Abstract
Cancer chemoprevention is fast becoming a lucrative approach for controlling cancer. Carcinogenesis being a complex multi-step, multi-factorial process, a number of chemopreventive interventions can be employed. These strategies are generally directed against two broad events of carcinogenesis viz., initiation and promotion/progression. Anti-initiation interventions principally involve inhibition of carcinogen activation, scavenging of free radicals and reactive carcinogen metabolites along with enhanced detoxification of carcinogens by modulating cellular metabolism. Anti-promotion strategies involve attenuation of enhanced cellular proliferation along with induction of cellular apoptosis and differentiation. Dietary agents or herbal anti-oxidants due to low toxicity and relative safety are promising chemopreventive agents. These agents after emerging successful through a series of in vitro and in vivo assays enter clinical trials. Many dietary compounds have emerged as promising chemopreventive agents in empirical experiments. However, in clinical trials these compounds have met with limited success. This emphasizes the need for further detailed research on the mechanisms of observed chemoprevention and choice, dose, duration and bioavailability of chemopreventive agent used. Complex issues such as choice and nutritional status of target population, genetic variation, gene-environment interactions and relevance of biomarkers analyzed also warrant further research and analyses.
Keywords: carcinogenesis, herbal antioxidant, screening assays, chemoprevention, current status
Introduction
Majority of human cancers are caused, mediated or modified by exogenous/endogenous environmental factors. Epidemiological studies have successfully demonstrated that certain well-defined exposures (e.g. tobacco, alcohol, ionizing radiation, occupational carcinogens and viruses etc.) increase the risk of cancers at specific sites, which have also been supported by in vivo experimental studies. A few associations like tobacco use and oral cancer have been established to be causative while for many other associations, their role in causation of cancer still remains to be established. Further, the identification of specific causative factors and evaluation of their relative importance has proved to be rather difficult since majority of cancers result from complex interactions between environmental and host factors [1]. Efforts to eliminate known human carcinogens like tobacco from the environment and current treatment approaches have met with limited success. Based on the experience with some infectious diseases and the recent progress in cardiology, prevention of diseases appears to be one of the achievable, cost effective and attractive approaches. Thus, cancer prevention serves as a promising approach to decrease cancer burden.
Cancer chemoprevention can be defined as the use of natural or synthetic compounds to prevent, suppress or delay the development of invasive carcinoma. Chemoprevention offers a promising approach to primary cancer prevention for a variety of organ systems. Based on empirical experiments and clinical evaluations many compounds belonging to diverse chemical classes have been identified as potential chemopreventive agents [2]. These include vitamins, minerals, naturally occurring phytochemicals and synthetic compounds.
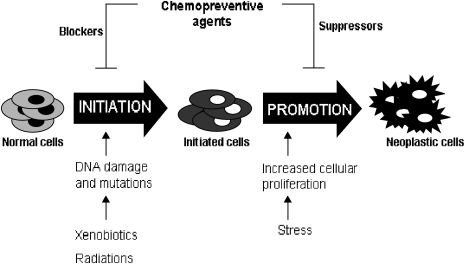
The scientific rationale for the use of cancer chemoprevention is based on the fundamental concept of multi-step carcinogenesis. Carcinogenesis is a long evolving process which can be broadly divided into two principal steps viz., initiation where normal cells acquire mutations via environmental mutagens, viruses etc. followed by promotion/progression where the initiated/mutant cells clonally expand and further transform to give rise to neoplasia. Thus inhibition of any stage of carcinogenesis by any agent can potentially prevent cancer [3] (Fig. 1).
Fig. 1.
Chemopreventive agents can prevent cancer by inhibiting the process of carcinogenesis either by inhibiting initiation and/or promotion event.
Cellular Metabolism and Carcinogenesis
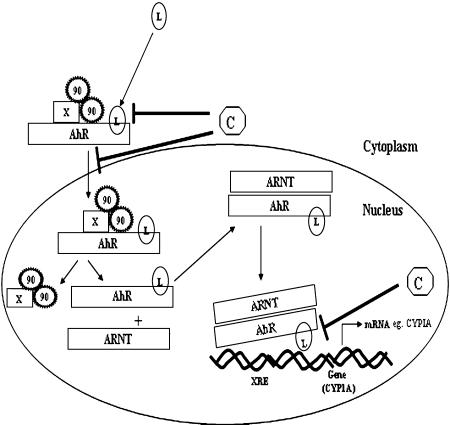
Cellular metabolism plays a very critical role in the process of initiation during carcinogenesis [4]. Xenobiotics entering the cellular environment are metabolized by phase I and phase II enzymes. Phase I enzymes, predominantly Cytochrome P450s (CYP450s—a super family of heme-thiolate enzymes), are involved in the first step of metabolism where xenobiotics are processed to more electrophilic moieties for further detoxification by phase II enzymes [5]. This step can be termed as bioactivation which renders pro-carcinogens to reactive intermediates and these in turn can form bio-molecular adducts e.g. DNA-adducts, protein-adducts etc. which mark the process of initiation. Thus, decreased activation of carcinogens due to modulation of the CYP 450 enzymes could be one of the plausible targets for chemoprevention to prevent the cancer initiation process. CYP1-CYP9 family of enzymes which are principally involved in xenobiotic metabolism have distinct substrate specificities and are differentially regulated by different ligands which interact with endogenous receptors like aryl hydrocarbon receptor (AhR) and pregnane X receptor (PXR). AhR is one of the well studied receptors involved in induction of CYP1A sub-family enzymes which are primarily involved in metabolizing xenobiotics like poly aromatic hydrocarbons (PAHs). AhR is a ligand dependent transcription factor and member of the basic-helix-loop-helix family. In an unliganded or inactive state the receptor is present in the cytoplasm in a complex with two molecules of molecular chaperones, hsp90, an immunophilin like protein, XAP-2 and the hsp90 interacting protein, p23. Upon ligand binding, the ligand-AhR-hsp90-XAP2-p23 complex translocates to nucleus where hsp90-XAP2-p23 dissociates from the ligand-AhR complex. This ligand-AhR complex further associates with a structurally related protein, AhR nuclear translocator (ARNT). This ligand bound AhR-ARNT complex recognizes consensus sequences termed xenobiotic response elements (XREs) to modulate the expression of downstream genes. Certain chemopreventive agents, like plant flavanoids, have shown to act as natural ligands of AhR and hence not only compete for binding with PAHs but also block subsequent nuclear translocation and DNA binding followed by transactivation of CYP genes [6]. Such agents, that can block pro-carcinogen activation, can serve as potential blockers of initiation of carcinogenesis (Fig. 2).
Fig. 2.
Schematic presentations of steps where chemopreventive agents can inhibit phase I enzyme induction. Chemopreventive agents can block the process of initiation by inhibiting xenobiotic induced transcriptional upregulation of phase I enzymes either by competing for receptor, inhibiting nuclear translocation of ligand and receptor complex or by inhibiting the binding of receptor ligand complex to specific promoter element. X = XAP2; 90 = heat shock protein 90; L = ligand (exogenous/endogenous); XRE = xenobiotic response element; C = chemopreventive agent.
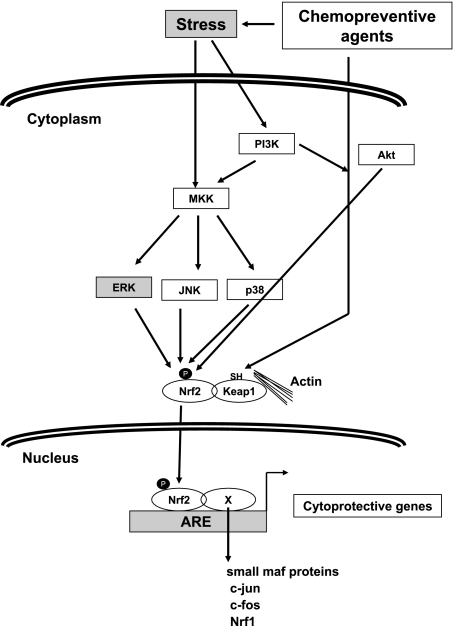
In the next step of metabolism, detoxification by phase II enzymes decrease the burden of bio-molecular adducts by eliminating the reactive-intermediates from cellular environment. Activated pro-carcinogens are conjugated with endogenous bio-molecules like glutathione (GSH) or glucuronic acid by phase II enzymes rendering them less toxic and more water soluble, thus blocking the process of initiation [5]. Hence, any agent that can alter this cellular metabolism by inducing phase II enzymes to block the process of initiation of carcinogenesis can be employed as a potential chemopreventive agent. Unlike phase I enzymes, phase II enzymes or more frequently referred as cyto-protective enzymes are regulated by common upstream promoter regulatory element called ARE (anti-oxidant response element) [7]. Thus, many enzymes regulated by this promoter element are referred as phase II enzymes and their inducibility can be exploited as potential chemopreventive strategy. Some of the most commonly exploited detoxifying enzymes are glutathione S-transferases (GSTs), UDP-glucuronosyltransferases, NADPH quinone oxidoreductase I (NQO1) and certain other anti-oxidant enzymes like superoxide dismutase, catalase etc. Agents that induce phase II enzymes bring about activation of ARE by activating an obligate transcription factor Nuclear Erythroid Factor 2 – Related Factor 2 (Nrf2), member of the Cap ‘n’ Collar family of basic region leucine b-ZIP transcription factor. Nrf2 heterodimerizes with array of leucine b-zip family members like small maf proteins, jun, fos etc. to either upregulate or inhibit transcription through ARE. Nrf2 under normal uninduced cellular environment is strictly regulated by its cytosolic inhibitor Keap1 which in turn is bound to actin cytoskeleton. Upon activation Nrf2 is released from Keap1 and translocates to nucleus where it binds to ARE after heterodimerizing to other leucine zipper proteins to transciptionally activate the downstream genes. Activating signals are generally regulated by ROS modulation in cells where a number of signaling molecules might interplay in cell type specific manner [7]. Most chemopreventive agents are modulators of cellular ROS and hence activate Nrf2 pathway, which in turn induces phase II detoxifying enzymes thus blocking initiation event (Fig. 3).
Fig. 3.
Model representing putative signaling cascade modulated by chemopreventive agents for induction of phase II enzymes. ROS modulation brought about by chemopreventive agents activate signaling kinases which modify NRF2. Modified NRF2 dissociates from its inhibitor keap1 and translocates to nucleus where it heterodimerizes with other transcription factors and binds to ARE, transactivating down stream genes.
In addition to bioactivation of xenobiotics, oxidative damage to DNA, proteins and lipids in human body due to exposure to endogenous or exogenous chemical agents, chronic infections and inflammations, is also an important factor in carcinogenesis. Thus, quenching of these reactive intermediates or radicals by chemopreventive agents like plant derived anti-oxidants is another important strategy for attenuation of carcinogenesis. In addition to directly quenching reactive intermediates, chemopreventive agents also quench reactive intermediates by activating certain cellular anti-oxidant enzymes like catalase or ameliorating their chemical transformation to carcinogenic products. Furthermore, initiation event can be blocked by enhancing cellular DNA repair [8].
Cellular Proliferation and Apoptosis
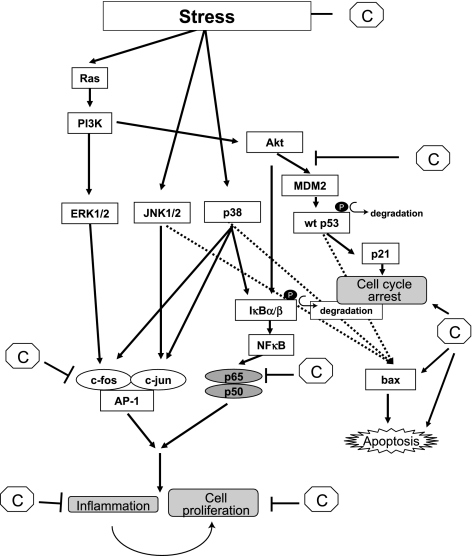
During carcinogenesis, initiated cells, after accumulating environmental insults and mutations, transform and clonally expand to give rise to tumor and this process is called promotion. This process is generally characterized by two important cellular events viz., cellular proliferation and apoptosis. Cellular proliferation, under normal conditions is a well regulated process where proliferation signals interplay with cell cycle checkpoint proteins. However, in transformed cells these regulatory processes are over-ridden to cause hyper proliferation under the influence of certain promotion signals like stress etc. Proliferation can be initiated by different endogenous and exogenous signals like mitogenic stimuli like growth factors, oxidative stress and hormones. Irrespective of the stimuli certain cellular pathways and down stream events remain similar in number of tissues and cell types. Few of such bio-molecules or effectors are protein kinase C (PKC family of proteins), PI3Kinase, MAP kinases like erk, jnk, p38 etc. These kinases upon activation upregulate a number of transcription factors like jun, fos, Nuclear factor kappa B (NF-kB), c-myc etc. that in turn regulate cell proliferation [9]. Thus, agents which can decrease activation of these signaling molecules can suppress the proliferation and hence promotion. Such agents are generally termed as suppressors.
Another important cellular event exploited in chemoprevention is apoptosis, which is characterized by cell shrinkage, membrane blebbing, chromatin condensation and DNA fragmentation. Induction of apoptosis or cell cycle arrest by elimination of cells under stress or genetically damaged cells may represent a protective mechanism by which chemopreventive agents can inhibit promotion/progression stages of carcinogenesis. General mechanisms for induction of apoptosis are hypothesized to be via stress signals elicited by chemopreventive agents that lead to the loss of mitochondrial membrane potential followed by release of cytochrome c. Consequently, an apoptosome is formed by the cytochrome c, apoptotic protease activating factor-1 (APAF-1) and caspase 9, which later results into activation of downstream effector caspases [10]. Loss of mitochondrial membrane potential is also inhibited by Bcl2 (anti-apoptotic protein) or induced by Bax (proapoptotic protein). Furthermore, dietary agent induced activation of p53 (activator of bax) can also mediate apoptosis in response to DNA damage [11]. Hence, chemopreventive agents can alter the trigger for apoptosis by altering the expression of anti/pro-apoptotic proteins, which in turn regulate mitochondrial membrane potential and caspase activation. Chemopreventive antioxidants can also induce apoptosis by modulating activation of certain transcription factors such as NF-kB and AP1 family members which are involved in induction of cell survival genes (Fig. 4).
Fig. 4.
Schematic presentations of signaling cascade where chemopreventive agents act as a suppressor. Chemopreventive agent can act as a suppressor either by inhibiting signaling kinases that activate transcription factor like AP-1 and NF-kB which in turn activate cell proliferation genes or by inducing apoptosis via activation of proapoptotic proteins like bax. C = chemopreventive agent; wt = wild type.
Herbal Compounds as Chemopreventive Agents
Among various chemopreventive compounds, dietary agents, due to their high tolerability and low toxicity are fast becoming lucrative targets. Based on experimental and epidemiological evidences, chemoprevention or dietary intervention especially employing herbal anti-oxidants is receiving increasing attention as they have shown anti-initiating and/or anti-promoting activities in experimental systems [4]. Tabulated below is a partial list of plants reported to possess chemopreventive potential along with probable active component(s) and their mode(s) of action (Table 1).
Table 1.
List of plants possessing chemopreventive activities
| No | Plant name [Reference] | Active Compound(s) | Mode of Action |
|---|---|---|---|
| 1 | Aegle marmelos [36] | Marmelosin | Anti-initiating |
| Luvangetin | Anti-proliferative | ||
| 2 | Allium sativum (Garlic) [37] | Diallyl sulphide | Anti-initiating |
| S-methyl cysteine | Anti-promoting | ||
| 3 | Allium cepa (Onion) [38] | Diallyl sulphide | Anti-initiating |
| S-methyl cysteine | Anti-promoting | ||
| Selenium | |||
| 4 | Aloe vera (Ghritakumari) [39] | Emodin | Anti-mutagenic |
| Salicylates | Anti-initiating | ||
| 5 | Alpinia officinarum (Galanga) [40] | Galangin | Anti-initiating |
| Anti-promoting | |||
| 6 | Andrographis paniculata (Kalmegh, Bhunimba) [41] | Andrographolide | Anti-initiating |
| Anti-promoting | |||
| 7 | Azadirachta indica (Neem) [42] | Anti-initiating | |
| Nimbolide | Anti-promoting | ||
| 8 | Boswellia serrata [43] | Boswellic acids | Anti-proliferative |
| 9 | Brassica juncea (Mustard and cruciferous vegetables) [44] | Sulforaphane | Anti-initiating |
| Indole 3-carbinol | |||
| 10 | Camellia sinensis (Green and Black tea) [45] | Flavonoids | Anti-initiating |
| Catechins | Anti-promoting | ||
| 11 | Capsicum annum (Red chilli) [46] | Capsaicin | Anti-promoting |
| 12 | Coriandrum sativum | Linalool | Anti mutagenic |
| (Coriander) [47] | Monoterpenes | ||
| 13 | Curcuma longa (Turmeric) [48] | Curcumin | Anti-initiating |
| (Curcuminoids) | Anti-promoting | ||
| 14 | Emblica officinalis (Amla) [49] | Tannins | Anti-promoting |
| Flavonoids | |||
| Pyrogallol | |||
| 15 | Eugenia caryophyllus (Clove) [50] | Eugenol | Anti-promoting |
| Isoeugenol | |||
| 16 | Foeniculum vulgare (Sweet fennel) [51] | Anethole | Anti-promoting |
| 17 | Garcinia indica (kokum) [52] | Garcinol | Anti-promoting |
| Anti-oxidant | |||
| 18 | Glycyrrhiza glabra (Licorice, Yastimadhu) [53] | Glycyrrhizin | Anti-promoting |
| Glycyrrhizic acid | |||
| 19 | Commiphora mukul (Guggulu) [54] | Guggulsterone | Anti-promoting |
| 20 | Glycine max (Soyabean) [55] | Genistein | Anti-promoting |
| 21 | Nerium oleander (Oleander) [56] | Oleandrin | Anti-promoting |
| 22 | Ocimum sanctum (Tulsi) [57] | Ursolic acid | Anti-promoting |
| Eugenol | |||
| 23 | Picrorhiza kurroa (Kutki) [58] | Picroliv | Anti initiating |
| 24 | Pinecone ginger (Zingiber zerumbet) [59] | Zerumbone | Anti-initiating |
| Anti-promoting | |||
| 25 | Punica granatum (Pomegranate) [60] | Elagic acid | Anti-promoting |
| 26 | Silybum marianum (Silymarin) [61] | Silibinin | Anti-initiating |
| Anti-promoting | |||
| 27 | Vitis vinifera (Grapes) [62] | Resveratrol | Anti-initiating |
| Anti-promoting | |||
| 28 | Withania somnifera (Ashwagandha) [63] | Withonalides | Anti-initiating |
| Anti-promoting | |||
| 29 | Zingiber officinale (Ginger) [64] | Gingerol | Anti-promoting |
| Paradol |
Few of the compounds like epigallocatechin gallate (green tea catechin), curcumin, indole-3-carbinol, resveratrol, etc. mentioned in Table 1 are currently undergoing clinical trials [12]. Of the many chemopreventive agents listed in the Table 1, we have focused our studies on turmeric and black tea.
Studies on Chemopreventive Efficacies of Turmeric
The powdered rhizome of the plant Curcuma longa known as turmeric has number of medicinal properties. Studies in our lab have shown turmeric and its active compound curcumin, to inhibit carcinogen induced mutation in the Ames assay and tumorigenesis in several experimental systems suggesting anti-initiating and/or anti-promoting activity against several chemical carcinogens [13–15]. Both in vitro and in vivo studies have shown the efficacy of turmeric/curcumin in inhibiting the carcinogen induced activity of various CYP450s isozymes [16, 17] and enhancement of phase II enzymes [18] resulting in decreased levels of carcinogen derived DNA adducts [17, 19]. However, the molecular basis of observed antiinitiation mechanism needs to be evaluated further.
Chemoprevention and Black Tea
Tea is fast emerging as a potential chemopreventive beverage and green tea is now a well established chemopreventive beverage and its biological activities are attributed to certain active flavanols like epigallocatechin gallate (EGCG) etc. Similar chemopreventive efficacy needs to be investigated for black tea (and its polyphenols), which is a widely consumed beverage. Since polymeric black tea polyphenols (PBPs) or thearubigins (TRs) comprise almost 54% of the total polyphenols in black tea, studies on chemoprevention in our lab are focused on these fractions. PBPs were isolated from black tea brew as five different fractions (PBP1 to PBP5) by liquid-liquid and solid-liquid extraction by exploiting their property of selective solubility in different solvents and were partially characterized by NMR and FTIR analysis [20]. In vivo studies indicated that, like green tea polyphenols, PBPs can inhibit carcinogen-induced up regulation of phase I enzymes like CYP450s and induce phase II detoxifying enzymes thus decreasing the load of DNA adduct formation [21–24]. Furthermore, PBPs have also shown anti-promoting properties in experimental skin carcinogenesis [unpublished observations].
Screening for Chemopreventive Efficacy
Putative chemopreventive agents are subjected to rigorous in vitro and in vivo screening assays to determine their efficacies against different stages of carcinogenesis, in defined model systems and investigate the mechanism(s) of chemomodulation [25]. In vitro assays are employed as rapid screens for determining the chemopreventive efficacy based on modulation of different events presumed to be mechanistically linked to carcinogenesis. In these evaluations, biochemical assays, bacterial or mammalian cell systems, cell lines, cell free extracts etc. are employed. Chemopreventive agents are screened for their abilities to inhibit carcinogen/mutagen-induced effects (mutagenic/clastogenic effects, adduct and free radical formation, effect on metabolic and repair enzymes etc.) or to inhibit cell proliferation or to enhance cell differentiation and apoptosis [26, 27].
These tests have generated voluminous and useful information about the chemopreventive properties and mechanism of action of large number of environmental agents (under defined conditions). However, these properties are not always reproducible in in vivo systems. Probable reasons for these differences are: (a) doses of chemopreventive agent(s) employed in in vitro studies are not achievable in vivo, (b) metabolic incompetance and lower inducibility of metabolic enzymes in cell lines as compared to tissues, (c) pharmacokinetics in the two system(s) and (d) lack of proper controls (e.g. normal cell counterpart for studies on cancer cell lines). Therefore, in vivo assay systems are essential for evaluation of chemopreventive potential.
Animal models are central to the theme of disease prevention as the data of short-term or long-term exposure of chemopreventive agents can be accrued by exploiting the short lifespan of animal models. In vivo studies allow us to take a closer look at signaling mechanisms and drug metabolism, forming an integral part of preclinical studies. Many animal models are available for testing the efficacy of chemopreventive agents.
Selection of model system is generally governed by their rapidity, expression of multi stage carcinogenesis, organ/tissue specificity, hormone responsiveness, invasiveness, slow/fast growth of tumor, histological types and particular relevance to most common human cancers.
In these assays, chemopreventive activity of an agent is generally investigated against carcinogen(s)-induced tumors or appropriate biomarkers in experimental animals. In experimental animals chemopreventive activity is judged either by increase in latency period or decrease in incidence and multiplicity of carcinogen or spontaneously induced tumors or by inhibition of carcinogen-induced alterations or development of pre-malignant lesions [25]. In some instances regression of tumor or attenuation of xenograft growth and metastasis is also investigated [28, 29].
Genetically engineered mouse models have also been employed to develop specific types of cancer or to substantially mimic human cancers e.g. APCmin, carries a germline truncation of one APC allele and develop multiple intestinal adenomas [30]. This approach is very helpful in demonstrating the role of defined molecular target or pathway in carcinogenesis and chemoprevention.
Studies employing animal models have contributed significantly in identification of number of chemopreventive agents and also helped in understanding the complexity of gene-environment interactions. However, a major drawback of assays employing animal models to study chemopreventive efficacies is shorter experimental duration than the inherent lifespan of animals. Due to this, adverse effects (if any) of chemopreventive agents exhibited at later time point may be missed. Several experimental studies have shown that some of the chemopreventive agent(s) have been observed to be carcinogenic under certain experimental conditions while some other agent(s) have been observed to be protective in one organ and enhance the risk in another organ [31]. Based on several of these observations, multi-organ model(s) for evaluation of chemopreventive activity of environmental agent(s) have been proposed where multi-organ carcinogenesis is initiated using multiple organ-specific carcinogens along with exposure to chemopreventive test compound prior, during and after carcinogen exposure to evaluate its chemopreventive effects against different carcinogens in different organs. In few, such studies it was observed that certain chemopreventive agents might promote carcinogenesis in animals challenged with more than one carcinogen as compared to animals challenged with only one specific carcinogen. Caution needs to be exercised while executing and analyzing such experimental data since over burdening animals with number of carcinogens at optimum carcinogenic doses might render certain organs like liver under excess stress which might be one of the reasons of promoting carcinogenesis [31]. Such drawbacks can be dealt with by subjecting the animals to sub-carcinogenic doses of different carcinogens and analyzing molecular markers instead of frank tumors as the end point. However, rodents differ from humans in many ways that are relevant to cancer such as oncogenic signaling, length of telomeres, extent of karyotypic abnormalities, basal metabolic rate and non-epithelial origin of murine malignancies as compared to epithelial origin of human malignancies. These suggest rodent models may not accurately reflect the cellular carcinogenesis and may require caution in extrapolating data from murine neoplasia studies directly to humans. Nonetheless, animal studies are of prime importance not only as confirmatory studies employing various models with different dose regimes but also for better understanding of molecular pathways.
Chemoprevention: Current Status and Future Perspectives
After establishment of pre-clinical efficacy of an agent, it further undergoes phase I, II and III clinical trials to test their safety, and efficacy in human situation [25]. Being relatively less toxic, dietary agents have advantage over other synthetic agents. But inspite of this most of the agents, that have emerged highly promising after pre-clinical safety and efficacy studies, have failed in human trials [32].
These failures broadly can be grouped into two distinct outcomes viz., adverse effects or null effect. The failure of the beta carotene trial for prevention of lung cancer in high risk population has chanalized rest of the preclinical studies on putative chemopreventive agents towards mechanistic aspects of the observed anticarcinogenic effects rather than focusing only on end points. The advent of more sensitive assays like expressional arrays enables a bird’s eye view of effect of particular chemopreventive agent at cellular, organ and entire organism level which will not only impart better understanding of the safety but also aid in identifying appropriate biomarker. The null effects of potential chemopreventive agents in clinical trials can be attributed to number of issues, which need increased attention. One such issue is individual differences in host susceptibility which in turn are governed by genetic constitution. Certain families of genes associated with xenobiotic metabolism, DNA repair etc. have functional single nucleotide polymorphisms, which may influence carcinogenesis and hence even chemoprevention [32]. The choice of target population for chemoprevention is another such issue as considering risk to benefit ratio, most logical population as target for chemoprevention would be high risk individuals. However, identification of such individuals for most cancers, such as oral cancer, is highly challenging due to diverse complexities of individual variations and gene-environment interactions. Moreover, anti-oxidants used as chemopreventive agents depending on the cellular milieu, can also act as pro-oxidants [32]. Choice of dietary chemopreventive agents as active compounds or whole plant extracts, dose, duration and mode of application for particular target population against specific cancers is also very critical. Use of dietary agents for chemoprevention also emphasizes nutritional studies, which encompass certain aspects like interactions of chemopreventive supplements with normal diet and host factors. Thus, clinical evaluation based on mechanistic studies, gene-environment interactions and complementing nutritional studies constitute the major thrust areas in chemoprevention research [33–35].
Acknowledgments
The authors thank the Indian Council of Medical Research (ICMR) and National Tea Research Foundation (NTRF) for their partial financial support to their research project(s) and to Lady Tata Memorial Trust for the award of senior scholarship to Ms. Rachana Patel and to the Council for Scientific and Industrial Research (CSIR) for the award of senior research fellowships to Ms. Suvarna Erande and Ms. Rachana Garg.
References
- 1.Harris C.C. In: Concluding remarks: role of carcinogens, co-carcinogens and host factors in cancer risk, in Human Carcinogenesis. Harris C.C., Autrup H., editors. Academic Press; New York: 1983. pp. 941–970. [Google Scholar]
- 2.Tsao A.S., Kim E.S., Hong W.K. Chemoprevention of cancer. CA. Cancer J. Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 3.Smith J.J., Tully P., Padberg R.M. Chemoprevention: a primary cancer prevention strategy. Semin. Oncol. Nurs. 2005;21:243–251. doi: 10.1016/j.soncn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Surh Y.H. Cancer chemoprevention with dietary phytochemicals. Nature Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 5.Moon Y.J., Wang X., Morris M.E. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol. in Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Ciolino H.P., Daschner P.J., Wang T.T.Y., Yeh G.C. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P4501A1 in MCF-7 human breast carcinoma cells. Biochem. Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 7.Hayes J.D., McMahon M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001;174:103–113. doi: 10.1016/s0304-3835(01)00695-4. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty S., Roy M., Bhattacharya R.K. Prevention and repair of DNA damage by selected phytochemicals as measured by single cell gel electrophoresis. J. Envt. Pathol. Toxicol. Oncol. 2004;23:215–226. doi: 10.1615/jenvpathtoxoncol.v23.i3.50. [DOI] [PubMed] [Google Scholar]
- 9.Manson M.M. Inhibition of survival signaling by dietary polyphenols and indole-3-carbinol. Eur. J. Cancer. 2005;41:1842–1853. doi: 10.1016/j.ejca.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell Mol. Life Sci. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 11.Choudhuri T., Pal S., Agarwal M.L., Das T., Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald P. Clinical trials in cancer prevention: current results and perspectives for the future. J. Nutr. 2004;134:3507S–3512S. doi: 10.1093/jn/134.12.3507S. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande S.S., Ingle A.D., Maru G.B. Inhibitory effects of curcumin free aqueous turmeric extract on benzo(a)pyrene induced stomach papilomas in mice. Cancer Lett. 1997;118:79–85. doi: 10.1016/s0304-3835(97)00238-3. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande S.S., Ingle A.D., Maru G.B. Chemopreventive efficacy of curcumin-free aqueous turmeric extract on 7, 12 dimethylbenz(a)anthraceneinduced rat mammary tumorigenesis. Cancer Lett. 1998;123:35–40. doi: 10.1016/s0304-3835(97)00400-x. [DOI] [PubMed] [Google Scholar]
- 15.Thapliyal R., Dolas S.S., Pakhale S.S., Maru G.B. Evaluation of DNA damage in mice topically exposed to total particulate matter from mainstream and sidestream smoke from cigarettes and bidis. Mutagenesis. 2004;19:413–421. doi: 10.1093/mutage/geh051. [DOI] [PubMed] [Google Scholar]
- 16.Thapliyal R., Deshpande S.S., Maru G.B. Effect(s) of turmeric on the activities of benzo(a)pyrene induced cytochrome P450 isozymes. J. Envt. Pathol. Toxicol. Oncol. 2001;20:59–63. [PubMed] [Google Scholar]
- 17.Deshpande S.S., Maru G.B. Effect(s) of curcumin on the formation of benzo(a)pyrene derived DNA adducts. Cancer Lett. 1995;96:71–80. doi: 10.1016/0304-3835(95)03903-a. [DOI] [PubMed] [Google Scholar]
- 18.Thapliyal R., Maru G.B. Inhibition of cytochrome P450 isozymes by curcumin(s) in vitro and in vivo. Food Chem. Toxicol. 2001;39:541–547. doi: 10.1016/s0278-6915(00)00165-4. [DOI] [PubMed] [Google Scholar]
- 19.Thapliyal R., Deshpande S.S., Maru G.B. Mechanism(s) of turmeric-mediated protective effects against benzo(a)pyrene-derived DNA adducts. Cancer Lett. 2002;175:79–88. doi: 10.1016/s0304-3835(01)00675-9. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan R., Maru G.B. Isolation and analyses of polymeric polyphenol fractions from black tea. Food Chem. 2006;94:331–340. [Google Scholar]
- 21.Krishnan R., Raghunathan R., Maru G.B. Effect of polymeric black tea polyphenols on benzo(a)pyrene [B(a)P]-induced cytochrome P4501A1 and 1A2 in mice. Xenobiotica. 2005;35:671–682. doi: 10.1080/00498250500202155. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan R., Maru G.B. Inhibitory effect(s) of polymeric black tea polyphenol fractions on the formation of [3H]-B(a)P-derived DNA adducts. J. Agric. Food Chem. 2004;52:4261–4269. doi: 10.1021/jf049979o. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan R., Maru G.B. Inhibitory effect(s) of polymeric black tea polyphenols on the formation of B(a)P-derived DNA adducts in mouse skin. J. Envt. Pathol. Toxicol. Oncol. 2005;24:103–114. doi: 10.1615/jenvpathtoxoncol.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan R., Patel R.R., Ramchandani A., Maru G.B. In: Cancer chemoprevention by tea polyphenols, in Medicinal properties of tea. Banerjee B., Chaudhuri T.C., editors. Oxford and IBH Publishing Co Pvt Ltd; New Delhi: 2005. pp. 133–170. [Google Scholar]
- 25.Crowell J. The chemopreventive agent development research program in the Division of Cancer Prevention of the US National Cancer Institute: an overview. Eur. J. Cancer. 2005;41:1889–1910. doi: 10.1016/j.ejca.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Gerhauser C., Klimo K., Heiss E., Neumann I., Gamal-Eldeen A., Knauft J., Liu G., Sitthimonchai S., Frank N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003;523–524:163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 27.Steele V., Sharma S., Mehta R., Elmore E., Redpath L., Rudd C., Bagheri D., Sigman C., Kelloff G. Use of in vitro assays to predict the efficacy of chemopreventive agents in whole animals. J. Cell Biochem. Suppl. 1996;26:29–53. doi: 10.1002/jcb.240630704. [DOI] [PubMed] [Google Scholar]
- 28.Saleem M., Kweon M.H., Yun J.M., Adhami V.M., Khan N., Syed D.N., Mukhtar H. A novel dietary triterpene lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–11213. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Che M., Bhagat S., Ellis K.L., Kucuk O., Doerge D.R., Abrams J., Cher M.L., Sarkar F.H. Regulation of gene expression and inhibition of experimental prostate cancer bone metastasis by dietary genistein. Neoplasia. 2004;6:354–363. doi: 10.1593/neo.03478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao C., Cooma I., Rodriguez J., Simi B., El-Bayoumy K., Reddy B. Chemoprevention of familial adenomatous polyposis development in the APC(min) mouse model by 1,4-phenylene bis(methylene)selenocyanate. Carcinogenesis. 2000;21:617–621. doi: 10.1093/carcin/21.4.617. [DOI] [PubMed] [Google Scholar]
- 31.Stoner G., Casto B., Ralston S., Roebuck B., Pereira C., Bailey G. Development of multi-organ rat model for evaluating chemopreventive agents: efficacy of indole-3-carbinol. Carcinogenesis. 2002;23:265–272. doi: 10.1093/carcin/23.2.265. [DOI] [PubMed] [Google Scholar]
- 32.Seifried H.E., Mcdonald S.S., Anderson D.E., Greenwald P., Milner J.A. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298. [PubMed] [Google Scholar]
- 33.Lippman S.M., Hong W.K. Cancer prevention science and practice. Cancer Res. 2002;62:5119–5125. [PubMed] [Google Scholar]
- 34.Greenwald P. Science, medicine and the future: cancer chemoprevention. Br. Med. J. 2002;324:714–720. doi: 10.1136/bmj.324.7339.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sporn M.B. Carcinogenesis and cancer: different perspectives on the same disease. Cancer Res. 1991;51:6215–6220. [PubMed] [Google Scholar]
- 36.Singh R.P., Banerjee S., Rao A.R. Effect of Aegle marmelos on biotransformation enzyme systems and protection against free-radical-mediated damage in mice. J. Pharm. Pharmacol. 2000;52:991–1000. doi: 10.1211/0022357001774714. [DOI] [PubMed] [Google Scholar]
- 37.Yang C.S., Chhabra S.K., Hong J.Y., Smith T.J. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J. Nutr. 2001;131:1041–1045. doi: 10.1093/jn/131.3.1041S. [DOI] [PubMed] [Google Scholar]
- 38.Belman S. Onion and garlic oils inhibit tumor promotion. Carcinogenesis. 1983;4:1063–1065. doi: 10.1093/carcin/4.8.1063. [DOI] [PubMed] [Google Scholar]
- 39.Kim H.K., Lee B.M. Inhibition of benzo[a]pyrene–DNA adduct formation by Aloe barbadensis Miller. Carcinogenesis. 1997;18:771–776. doi: 10.1093/carcin/18.4.771. [DOI] [PubMed] [Google Scholar]
- 40.Heo M.Y., Sohn S.J., Auc W.W. Anti-genotoxicity of galangin as a cancer chemopreventive agent candidate. Mutat. Res. 2001;488:135–150. doi: 10.1016/s1383-5742(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 41.Singh R.P., Banerjee S., Rao A.R. Modulatory influence of Andrographis paniculata on mouse hepatic and extrahepatic carcinogen metabolizing enzymes and antioxidant status. Phytother. Res. 2001;15:382–390. doi: 10.1002/ptr.730. [DOI] [PubMed] [Google Scholar]
- 42.Dasgupta T., Banerjee S., Yadava P.K., Rao A.R. Chemopreventive potential of Azadirachta indica (Neem) leaf extract in murine carcinogenesis model systems. J. Ethnopharmacol. 2004;92:23–36. doi: 10.1016/j.jep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Liu J.J., Nilsson A.N., Oredsson S., Badmaev V., Duan R.D. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in HepG2 cells via a caspase-8 dependent pathway. Int. J. Mol. Med. 2002;10:501–505. [PubMed] [Google Scholar]
- 44.Kensler T.W., Chen G.J., Egner P.A., Fahey J.W., Jacobson L.P., Stephenson K.K., Ye L., Coady J.L., Wang J.B., Wu Y., Sun Y., Zhang Q.N., Zhang B.C., Zhu Y.R., Qian G.S., Carmella S.G., Hecht S.S., Benning L., Gange S.J., Groopman J.D., Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol. Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 45.Lambert J.D., Yang C.S. Mechanisms of cancer prevention by tea constituents. J. Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 46.Surh Y.J. More than spice: capsaicin in hot chili peppers makes tumor cells commit suicide. J. Natl. Cancer Inst. 2002;94:1263–1265. doi: 10.1093/jnci/94.17.1263. [DOI] [PubMed] [Google Scholar]
- 47.Eslava J.C., Arroyo S.G., Pietrini R.V., Aguirre J.J.E. Antimutagenicity of coriander (Coriandrum sativum) juice on the mutagenesis produced by plant metabolites of aromatic amines. Toxicol. Lett. 2004;153:283–292. doi: 10.1016/j.toxlet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Azuine M.A., Bhide S.V. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr. Cancer. 1992;17:77–83. doi: 10.1080/01635589209514174. [DOI] [PubMed] [Google Scholar]
- 49.Jose J.K., Kuttan G., Kuttan R. Antitumour activity of Emblica officinalis. J. Ethnopharmacol. 2001;75:65–69. doi: 10.1016/s0378-8741(00)00378-0. [DOI] [PubMed] [Google Scholar]
- 50.Zheng G.Q., Kenney P.M., Lam L.K. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J. Nat. Prod. 1992;55:999–1003. doi: 10.1021/np50085a029. [DOI] [PubMed] [Google Scholar]
- 51.Ruberto G., Baratta M.T., Deans S.G., Dorman H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66:687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi F., Saito M., Ariga T., Yoshimura Y., Nakazawa H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000;48:2320–2325. doi: 10.1021/jf990908c. [DOI] [PubMed] [Google Scholar]
- 53.Jo E.H., Kim S.H., Ra J.C., Kim S.R., Cho S.D., Jung J.W., Yang S.R., Park J.S., Hwang J.W., Aruoma O.I., Kim T.Y., Lee Y.S., Kang K.S. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230:239–247. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 54.Shishodia S., Aggarwal B.B. Guggulsterone inhibits NF-κB and IκB kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J. Biol. Chem. 2004;279:47148–47158. doi: 10.1074/jbc.M408093200. [DOI] [PubMed] [Google Scholar]
- 55.Wanga J., Eltoumb I.E., Lamartinierea C.A. Dietary genistein suppresses chemically induced prostate cancer in Lobund–Wistar rats. Cancer Lett. 2002;186:11–18. doi: 10.1016/s0304-3835(01)00811-4. [DOI] [PubMed] [Google Scholar]
- 56.Afaq F., Saleem M., Aziz M.H., Mukhtar H. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion markers in CD-1 mouse skin by oleandrin. Toxicol. Appl. Pharmacol. 2004;195:361–369. doi: 10.1016/j.taap.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 57.Karthikeyan K., Ravichandran P., Govindasamy S. Chemopreventive effect of Ocimum sanctum on DMBA-induced hamster buccal pouch carcinogenesis. Oral Oncol. 1999;35:112–119. doi: 10.1016/s1368-8375(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 58.Rajeshkumar N.V., Kuttan R. Protective effect of picroliv, the active constituent of Picrorhiza kurroa, against chemical carcinogenesis in mice. Teratog. Carcinog. Mutagen. 2001;21:303–313. doi: 10.1002/tcm.1018. [DOI] [PubMed] [Google Scholar]
- 59.Murakami A., Takahashi D., Kinoshita T., Koshimizu K., Kim H.W., Yoshihiro A., Nakamura Y., Jiwajinda S., Terao J., Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the alpha, beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23:795–802. doi: 10.1093/carcin/23.5.795. [DOI] [PubMed] [Google Scholar]
- 60.Malik A., Afaq F., Sarfaraz S., Adhami V.M., Syed N.D., Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh R.P., Dhanalakshmi S., Tyagi A.K., Chan D.C.F., Agarwal C., Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor binding protein-3 levels. Cancer Res. 2002;62:3063–3069. [PubMed] [Google Scholar]
- 62.Aziz M.H., Kumar R., Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms. Int. J. Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 63.Prakash J., Gupta S.K., Dinda A.K. Withania somnifera root extract prevents DMBA-induced squamous cell carcinoma of skin in Swiss albino mice. Nutr. Cancer. 2002;42:91–97. doi: 10.1207/S15327914NC421_12. [DOI] [PubMed] [Google Scholar]
- 64.Murakami A., Tanaka T., Lee J.Y., Surh Y.J., Kim H.W., Kawabata K., Nakamura Y., Jiwajinda S., Ohigashi H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int. J. Cancer. 2004;110:481–490. doi: 10.1002/ijc.20175. [DOI] [PubMed] [Google Scholar]