Abstract
Recently the finding of gastric cancer in Helicobacter pylori (H. pylori)-infected mouse models was reported. Studies of humans and animal models have shown that H. pylori infection stimulates gastric epithelial cell proliferation and apoptosis. Polyphenols contained in green tea and related compounds were reported to have a variety anti-tumor effects and bactericidal properties. We studied the effect of green tea polyphenols on gastric cell proliferation and apoptosis in an H. pylori-infected mouse model. This model was prepared by inoculating Balb/c mice with 108 cfu of H. pylori (NCTC 11637 strain) by gavage. Beginning 18 weeks after inoculation, 0.5% polyphenols were given in drinking water every day for 2 weeks. Mice were sacrificed 1 h after bromodeoxyuridine (BrdU) was given i.p. for preparation of paraffin-embedded specimens. Cell proliferation and apoptosis were examined by the avidin-biotin complex method using anti-BrdU antibody and the TUNEL method, respectively. H. pylori infection resulted in increased BrdU-labeled cells in both the antrum and the bodies. Administration of polyphenols suppressed this increased proliferation. H. pylori infection increased apoptotic cells in both the antrum and the corpus in comparison with controls. This increase was not seen in H. pylori-infected mice given polyphenols. We conclude the administration with polyphenols might suppress gastric carcinogenesis that is in part related to H. pylori infection.
Keywords: Helicobacter pylori, green tea polyphenols, gastric cell proliferation, apoptosis
Introduction
Helicobacter pylori (H. pylori) infection has been proven to cause chronic gastritis [1–5], and is considered to be a factor in gastroduodenal ulceration. Furthermore, gastric cancer was observed in Mongolian gerbils infected with H. pylori [6], and H. pylori was designated as a class I carcinogen for stomach cancer by the World Health Organization (WHO) [7]. On this basis, attempts have been made worldwide to eradicate H. pylori.
Gastric epithelium is continuously injured by a variety of factors, resulting in a loss of epithelial cells. However, gastric mucosa has a remarkable reproductive capacity, and mucosal integrity is maintained by continuous reproduction and migration of new cells from the epithelial proliferative zone. Mucosal integrity is maintained by striking a balance between the number of apoptotic cells, designated as undergoing physiological death, and those reproducing from the proliferative zone. But when cell proliferation exceeds apoptosis, excessive growth of the gastric mucosa occurs, which may induce carcinogenesis. On the other hand, when cell death exceeds reproduction, the decrease in cell numbers results in disintegration of the mucosa, forming erosions and ulceration. In studies on humans and animal models, H. pylori infection has been recognized to stimulate cell proliferation in the gastric epithelium [8–12], and also apoptosis is stimulated in the gastric mucosa infected with H. pylori [13, 14]. But the mechanisms of the relationship between increased cell proliferation and apoptosis caused by H. pylori have not been elucidated well.
Infection with H. pylori is known as a factor in chronic gastritis, peptic ulcer and gastric cancer. Chronic inflammation is dominated by neutrophils, macrophages, lymphocytes and plasma cells. Interleukin-8 (IL-8) is a potent neutrophil-activating chemokine and is central to immunopathogenesis of H. pylori-induced injury [15].
Green tea polyphenols (GTP), which are compounds in Japanese green tea, are reported to have bactericidal properties against pathogenic bacteria in food and also have anti-tumor effects; attention has been given to their application as a new treatment for infectious diseases and malignant neoplasm [16, 17]. In this study, we examined the effects of GTP on gastric proliferation and apoptosis in an H. pylori-infected mouse model. Further, we studied the effect of GTP on the expression of IL-8, in H. pylori-infected gastric mucosa and the effect of GTP on H. pylori growth on mouse gastric mucosa.
Materials and Methods
Preparation of mouse model
Four-week-old Balb/C mice (Sankyo Lab, Tokyo, Japan) were inoculated orally with 108 cfu/mouse of H. pylori (NCTC 11637 strain) for 2 days as preparation for the infection model. Beginning 18 weeks after inoculation, GTP (Thea-flan, Itoen, Tokyo, Japan) resolved in distilled water at a final concentration of 0.5% was given ad libitum every day for 2 weeks. On day 14 and day 28 after GTP administration, mice were sacrificed and stomachs were fixed with formaldehyde for 24 h.
Cell proliferation and apoptosis
Cell proliferation was examined by the avidin-biotin complex ABC method using anti-bromodeoxyuridine (BrdU) antibody. Mice were given 0.2 mg/kg of BrdU intraperitoneally and sacrificed 1 h later. After the stomach was fixed with Carnoy’s solution (ethanol: chloroform: acetic acid, 6:3:1), paraffin-embedded specimens were prepared. Specimens were deparaffinized with xylene, and hydrated with graded ethanol. DNA in the specimens was degenerated by 4 M HCl for 30 min, and the intrinsic peroxidase was blocked with 0.3% hydrogen peroxide in methanol. Non-specific binding was blocked with phosphate buffered saline (PBS) containing 3% skimmed milk (Wako, Tokyo, Japan) for 30 min. After the specimens were incubated with anti-BrdU antibody diluted 20-fold (Dako, Copenhagen Denmark) overnight at 4°C, they were washed with PBS, followed by incubation with biotinylated secondary antibody diluted 20-fold (Dako, Copenhagen, Denmark) for 1 h. Then they were incubated with ABC solution (Vector Laboratories, Burlingame, CA) for 30 min and visualized with DAB (Wako, Tokyo, Japan). A specimen with all mucosal layers intact without defect was used as a control.
Apoptosis was examined by the terminal uridine deoxynucleotide nick end-labeling (TUNEL) method. After paraffin-embedded specimens were deparaffinized, they were treated with 20 µg/ml of proteinase K at 37°C for 15 min. Then, the intrinsic peroxidase was blocked with methanol containing 3% hydrogen peroxide and specimens were incubated at pH 6.6 for 10 min. The terminal transferase buffer was prepared by mixing 200 mM of potassium cacodylate, 0.2 mM EDTA, 25 mM Tris-HCl, and 0.25 mg/ml of bovine serum albumin (BSA), and slides were incubated in the terminal transferase buffer containing 1 mM of cobalt-HCl, 0.5 U/l terminal transferase, and 0.4 µM of digoxigenin-11-deoxyuridine triphosphate (dUTP) at 37°C for 90 min. The reaction was stopped with 300 mM NaCl solution with 30 nM sodium citrate, and slides were washed with PBS containing 2% BSA. To detect the binding of dioxigenin-11-dUTP, slides were incubated at room temperature for 30 min at pH 7.5 with the Fab fragment of anti-dioxigenin (Boehringer, Indianapolis, IN) in the reaction containing 100 mM Tris-HCl and 150 mM sodium chloride. Slides were washed with distilled water, and later with 0.1 M acetate buffer at pH 6. After visualization with DAB, cell nuclei were counterstained with hematoxylin.
Expression of IL-8 and effect of GTP on H. pylori growth
The expression of IL-8 on mouse gastric mucosa was studied by the ABC method with anti-KC goat polyclonal antibody (Santa Cruz, CA). Slides were incubated with anti-KC antibody and 100-fold polyclonal rabbit anti-H. pylori antibody (Dako, Copenhagen, Denmark) overnight at 4°C. After washing with PBS, slides were incubated with biotinylated secondary antibody diluted 20-fold (Dako Copenhagen, Denmark). Slides were incubated with ABC solution for 30 min and visualized with DAB.
Immunohistochemical analysis
Twenty specimens per mouse from the antrum and body were examined. BrdU-labeled cells were counted from the deepest part to the luminal surface of the crypt. The total number of cells in the proliferative zone (PZ cells) was counted from the BrdU-labeled cell closest to the serosa to the BrdU-labeled cell uppermost on the luminal side. The number of cells in the crypt was counted, and the percentage of cells in the proliferative zone (PZ cells/total cells ratio) was calculated. Apoptosis was expressed as TUNEL-positive cells in 300 crypt cells. The expression of KC was expressed by the percentage of staining per mm2 using Image-pro-plus system (Planetron Inc, Tokyo, Japan). For all parameters, 10 sites were selected per mouse. The number of H. pylori was counted by a blinded pathologist, and scored from 1 to 3. All data was studied by ANOVA test. A p value of <0.05 was considered to be statistically significant.
Results
Number of BrdU-labeled cells
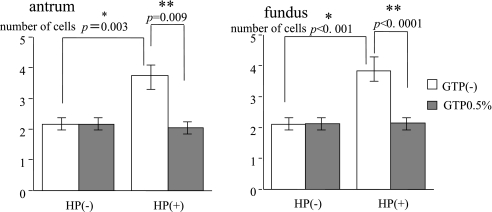
Cells with positive staining for BrdU were counted vertically from the epithelium to the submucosal layer under light microscopy (Fig. 1, 2, 3). The number of BrdU labeled cells was increased over the control by H. pylori infection in the antrum (control 2.15 ± 0.15 vs H. pylori 3.74 ± 1.01; p = 0.003). The administration of GTP decreased the number of labeled cells, which was increased by H. pylori infection. (H. pylori + GTP 2.05 ± 0.09 vs H. pylori only 3.74 ± 1.01; p = 0.009). In comparing the number of labeled cells between the antrum of animals infected with H. pylori and those infected with H. pylori and later administered GTP, the number of labeled cells was reduced (H. pylori + GTP 2.05 ± 0.09 vs H. pylori only 3.74 ± 1.01; p = 0.009. In bodies, the number of labeled cells also was greater than in the control in the presence of H. pylori infection (control 2.11 + 0.09 vs H. pylori only 3.82 ± 0.67; p<0.0001), but this increase was not as great in those administered GTP additionally (H. pylori + GTP 2.14 ± 0.15 vs H. pylori only 3.82 ± 0.67; p<0.0001) (Fig. 4).
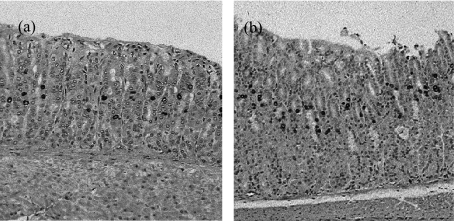
Fig. 1.
Immunohistochemistry for BrdU staining with the ABC method on gastric mucosal tissue of Balb/c mice (untreated mice): (a), antrum; (b), the body.
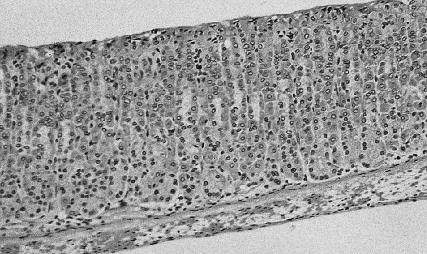
Fig. 2.
Gastric mucosal tissue of Balb/c mice (untreated mice) stained for apoptotic cells by the TUNEL method.
Fig. 3.
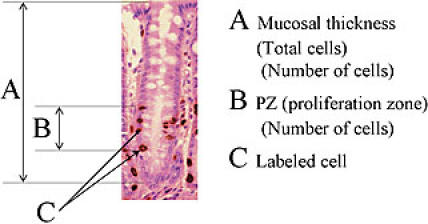
Anti BrdU immunohistochemical staining on gastric mucosa of Balb/c mouse: (A) showed the Mucosal thickness, (B) proliferation zone, (C) Labeled cell.
Fig. 4.
H. pylori (HP) infection significantly increased BrdU-labeled cells. Administration of green tea polyphenols (GTP) significantly suppressed the increase in BrdU-labeled cells.
*significant difference between with and without H. pylori challenge.
**significant difference between with and without green tea polyphenols.
Number of cells in proliferative zone
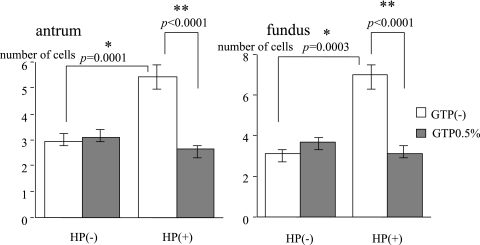
In the antrum, the number of PZ cells was increased by H. pylori infection (control 2.93 ± 0.37 vs H. pylori 5.43 ± 0.96; p = 0.0001), but further administration of GTP showed fewer PZ cells than with H. pylori infection alone (H. pylori + GTP 2.64 ± 0.35 vs H. pylori only 5.43 ± 0.96; p<0.0001). In the bodies, number of PZ cells was also increased in the presence of H. pylori infection (control 3.13 ± 0.56 vs H. pylori 7.00 ± 1.70; p = 0.0003), but this value was less with the additional administration of GTP (H. pylori + GTP 3.14 ± 0.31 vs H. pylori only 7.00 ± 1.70, p<0.0001) (Fig. 5).
Fig. 5.
H. pylori (HP) infection significantly increased cells in the proliferative zone. Green tea polyphenols (GTP) significantly suppressed the increase in cells in the proliferative zone.
*significant difference between with and without H. pylori challenge.
**significant difference between with and without green tea polyphenols.
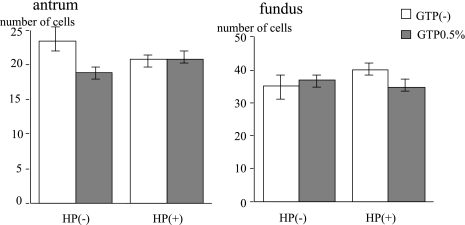
Total number of crypt cells
There was no significant difference in the number of total crypt cells between the H. pylori-inoculated group and the control. Even with GTP administration the total cell numbers were not very different (antrum: control 20.40 ± 3.66, H. pylori 20.93 ± 2.93, H. pylori + GTP 18.83 ± 2.66, bodies: control 35.08 ± 7.64, H. pylori 40.16 ± 3.88, H. pylori + GTP 36.77 ± 4.14) (Fig. 6).
Fig. 6.
There was no difference in the total number of cells in the crypt between the H. pylori (HP) infected group and the control. Green tea polyphenols (GTP) did not significantly change total cell number.
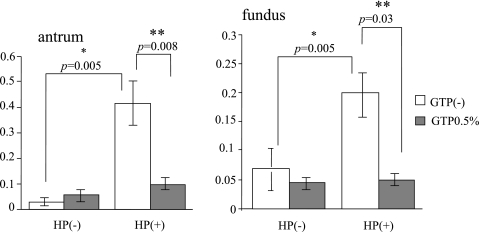
Number of apoptotic cells
TUNEL-positive cells were counted microscopically, with the specimen without a mucosal defect as a control (Fig. 2). The number of apoptotic cells was greater than in the control in the presence of H. pylori infection (control 0.0.26 ± 0.065 vs H. pylori 0.414 ± 0.266, p = 0.005) in the antrum. With the additional administration of GTP the number of apoptotic cells was less than with H. pylori alone (H. pylori + GTP 0.092 ± 0.042 vs H. pylori 0.414 ± 0.266; p = 0.008) Also, in the bodies, the number of apoptosis cells was greater by infection with H. pylori than in controls (control 0.067 ± 0.090 vs H. pylori 0.198 ± 0.163; p = 0.005), but apoptosis appeared to be suppressed by GTP administration (H. pylori + GTP 0.048 ± 0.027 vs H. pylori 0.198 ± 0.163; p = 0.03) (Fig. 7).
Fig. 7.
Apoptotic cells significantly increased in the H. pylori (HP) -infected group. The increased apoptosis was suppressed by the administration of green tea polyphenols (GTP).
*significant difference between with and without H. pylori challenge.
**significant difference between with and without green tea polyphenols.
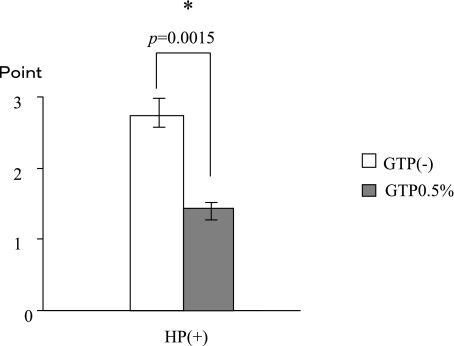
H. pylori growth
The administration of GTP suppressed H. pylori growth on Balb/C mouse gastric mucosa. (H. pylori + GTP 1.47 ± 0.71vs. H. pylori only 2.81 ± 0.26; p = 0.0015) (Fig. 8).
Fig. 8.
The H. pylori growth was reduced by the administration of GTP.
*significant difference between with and without H. pylori Challenge.
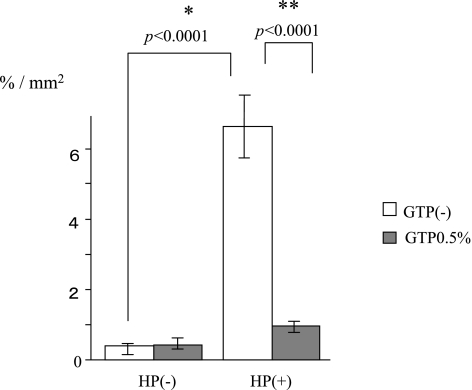
Expression of KC
Expressions of KC were increased by H. pylori infection (control 0.4 ± 0.3% vs H. pylori 6.3 ± 1.5%, p<0.0001). However, with the administration of GTP the expression of KC was reduced compared with values for H. pylori infection alone (H. pylori + GTP 0.6 ± 0.3% vs H. pylori 6.3 ± 1.5%; p<0.0001) (Fig. 9).
Fig. 9.
The expression of KC was increased by H. pylori (HP) infection. Administration of green tea polyphenols (GTP) reduced the expression of KC increased by H. pylori infection.
*significant difference between with and without H. pylori challenge.
**significant difference between with and without green tea polyphenols.
Discussion
It is widely known that tumors appear due to the disruption of the integrity maintained by two mechanisms, cell proliferation and cell death [18, 19] .The integrity of normal gastric mucosa is maintained by the balance in cell numbers between apoptosis, defined as cell suicide, and cell proliferation. However, excessive cell proliferation over cell death results in the occurrence of malignant neoplasm [20]. Otherwise, excessive cell death over proliferation results in gastric ulceration or erosions. H. pylori is related to the occurrence of gastric cancer and was designated as a class I carcinogen by WHO in 1994 [7]. It has been confirmed that Mongolian gerbils developed gastric cancer after long-term infection with H. pylori [6]. Clinical studies indicated that H. pylori infection increased cell proliferation in the gastric epithelium, but the increase was suppressed by H. pylori eradication [18, 21]. Our results demonstrated that cell proliferation and apoptosis were both stimulated from 18 weeks after the H. pylori infection.
We previously reported the relationship between cell turnover and apoptosis in the gastric mucosa over time in mice infected with H. pylori [19]. We showed that apoptosis was increased from 6 weeks after infection, but that cell proliferation was stimulated from 12 weeks after incubation with H. pylori [22]. Apoptosis is increased in the gastric mucosa infected with H. pylori and subsequently the phenomenon of increased cell proliferation was observed.
In the present study, H. pylori infection did not increase the total number of cells in gastric mucosa in our mouse model. The reason may be that we only studied gastric epithelial cell proliferation until 18 weeks after H. pylori infection. In human patients who had been infected with H. pylori since childhood it took many years to develop cancer. Long-term infection with H. pylori may cause an increase in the total number of cells in gastric mucosa over 18 weeks. It has been speculated that one reason why H. pylori stimulates cell proliferation in gastric mucosa is that the infiltration of inflammatory cells into the mucosa by the infection induces cell proliferation [10]. It is considered that the stimulated cell proliferation is a secondary reaction to the inflammation caused by infection. Histologically, inflammatory cells, including polynuclear neutrophils and lymphocytes, are present in H. pylori-infected gastric mucosa.
Yoshino et al. [23] reported that H. pylori infection induces Reg protein expression in human gastric mucosa, which is significantly up regulated by IL-8. IL-8 can be a stimulator of gastric cell proliferation by H. pylori infection through expression of Reg protein. We also demonstrated that H. pylori infection increased cell proliferation, and also that H. pylori infection increased the expression of IL-8. From these findings, the expression of IL-8 can be considered a mediator for cell proliferation in our model.
Green tea, which has polyphenols as a principal constituent, has been drunk widely in Japan and China for more than 5000 years [24]. It has been reported that the polyphenols in green tea have anti-tumor effects [16, 17], and epidemiologically those who drink green tea regularly have a lower risk of cancer. Those who drink 10 cups or more of green tea every day have a lower risk of gastric cancer compared with those having a lesser intake[25]. Also, those who live in Shizuoka prefecture in Japan, where green tea leaves are grown, have lower risks of all cancer, including gastric cancer [26]. Polyphenols are elements also found in red wine and cacao, and a variety of their properties have been reported recently. Briviba et al. [25] reported that the polyphenols in red wine suppress proliferation of colon cancer cells.
In our study, a 14-day administration of GTP significantly suppressed cell proliferation and apoptosis stimulated by H. pylori infection. This suppression was at the same level as in mice without infection. Mabe et al. [21] reported that green tea itself has antibacterial and bactericidal properties, and its benefit in eradication of H. pylori in mouse models has been reported. We demonstrated that the number of H. pylori organisms was reduced by GTP administration. The eradicative effect of GTP on H. pylori seemed to be one reason for the for decrease in the gastric cell proliferation and apoptosis that were stimulated by infection. GTP suppressed the expression of EGF in JB6 mouse epithelial cells [27], and it can be speculated that GTP administration suppressed the increased cell proliferation caused by H. pylori infection through activity to suppress the induction of growth factors in the gastric mucosa.
IL-8 is a potent neutrophil-activating chemokine, central to the immunopathogenesis of H. pylori. In this study, we measured mouse KC as a marker for inflammation. Mouse KC is a neutrophil-attracting chemokine and has a function corresponding to human IL-8 as well as MIP-2 in mice. We showed that the expression of KC protein was stimulated by H. pylori infection and that the administration of GTP reduced the stimulated KC expression. Naito et al. [28] reported that oxidative and nitrosative stress associated with inflammation plays an important role in gastric carcinogenesis as a mediator of carcinogenic compound formation, DNA damage, and cell proliferation. IL-8 expression is reported as the most important factor for inflammation and/or the occurrence of gastric cancer. Joh et al. [29] reported that H. pylori and IL-8 significantly stimulate EGFR phosphorylation. The inhibition of EGFR phosphorylation by H. pylori and also IL-8 reduced cell growth of gastric cells. Administration with GTP reduced IL-8 expression and also cell proliferation. From these data, the effect of GTP was a reduction of the expression of IL-8 on gastric mucosa, and IL-8 may reduce cell proliferation through the phosphorylation of growth factors and these receptors. Likewise, in the gastric mucosa, GTP administration may suppress the increased cell proliferation caused by H. pylori infection by inhibiting cytokine production and preventing the spread of inflammation in the gastric mucosa.
The effect of GTP on apoptosis has been examined in various organs, and most results indicate that the effect is proapoptotic. However, in our present study, administration of GTP tended to suppress the apoptosis stimulated by H. pylori infection. This difference, first of all, may be ascribed to the existence of a mechanism in the gastric mucosa to maintain its integrity, and when GTP administration suppresses proliferation excessively, injury to the epithelium may appear. However, the mechanism to maintain integrity may affect the degree of apoptosis induced by GTP administration. This possibility is conceivable because our result showed that the total cell number in gastric mucosa was not affected.
We presented data that H. pylori infection stimulated cell proliferation and apoptosis in a mouse model, which was in turn inhibited by GTP administration. This indicates that administration of GTP may inhibit carcinogenesis in the stomach, which is partially related to H. pylori infection.
References
- 1.Parsonnet J., Friedman G.D., Vandersteen D.P., Chang Y., Vogelman J.H., Orentreich N., Siblev R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 2.Cover T.L., Blaser M.J. Helicobacter pylori and gastroduodenal disease. Annu. Rev. Med. 1992;43:135–145. doi: 10.1146/annurev.me.43.020192.001031. [DOI] [PubMed] [Google Scholar]
- 3.Talley N.J., Zinsmeister A.R., Weaver A., DiMagno E.P., Carpenter H.A., Perez-Perez G.I., Blaser M.J. Gastric adenocarcinoma and Helicobacter pylori infection. J. Natl. Cancer Inst. 1991;83:1734–1739. doi: 10.1093/jnci/83.23.1734. [DOI] [PubMed] [Google Scholar]
- 4.Hansson L.E., Engstrand L., Nyren O., Evans D.J. Jr., Lindgren A., Bergstrom R., Andersson B., Athlin L., Bendtsen O., Tracz P. Helicobacter pylori infection: independent risk indicator of gastric adenocarcinoma. Gastroenterology. 1994;106:1398–1400. doi: 10.1016/0016-5085(93)90954-b. [DOI] [PubMed] [Google Scholar]
- 5.Group E.S. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 6.Honda S., Fujioka T., Tokieda M., Satoh R., Nishizono A., Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. IARC monographs on the evaluation of carcinogenic risks to humans. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 8.Sobala G.M., Schorah C.J., Shires S., Lynch D.A., Gallacher B., Dixon M.F., Axon A.T. Effect of eradication of Helicobacter pylori on gastric juice ascorbic acid concentrations. Gut. 1993;34:1038–1041. doi: 10.1136/gut.34.8.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch D.A., Mapstone N.P., Clarke A.M., Sobala G.M., Jackson P., Morrison L., Dixon M.F., Quirke P., Axon A.T. Cell proliferation in Helicobacter pylori associated gastritis and the effect of eradication therapy. Gut. 1995;36:346–350. doi: 10.1136/gut.36.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenes F., Ruiz B., Correa P., Hunter F., Rhamakrishnan T., Fontham E., Shi T.Y. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre-and post-eradication indices of proliferating cell nuclear antigen. Am. J. Gastroenterol. 1993;88:1870–1875. [PubMed] [Google Scholar]
- 11.Cahill R., Sant S., Beattie S., Hamilton H., O’Morain C. Helicobacter pylori and increased epithelial cell proliferation: a risk factor for cancer. Eur. J. Gastroenterol. Hepatol. 1994;6:1123–1127. [Google Scholar]
- 12.Cahill R.J., Kilgallen C., Beattie S., Hamilton H., O’Morain C. Gastic epithelial cell kinetics in the progression from normal mucosa to gastric carcinoma. Gut. 1996;38:177–181. doi: 10.1136/gut.38.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss S., Calam J., Agarwal B., Wang S., Holt P. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannick E.E., Bravo L.E., Zarama G., Realpe J.L., Zhang X.J., Ruiz B., Fontham E.T., Mera R., Miller M.J., Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 15.Crabtree J., Lindley I.Mucosal interleukin-8 and H. pylori-associated gastroduodenal disease Eur. J. Gastroenterol. Hepatol., Suppl 1S33–8.1994 [PubMed] [Google Scholar]
- 16.Smith D.M., Wang Z., Kazi A., Li L.H., Chan T.H., Dou Q.P. Synthetic analogs of green tea polyphenols as proteasome inhibitors. Mol. Med. 2002;8:382–392. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B. The health effects of tea polyphenols and their antioxidant mechanism. J. Clin. Biochem. Nutr. 2006;38:59–68. [Google Scholar]
- 18.Schulte-Hermann R., Bursch W., Grasl-Kraupp B., Torok L., Ellinger A., Mullauer L. Role of active cell death (apoptosis) in multi stage carcinogenesis. Toxicol Lett. 1995;82/83:143–148. doi: 10.1016/0378-4274(95)03550-8. [DOI] [PubMed] [Google Scholar]
- 19.Hall P.A., Coates P.J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J. Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 20.Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 21.Mabe K., Yamada M., Oguni I., Takahashi T. In vitro and in vivo activities of tea catechins against Helicobactor pylori. Antimicrob. Agents Chemother. 1999;43:1788–1791. doi: 10.1128/aac.43.7.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi T., Nakajima N., Kuwayama H., Ito Y., Iwasaki A., Arakawa Y. Gastric epithelial cell proliferation and apoptosis in Helicobacter pylori-infected mice. Aliment. Pharmacol. Ther. 2000;14(suppl. 1):68–73. doi: 10.1046/j.1365-2036.2000.014s1068.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino N., Ishihara S., Rumi M.A.K., Ortega-Cava C.F., Yuki T., Kazunori H., Takazawa S., Okamoto H., Kadowaki Y., Kinoshita Y. Interleukin-8 regulates expression of Reg protein in Helicobacter plyori-infected gastric mucosa. Am. J. Gastroenterol. 2005;200:2157–2166. doi: 10.1111/j.1572-0241.2005.41915.x. [DOI] [PubMed] [Google Scholar]
- 24.Weisburger J.H., Comer J., Kiple F.K., Ornelas K.C. The cambridge world history of food. Cambridge University Press; New York: 1980. pp. 712–720. [Google Scholar]
- 25.Brivida K., Pan L. Rechkemmer G. Red wine polyphenols inhibited the growth of colon carcinoma cells and modulated the activation pattern of mitogen-activated protein kinases. J. Nutr. 2002;132:2814–2818. doi: 10.1093/jn/132.9.2814. [DOI] [PubMed] [Google Scholar]
- 26.Oguni I., Cheng S.J., Lin P.Z. Protection against cancer risk by Japanese green tea. Prev. Med. 1992;21:332. [Google Scholar]
- 27.Yang F., de Villiers W.J., McClain C.J., Varilek G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-α production and lethality in a murine model. J. Nutr. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y., Yoshikawa T. Carcinogenesis and chemoprevention in gastric cancer associated with Helicobacter pylori infection: role of oxidants an antioxidants. J. Clin. Biochem. Nutr. 2005;36:37–49. [Google Scholar]
- 29.Joh T., Kataoka H., Tanida S., Watanabe K., Ohshima T., Sasaki M., Nakao H., Ohhara H., Higashiyama S., Itoh M. Helicobacter pylori-stimulated inerleukin-8 (IL-8) promotes cell proliferation through transactivation of epidermal growth factor receptor (EGFR) by disintegrin and metalloproteinase (ADAM) actibvation. Dig. Dis. Sci. 2005;50:2081–2089. doi: 10.1007/s10620-005-3011-0. [DOI] [PubMed] [Google Scholar]