 | 29 | 0.2633 | |
| 2 | 27 | 17 | 0.2633 | |
| 3 | 12 | 15 | 0.2633 | |
| 4 | 1 | 5 | 0.2633 | |
| 5 | 0 | 0 | 0.2633 | |
| Countries | China | 86 | 88 | |
| Korea | 76 | 77 | ||
| Thailand | 45 | 38 |
MLS: modified Lanza score, statistical analysis by chi-square test
Table 2.
Daily minimum NSAID dosages
| NSAID | Minimum daily dosage | Rebamipide n (%) | Misoprostol n (%) | All patients n (%) | p value |
|---|---|---|---|---|---|
| Diclofenac | 75 mg/day | 75 (36.2) | 76 (37.4) | 151 (36.8) | n.s. |
| Aceclofenac | 100 mg/day | 86 (41.5) | 57 (28.1) | 143 (34.9) | n.s. |
| Naproxen | 500 mg/day | 13 (6.3) | 19 (9.4) | 32 (7.8) | n.s. |
| Sulindac | 100 mg/day | 6 (2.9) | 10 (4.9) | 16 (3.9) | n.s. |
| Ibuprofen | 400 mg/day | 5 (2.4) | 7 (3.4) | 12 (2.9) | n.s. |
| Fenoprofen | 600 mg/day | 3 (1.4) | 5 (2.5) | 8 (2.0) | n.s. |
| Mixed | 19 (9.1) | 29 (14.3) | 48 (11.7) | n.s. |
Mixed: More than 2 NSAIDs
Statistical analysis by chi-square test (n.s.: not significant)
Evaluation of the incidence of gastrointestinal ulcers and adverse events included 207 patients in the rebamipide group and 203 in the misoprostol group. For this analysis, 31 patients were excluded from the rebamipide group and 47 from the misoprostol group because of adverse events, patient’s request, or other reasons (Fig. 2).
Prevalence of peptic ulcer
The prevalences of gastric ulcer during the 12-week study period were 7/176 (3.9%) in the rebamipide group and 3/156 (1.9%) in the misoprostol group. The prevalences of duodenal ulcer were 1/176 (0.5%) in the rebamipide group and 4/156 (2.5%) in the misoprostol group. Peptic ulcer was found in 8/176 (4.5%) subjects in the rebamipide group and 7/156 (4.4%) in the misoprostol group. The cumulative incidences of peptic ulcer in the rebamipide and misoprostol groups did not differ (Odds ratio: 0.98, p = 0.9796) (Table 3). Gastric mucosal damage, as evaluated by modified Lanza score, did not differ between the misoprostol and rebamipide groups (p = 0.2284) (Table 3). The high-risk subgroup included patients with age more than 65 years, concurrent anticoagulation treatment, history of peptic ulcer or bleeding, concurrent corticosteroid therapy, and more than double the standard dose of NSAID. The cumulative incidence of peptic ulcer in this subgroup was 4.0% for rebamipide and 3.9% for misoprostol. Prevention of NSAID-induced peptic ulcer did not differ between the rebamipide and misoprostol groups (Table 4).
Table 3.
Effects of rebamipide on endoscopic appearance of NSAID-induced gastric mucosal injury
| Rebamipide n = 176 (%) | Misoprostol n = 156 (%) | OR (95% CI) | p value | |
|---|---|---|---|---|
| Gastric ulcer | 7 (3.9) | 3 (1.9) | 0.47 (0.10–1.73) | 0.2847 |
| Duodenal ulcer | 1 (0.5) | 4 (2.5) | 4.60 (0.67–90.54) | 0.1741 |
| Peptic ulcer | 8 (4.5) | 7 (4.4) | 0.98 (0.33–2.81) | 0.9796 |
| MLS 0/1/2 | 99/25/23 (83.5) | 102/26/12 (89.7) | 0.2284 | |
| MLS 3/4/5 | 18/4/7 (16.5) | 13/0/3 (10.3) | 0.2284 | |
OR: Odds ratio
95%CI: 95% Confidence Interval
Ulcer: A and H stage ulcer only
Table 4.
Effect of rebamipide on endoscopic appearance of NSAID-induced gastric mucosal injury in high-risk subjects
| Rebamipide n = 151 (%) | Misoprostol n = 154 (%) | OR(95%CI) | p value | |
|---|---|---|---|---|
| Gastric ulcer | 5 (3.3) | 2 (1.3) | 0.38 (0.05–1.81) | 0.2574 |
| Duodenal ulcer | 1 (0.7) | 4 (2.6) | 3.99 (0.58–78.68) | 0.2174 |
| Peptic ulcer | 6 (4.0) | 6 (3.9) | 0.97 (0.30–3.19) | 0.9723 |
OR: Odds ratio
95%CI: 95% Confidence Interval
Ulcer: A and H stage ulcer only
Cumulative incidences of dyspeptic and abdominal symptoms
Diarrhea was the most frequent adverse event, and was significantly more common in the misoprostol group (21.2%) than in the rebamipide group (1.9%). Lower abdominal pain and bloating were significantly more common in the misoprostol group than in the rebamipide group, (7.7% vs. 14.8%, p = 0.0228 and 10.1% vs 20.7%, p = 0.00229). The cumulative incidence of dyspeptic and abdominal symptoms such as diarrhea and lower abdominal pain in the rebamipide group was significantly lower than in the misoprostol group (Table 5).
Table 5.
Cumulative incidences of dyspeptic and abdominal symptoms determined from patients diaries
| Number of dyspeptic and abdominal symptoms | Rebamipide (%) | Misoprostol (%) | p value |
|---|---|---|---|
| Diarrhea | 4 (1.9) | 43 (21.2) | <.0001 |
| Low abdominal pain | 16 (7.7) | 30 (14.8) | 0.0228 |
| Low abdominal symptoms | 20 | 73 | <.0001 |
| Nausea | 20 (9.7) | 20 (9.9) | n.s. |
| Bloating | 21 (10.1) | 42 (20.7) | 0.0029 |
| Satiety | 19 (9.2) | 18 (8.9) | n.s. |
| Fullness | 21 (10.1) | 32 (15.8) | n.s. |
| Vomiting | 5 (2.4) | 9 (4.4) | n.s. |
| Epigastric pain | 28 (13.5) | 40 (19.7) | n.s. |
| Acid regurgitation | 11 (5.3) | 11 (5.4) | n.s. |
| Total dyspeptic symptoms | 125 | 172 | 0.0509 |
| Total | 145 | 245 | 0.0083 |
Statistical analysis by chi-square test (n.s.: not significant)
Discussion
NSAIDs are widely used for the relief of pain and inflammation in patients with rheumatoid arthritis or other inflammatory diseases. NSAIDs, which can induce gastropathies such as dyspepsia and epigastric pain due to peptic ulceration, can unfortunately worsen the quality of life of patients, and result in serious, life-threatening ulcers. NSAIDs inhibit prostaglandin synthesis; this is the principal mechanism responsible for both their anti-inflammatory effects and gastrointestinal toxicity. Inhibition of PG synthesis abrogates a number of PG-dependent mucosal defense mechanisms such as mucosal blood flow and bicarbonate secretion [11].
This study has certain limitations: it was not performed in double-blind fashion, and multiple agents rather than a single agent were used. The main reason we performed a placebo study instead of a comparative study is that it was difficult to obtain permission to perform the study from the Ethics Committees of each center of the three Asian countries involved. In addition, 12-week administration resulted in a relatively high drop-out rate: 33 patients (15.9%) in the rebamipide group and 48 patients (23.6%) in the misoprostol group. The major reason for patient drop-out was request by patients for discontinuation of participation in the study: 20.9% of patients discontinued the study since they exhibited improvement and therefore no longer needed administration of NSAIDs. This was followed by low level of compliance with test drugs, and abdominal symptoms. In particular, 16 subjects (including 12 patients in the misoprostol group) dropped out due to interaction of dyspeptic and abdominal symptoms (Fig. 2).
In this study, 6 NSAIDs were administered at the minimum daily dose. If subjects were administered less than minimum daily dose, they were not included in analysis due to low level of drug compliance. Various NSAIDs were used in each center in Korea, China, and Thailand. Thus, a total of 6 types of NSAIDs with similar propensity to induce gastrointestinal complications were used in this study.
The subjects of this study included patients who re-started administration of NSAIDs after more than 4 weeks of discontinuation of NSAIDs, as well as those who received NSAIDs for the first time. Patients who had been taking NSAIDs were excluded from the study, since they were vulnerable to dyspepsia, and this could make it difficult to clearly determine the incidence of dyspepsia, the purpose of this study.
Several studies have been performed in attempts to identify agents that can be coadministered to prevent NSAID-induced ulcers and ulcer complications. Recently, administration of proton pump inhibitors has been reported to be associated with acceleration of ulcer healing and prevention of ulcer relapse among long-term users of NSAIDs [12, 13]. Repeated use of the synthetic prostaglandin misoprostol as a form of replacement therapy has been shown to prevent NSAID-induced gastroduodenal ulcers and reduce the incidence of life-threatening ulcer complications [3, 10]. Misoprostol features poor compliance and adverse effects such as diarrhea, abdominal pain, and nausea during administration [14]. In our study, the misoprostol group had more adverse events such as diarrhea, abdominal pain, and bloating than did the rebamipide group. These adverse effects of misoprostol suggest that rebamipide is more useful for prevention of NSAID-induced gastrointestinal complications. These findings suggest that strong suppression of acid secretion and increase in PG production were effective in preventing NSAID-induced gastric ulcers.
For these reasons, we performed the present randomized, multicenter, controlled study of rebamipide and misoprostol. Both of these agents stimulate PG biosynthesis. In this study, the incidences of NSAID-induced peptic ulcer in the misoprostol and rebamipide groups were equivalent (Odds Ratio: 0.98, 95%CI = 0.33-2.81, p = 0.9796). The number of cases of gastric ulcer in the rebamipide group was seven, although this group included two patients with serious mucosal damage before initiation of the trial (grades 3 and 4, as determined using modified Lanza score). In this study, patients were relatively young, with a mean age in the rebamipide group of 48 and in the misoprostol group of 46 years. This was one of the reasons for the relatively low rates of induction of gastric ulcer in this trial. The high-risk group was defined to include patients with age above 65 years, concurrent use of anticoagulation, history of peptic ulcer or bleeding, concurrent use of corticosteroid therapy, and/or high-dose administration of NSAIDs >2 times. The results for this group are shown in Table 4, and were similar to those for the group of all patients.
In addition, rebamipide was better tolerated than misoprostol. The cumulative incidence of dyspeptic symptoms in the rebamipide group was significantly lower than that in the misoprostol group (diarrhea p<0.0001, low abdominal pain p = 0.0228, and bloating p = 0.00229, respectively). Rebamipide stimulates the production of endogenous PGs [6]. This finding demonstrated the importance of PG induction in the prevention of NSAID-induced gastric ulcer. Blood flow is known to be regulated by prostaglandins. In our preliminary study on the prevention of ibuprofen-induced gastrointestinal complications for healthy volunteers, rebamipide did not decrease blood flow in the antrum [15].
In conclusion, in this randomized, multicenter, controlled trial, rebamipide prevented peptic ulcers as effectively as misoprostol in patients on long-term NSAID therapy. In addition, rebamipide decreased low abdominal symptoms more significantly than misoprostol. Rebamipide may thus be a useful therapeutic option for the prevention of NSAID-induced gastrointestinal ulcer because of its therapeutic effect and safety.
Acknowledgments
Acknowledgments
This study was supported by a grant from Korea OIAA (Otsuka International Asia Arab) Co., Ltd., Seoul, Korea.
Other investigators participating in this study
Xin-Guang Liu, De-An Tian, and He-Sheng Luo from China and Varocha Mahachai and Ong-ard Praisontarangkul from Thailand.
Abbreviations
- NSAID
nonsteroidal antiinflammatory drug
- PPI
proton pump inhibitor
- GERD
gastroesophageal reflux disease
- H2RA
histamine 2 receptor antagonist
- STORM
Study of NSAID-induced GI Toxicity Prevention by Rebamipide and Misoprostol
References
- 1.Maetzel A., Ferraz M.B., Bombardier C. The cost-effectiveness of misoprostol in preventing serious gastrointestinal events associated with the use of nonsteroidal anti-inflammatory drugs. Arthritis Rheum. 1998;41:16–25. doi: 10.1002/1529-0131(199801)41:1<16::AID-ART3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Raskin J.B., White R.H., Jackson J.E., Weaver A.L., Tindall E.A., Lies R.B., Stanton D.S. Misoprostol dosage in the prevention of nonsteroidal anti-inflammatory drug-induced gastric and duodenal ulcers: a comparison of three regimens. Ann. Intern. Med. 1995;123:344–350. doi: 10.7326/0003-4819-123-5-199509010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein F.E., Graham D.Y., Senior J.R., Davies H.W., Struthers B.J., Bittman R.M., Geis G.S. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. Ann. Intern. Med. 1995;123:241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Elliott S.L., Yeomans N.D., Buchanan R.R.C., Smallwood R.A. Efficacy of 12 months’ misoprostol as prophylaxis against NSAID-induced gastric ulcers. Scand. J. Rheumatol. 1994;23:171–176. doi: 10.3109/03009749409103056. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki K., Ishiyama H., Imaizumi T., Kanbe T., Yabuchi Y. Effect of OPC-12759, a novel antiulcer agent, on chronic and acute experimental gastric ulcer, and gastric secretion in rats. Jpn. J. Pharmacol. 1989;49:441–448. doi: 10.1254/jjp.49.441. [DOI] [PubMed] [Google Scholar]
- 6.Arakawa T. Rebamipide, a novel prostaglandin-inducer, accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer: comparison with cimetidine. Dig. Dis. Sci. 1995;40:2469–2472. doi: 10.1007/BF02063257. [DOI] [PubMed] [Google Scholar]
- 7.Zea-Iriarte W.L., Makiyama K., Goto S., Murase K., Urata Y., Sekine I., Hara K., Kondo T. Impairment of antioxidants in colonic epithelial cells isolated from trinitrobenzene Sulphonic acid-induced colitis rats. Scand. J. Gastroenterol. 1996;31:985–992. doi: 10.3109/00365529609003118. [DOI] [PubMed] [Google Scholar]
- 8.Tarnawski A., Arakawa T., Kobayashi K. Rebamipide treatment activates epidermal growth factor and its receptor expression in normal and ulcerated gastric mucosa in rats: one mechanism for its ulcer healing action? Dig. Dis. Sci. 1998;43:90S–98S. [PubMed] [Google Scholar]
- 9.Naito Y., Yoshikawa T., Iinuma S., Yagi N., Matsuyama K., Boku Y., Fujii T., Yoshida N., Kondo M., Sasaki E. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig. Dis. Sci. 1998;43:83S–89S. [PubMed] [Google Scholar]
- 10.Graham D.Y., White R.H., Moreland L.W., Schubert T.T., Katz R., Jaszawski R., Tindall E., Triadafilopoulos G., Stromatt S.C., Teoh L.S. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study Group. Ann. Intern. Med. 1993;119:257–262. doi: 10.7326/0003-4819-119-4-199308150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Gronbech J.E., Lacy E.R. Role of gastric blood flow in impaired defense and repair of aged rat stomachs. Am. J. Physiol. 1995;269:G737–G744. doi: 10.1152/ajpgi.1995.269.5.G737. [DOI] [PubMed] [Google Scholar]
- 12.Hawkey C.J., Karrasch J.A., Szczepanski L., Walkar D.G., Barkun A., Swannell A.J., Yeomans N.D. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs: Omeprazole versus Misoprostol for NSAID-Induced Ulcer Management (OMNIUM) Study Group. N. Engl. J. Med. 1998;338:727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- 13.Yeomans N.D., Tulassay Z., Juhasz L., Racz I., Howard J.M., van Rensburg C.J., Swannell A.J., Hawkey C.J. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. N. Engl. J. Med. 1998;338:719–726. doi: 10.1056/NEJM199803123381104. [DOI] [PubMed] [Google Scholar]
- 14.Weaver A.L., Gitlin N. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs. Arch. Intern. Med. 2002;162:2248–2249. doi: 10.1001/archinte.162.19.2248-a. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.K., Kim J.I., Kim J.K., Han J.Y., Park S.H., Choi K.Y., Chung I.S. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig. Dis. Sci. 2007 doi: 10.1007/s10620-006-9367-y. In press. [DOI] [PubMed] [Google Scholar]