Abstract
There has been considerable public and scientific interest in the use of phytochemicals derived from dietary components to combat human diseases. They are naturally occurring substances found in plants. Ferulic acid (FA) is a phytochemical commonly found in fruits and vegetables such as tomatoes, sweet corn and rice bran. It arises from metabolism of phenylalanine and tyrosine by Shikimate pathway in plants. It exhibits a wide range of therapeutic effects against various diseases like cancer, diabetes, cardiovascular and neurodegenerative. A wide spectrum of beneficial activity for human health has been advocated for this phenolic compound, at least in part, because of its strong antioxidant activity. FA, a phenolic compound is a strong membrane antioxidant and known to positively affect human health. FA is an effective scavenger of free radicals and it has been approved in certain countries as food additive to prevent lipid peroxidation. It effectively scavenges superoxide anion radical and inhibits the lipid peroxidation. It possesses antioxidant property by virtue of its phenolic hydroxyl group in its structure. The hydroxy and phenoxy groups of FA donate electrons to quench the free radicals. The phenolic radical in turn forms a quinone methide intermediate, which is excreted via the bile. The past few decades have been devoted to intense research on antioxidant property of FA. So, the present review deals with the mechanism of antioxidant property of FA and its possible role in therapeutic usage against various diseases.
Keywords: ferulic acid, oxidative stress, anti-inflammatory, radioprotector, antioxidant
Introduction
There is an emerging interest in the use of naturally occurring antioxidants for their therapeutic usage. Particularly, phenolics are considered as potential therapeutic agents against a wide range of ailments including neurodegenerative diseases, cancer, diabetes, cardiovascular dysfunction, inflammatory diseases and in ageing [1]. Phenolics are widely distributed in the plant kingdom and are therefore an integral part of the diet, with significant amounts being reported in vegetables, fruits and beverages [2]. Although the dietary intake of phenolics varies considerably among geographic regions, it is estimated that daily intake range from about 20 mg to 1 gm, which is higher than that for vitamin E [3] Phenolics exhibit a wide range of biological effects including antibacterial, anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, anticarcinogenic and vasodilatory actions [4]. Dietary plant phenolic compounds have been described to exert a variety of biological actions such as free radical scavenging, metal chelation, modulation of enzymatic activity and more recently to affect signal transduction, activation of transcription factors and gene expression. They received particular attention in the past 10 years because of their putative role in the prevention of several human diseases, particularly atherosclerosis and cancer [5].
Sources of Ferulic Acid (FA)
FA is a ubiquitous plant constituent that arises from the metabolism of phenylalanine and tyrosine. It occurs in seeds and leaves both in its free form and covalently linked to lignin and other biopolymers. In wheat, FA is ester linked to cell wall carbohydrates and occurs in higher concentration in the alcurone, pericarp and embryo cell walls. The trans-isomer predominates and accounts for 90% of the total phenolic acids in common flour [6]. FA is also a major constituent of fruits (e.g. orange), some vegetables (e.g. tomato, carrot), and sweet corn [7].
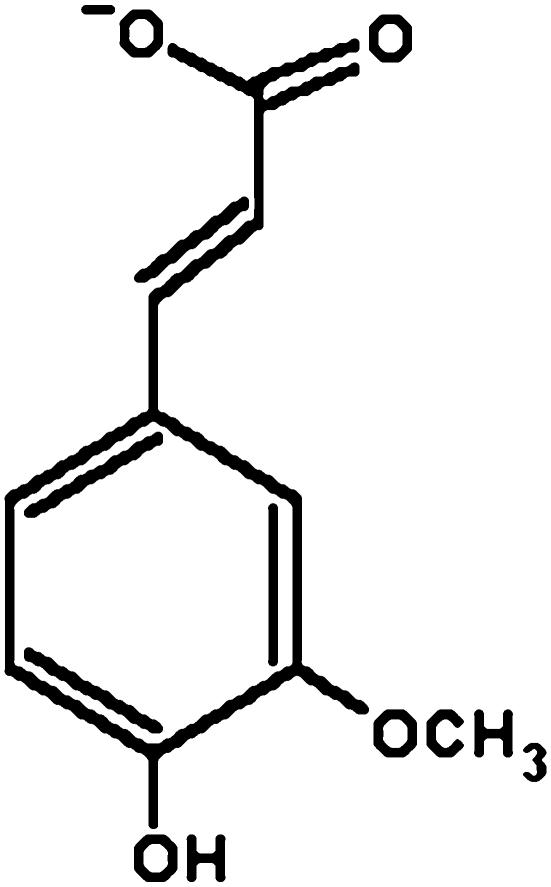
FA (4-hydroxy-3-methoxy cinnamic acid) (Fig. 1) is a phenolic compound it possesses three distinctive structural motifs that can possibly contribute to the free radical scavenging capability of this compound. The presence of electron donating groups on the benzene ring (3 methoxy and more importantly 4-hydroxyl) of FA gives the additional property of terminating free radical chain reactions. The next functionality-the carboxylic acid group in FA with an adjacent unsaturated C-C double bond-can provide additional attack sites for free radicals and thus prevent them from attacking the membrane. In addition, this carboxylic acid group also acts as an anchor of FA, by which it binds to the lipid bilayer, providing some protection against lipid peroxidation. Clearly, the presence of electron donating substituents enhances the antioxidant properties of FA [8].
Fig. 1.
Structure of FA
Mechanism of Antioxidant Action of FA
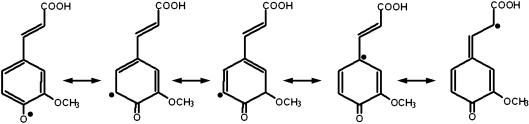
The antioxidant potential of FA can usually be attributed to its structural characteristics (Fig. 2). FA, because of its phenolic nucleus and unsaturated side chain can readily form a resonance stabilized phenoxy radical, which accounts for its potent antioxidant activity. Any reactive radical colliding with FA easily abstracts a hydrogen atom to form phenoxy radical. This radical is highly resonance stabilized since the unpaired electron may be present not only on the oxygen but it can be delocalized across the entire molecule. Additional stabilization of the phenoxy radical is provided by the extended conjugation in the unsaturated side chain. This resonance stabilization accounts for the effective antioxidant potential of FA. Moreover this phenoxy radical is unable to initate or propagate a radical chain reaction, and its most probable fate is a collision and condensation with another ferulate radical to yield the dimer curcumin. Such coupling may lead to a host of products, all of which still contain phenolic hydroxyl groups capable of radical scavenging. The presence of a second phenolic hydroxyl group substantially enhances the radical scavenging activity due to additional resonance stabilization and o-quinone formation [9].
Fig. 2.
Resonance Stabilization of FA radical
Metabolism and Absorption of FA
The absorption, metabolism and tissue distribution of FA has been extensively studied in rodents and humans. The metabolites of FA and their relative proportions will depend on many factors, including dose, route of administration and animal species. Ingestion of FA into humans is metabolized and excreted in urine as 3-hydroxyphenyl and 3-methoxy-4-hydroxy phenyl derivatives of phenyl propionic acid, hydracrylic acid and glycine conjugates. Feeding studies in rats with FA revealed metabolism to a dehydroxylated compound and the same hydroxy methoxy derivatives, as in the human studies, with FA itself being partly excreted as the glucuronide [10]. Intraperiotneal administration of FA to the rats is excreted as 3-hydroxy phenyl propionic acid a major urinary metabolite [11].
Bioavailability of FA
The physiological importance of FA and notably its antioxidant property depends upon its availability for absorption and subsequent interaction with target tissues [12]. It is more bioavailable than other dietary flavonoids and monophenolics so far studied [13]. FA stays in blood for longer than other antioxidants such as vitamin C. FA would therefore be expected to stay in the body long enough to help in keeping the free radicals at bay. Under normal conditions 56.1% of perfused FA enters the enterocytes by a yet unidentified mechanism. In these cells, FA is readily conjugated and the resulting metabolites leave the intestinal cells only towards the serosal side because no conjugated forms of FA are detected in the intestinal lumen. Under such conditions 56.1% of perfused FA, corresponding to the absorption, is recovered in the plasma mesenteric vein as conjugated derivative. A part of these conjugates enters into the hepatocytes and secreted in the bile (6%) and 49.9% of the perfused dose is distributed to the peripheral tissues and may have biological effects [14].
Anti-inflammatory Effect of FA
Chronic or acute inflammation is a multiple process, which is mediated by activated inflammatory or immune cells. From the immune system, macrophages play a central role in managing many different immunopathological phenomena such as the overproduction of pro-inflammatory cytokines and inflammatory mediators (reactive oxygen species (ROS), nitric oxide (NO) and prostaglandin E2) generated by activated inducible nitric oxide synthase (iNOS) and cyclooxygenase [15]. It has been reported that a number of antioxidants including FA and related ester derivatives decrease the levels of some inflammatory mediators, e.g., prostaglandin E2 and tumor necrosis factor-alpha [16] and iNOS expression and function [17] in cells stimulated by the bacterial endotoxin lipopolysaccharide. There is evidence that hydrophobic ester derivatives of FA have enhanced inhibitory activity on iNOS protein expression in lipopolysaccharide/interferon-γ (LPS/IFNγ) activated RAW 264.7 cells [18]. Hosada et al. [19] reported that feruloyl-myo-inositois the derivatives of FA suppressed the cyclooxygenase-2 promoter activity through the β-galactosidase reporter gene assay system in human colon cancer DLD-1 cells. It was also reported that FA inhibits the production of murine MIP-2, one of the members of chemokine superfamily, in LPS-stimulated RAW 264.7 cells in a dose dependent manner. These findings suggest that FA might have potential as an anti-inflammatory drug and reveals at least in part the mechanisms of its anti-inflammatory effect [20].
Therapeutic Usage of FA
FA is receiving greater attention nowadays in the research world because of its wide range of therapeutic effects. For example alkyl ferulate has an anti-carcinogenic potential and is a more effective cancer preventive agent. Another FA derivative in which geranyl group is attached to the phenolic hydroxyl group of ethyl ferulate, exhibits anti-colon carcinogenesis [21]. A novel polyphenol with FA and gallic acid is found to have higher activity as an anti-carcinogen than the original phytochemicals. The compounds consisting of FA and myo-inositol also have potential as cancer chemopreventive agents [22]. Most of its therapeutic potentials are ascribed to antioxidants and anti-inflammatory activity.
Antidiabetic Effect
Diabetes is the most common endocrine disorder characterized by the hyperglycemia, which causes over production of free radicals thereby result in oxidative stress [23]. This stress is defined as an imbalance between the levels of prooxidants and antioxidants in the biological systems, leading to cellular injury [24]. It has been reported that the blood glucose level in streptozotocin induced diabetic animals is reduced by the administration of FA. FA, which has been shown to have antioxidant properties, helps to neutralize the free radicals produced by streptozotocin in the pancreas and thereby decrease the toxicity of streptozotocin. This decreased oxidative stress/toxicity on the pancreas may help the beta cells to proliferate and secrete more insulin, which may have been reduced due to streptozotocin treatment. This increased insulin secretion can cause increased utilization of glucose by the extra hepatic tissues and thereby decrease the blood glucose level [25]. Nomura et al. [26] have also been reported that amide compounds of FA exhibited their stimulatory abilities on insulin secretion in rat pancreatic RIN-5F cells. Administration of FA at a dose of 0.01% and 0.1% of basal diet showed it can suppress the blood glucose levels in streptozotocin induced diabetic mice. In KK-Ay mice 0.05% of FA suppressed the blood glucose level effectively [27].
Anticancer Effects
Free radicals are considered as important factors in the etiology of cancer. Dietary components with antioxidant activity have been receiving particular attention as potential inhibitors of several cancers [28]. Phytochemicals can exert anti-cancer activities, partially based on their ability to quench ROS and thereby protecting critical cellular molecules (i.e. DNA, proteins and lipids) from oxidative insult [29]. Phytochemicals may also interfere with intracellular signaling pathways, such as those, which regulate proliferation, induction of apoptosis and response to oxidative stress [30]. Important mechanisms for the anticarcinogenic effects of polyphenols include the reduction of proliferative activity and the induction of apoptosis in cancer cells [31]. Studies have shown that FA exhibits anticarcinogenic effects against azoxymethane-induced colon carcinogenesis in F344 rats [32]. It has also been reported to depress 12-O-tetradecanoylphorbol-13-acetate (TPA)-promotion of skin tumorgenesis [33] as well as to inhibit occurrence of pulmonary cancers in mice [34]. Stich et al. [35] have reported that there was a significant decrease in urinary N-nitrosoproline levels in humans on treatment with FA. The mechanism suggesting that inhibition of nitrosation and endogenous formation of carcinogenic nitrosamines. Several plant phenolics are known to be potent inhibitors for mutagenesis and carcinognesis by polycyclic aromatic hydrocarbon [36]. They act as effective electrophilic trapping agents [37] and are also known to be blockers of nitrosamine formation [38].
Antiapoptotic Effect
Apoptosis is a certain type of cell death in multicellular organisms and involves a cascade of closely regulated intracellular events leading to cell suicide [39]. Reports suggest that phenolic compounds generally bring about the normal homeostasis by inducing apoptosis in various cancer cells [40]. Studies have shown the cytotoxic effects of these dietary polyphenols against different tumors, mediated through apoptosis [41]. Cells undergoing programmed cell death express phosphatidyl serine on their surface, which aids in their recognition and phagocytosis by macrophages, thereby limiting inflammation [42]. Externalization of phosphatidyl serine by hydrogen peroxide (H2O2) indicates pre-apoptotic stage of the peripheral blood mononuclear cells (PBMCS). The inhibition of externalization of phosphatidyl serine by FA indicates the anti-apoptotic activities of these polyphenols in human PBMCS. The decrease in H2O2-induced externalization of phosphatidyl serine in peripheral blood mononuclear cells pretreated with polyphenols indicates the involvement of scavenging of radicals by the phenolics and/or dissociation of phenolic-translocase enzyme binding due to oxidative stress [43]. Khanduja et al. [43] have reported that phenolic compounds like FA significantly exhibit anti-apoptotic activity in normal PBMCS exposed to H2O2 induced oxidative stress.
Anti-Ageing Effect
Acute and chronic exposure to sun rays promotes premature skin ageing, erythema, inflammation, immunodepression and photo-carcinogenesis [44]. Exposure of ultra violet (UV) radiation results in the generation of reactive oxygen/nitrogen species resulting in oxidative damage. These events can ultimately lead to diseases related to UV-radiation, such as irritation or sunburn, photoallergy, immunosuppression, photoageing and skin cancer [45]. Recently a great deal of focus has been shed on the antioxidant potentialities of FA. This is due to its phenolic nucleus and an extended side chain conjugation, FA readily forms a resonance stabilized phenoxyl radical, which accounts for its potent antioxidant potential. UV absorption by FA catalyses stable phenoxyl radical formation and thereby potentiates its ability to terminate free radical chain reactions. By virtue of effectively scavenging chain reactions and deleterious radicals and suppressing radiation-induced oxidative reactions, FA may also serve as an important antioxidant in preserving physiological integrity of cells exposed to both air and the impinging UV radiation [9].
Hepatoprotective Effect
Chronic alcohol ingestion is associated with a variety of pathological conditions varying from simple intoxication to severe, life threatening derangement of metabolism in liver [46]. Intake of alcohol results in excessive generation of free radicals [47], which alter the biomembrane and cause severe damage. FA works well in herbal antioxidant formula, vitamin and herbal health supplement, and body’s immune system can be benefited from FA. These reports heavily favour the idea that regular ingestion of FA may provide substantial protection against alcohol and polyunsaturated fatty acid (PUFA) induced toxicity and may provide the body with the ability to triumph over the deleterious effects of alcohol and PUFA [48]. Treatment with FA significantly decreased the activities of these enzymes in plasma. FA is shown to preserve physiological integrity of the cells exposed to various stress. This can be attributed to the effective antioxidant property of FA. Normally phenolic compounds act by scavenging free radicals and quenching the lipid peroxidative side chain. Phenolic compounds can act as free radical scavengers by virtue of their hydrogen donating ability and forming aryloxyl radicals [49]. It has been proposed that hydroxyl and hydroperoxyl radicals initiate H+ abstraction from a free phenolic substrate to form phenoxyl radical that can rearrange to quinonemethide radical intermediate [50], which is excreted via bile. Srinivasan et al. [51] have also been reported FA protects against carbon tetrachloride (CCl4) induced toxicity in an experimental animal model, which ascribed to antioxidant potential.
Neuroprotective Effect
Increasing experimental evidence indicates the importance of oxidative stress in pathology and neurotoxicity associated with aging and many neurodegenerative diseases such as Alzheimer’s disease. Alzheimer’s disease is an age associated dementing disorder, and many other neurodegenerative disorders, are characterized by free radical-mediated oxidative stress in brain [52]. The free radical mediated ROS and reactive nitrogen species (RNS) generated in brain can lead to protein, DNA and RNA oxidation, lipid peroxidation and neuronal dysfunction or death [53]. FA is reported as a potent scavenger of ROS and RNS and thereby reduces the chance of free radical attack on proteins and hence preventing their oxidative modification [53]. Kanski et al. [8] have reported that FA protects against free radical mediated changes in the conformation of synaptosomal membrane proteins as monitored by EPR spin labeling techniques. The presence of antioxidant and anti-inflammatory properties of FA may be potentially due to its ability to inihibit leukotriene production and reduce oxidative stress in brain [20]. The long term administration of FA at a dose of 300 µM effectively protects against amyloid beta-peptide (1-42) toxicity by inhibiting microglial activation in vivo [54]. Sultana et al. [55] showed that 10–50 µM of FA significantly protects against amyloid beta-peptide (1-42) toxicity by modulating oxidative stress directly and by inducing protecting genes in hippocampal cultures. FA ethylesters also exert neuroprotective effects by upregulating protective enzymes, such as hemeoxygenase-1 and heat shock protein 70 [56].
Radioprotective Effect
Radiation therapy is a form of cancer treatment that uses ionizing radiation to kill cancer cells and shrink tumors. It also injures or destroys the normal cells by damaging their genetic material, making it impossible for these cells to continue to grow and divide. Although radiation damages both cancer cells and normal cells, the goal of radiation therapy is to kill as many cancer cells as possible, while limiting the damage to nearby healthy tissue [57]. The deleterious effects of ionizing radiation in biological systems are mainly mediated through the generation ROS including superoxide anion (O2·–), hydroxyl radical (OH·) and hydrogen peroxide H2O2. These ROS are known to cause oxidative stress in several critical cellular molecules like DNA, proteins and lipid membranes [58]. Radiation-induced oxidative damage can lead to chromosomal aberration, lipid peroxidation and alterations in endogenous antioxidants [59]. It has been reported that pretreatment with FA to γ-irradiated lymphocytes resulted in decreased lipid peroxidation and improved antioxidant status preventing the damage to the lymphocytes. This may be due to the antioxidant sparing action of FA. Since FA prevents the formation of ROS, the syntheses of these enzymes are not affected [60]. FA scavenges superoxide anion radical and inhibits lipid peroxidation induced by superoxide and the effect of FA is similar to that of superoxide dismutase (SOD) [61]. In addition to its antioxidant activity, FA modulates phase II enzymes like glutathione s-transferase (GST) and the radioprotection of FA was also observed this may be attributed to the up regulation of antioxidant enzymes [62]. It was also be reported that FA induces intrinsic antioxidant mechanisms such as superoxide dismutase, catalase and glutathione reductase activities [63].
Pulmonary Protective Effect
Nicotine is one of the major hazardous compounds of cigarette smoke and thus mimics most of the deleterious effects of cigarette smoking. It increases lipid peroxidation and thus causes oxidative cellular injury, which is believed to play a major role in the pathogenesis of several smoking-related diseases [64]. Administration of FA reversed the damage induced by nicotine and increases the endogenous antioxidant defense system and protects the cells from oxidative damage. FA effectively quenches the free radicals, prevents them from attacking the membrane, protects the membrane, inhibits the leakage of marker enzymes into circulation, and improves the antioxidant status in circulation [65].
Hypotensive Effect
Hypertension is associated with an elevation of ROS and frequently also with an impairment of endogenous antioxidant mechanisms [66]. The ROS abundance in hypertension could result from increased production or impaired degradation. Many investigators have reported that spontaneously hypertensive rats (SHR) arteries have increased superoxide anion production compared to WKY rats, and that this reacts with NO, thereby effectively depleting NO in vascular endothelial cells [67, 68]. FA is reported to scavenge superoxide anions [65]; it might be that FA improves the bioavailability of NO in blood vessels in SHR. The possibility is that FA acts specifically on blood vessels in SHR to cause a relaxation response in vascular endothelial cells. Studies have reported that FA had a hypotensive effect and attenuated the elevation of blood pressure in spontaneously hypertensive rats. The significant inhibition of the depressive effect of FA by the intravenous injection of NG-nitro-L-arginine methyl ester (L-NAME) suggests that the hypotensive effect of FA was associated with nitric oxide-mediated vasodilatation [69].
Anti-Atherogenic Effect
The link between oxidative stress and atherosclerosis was originally derived from the oxidative modification hypothesis as a key step in the generation of macrophage derived foam cells and the initiation of atherosclerosis [70]. FA effectively reduced the lipid levels. This may be attributed to the effective antioxidant property of FA. Supplementation with FA decreased the levels of free fatty acids, triglycerides, cholesterol and phospholipids in streptozotocin induced hyperlipidemic diabetic rats [7]. Reports have shown that FA exerts hypolipidemic activity by decreasing the HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis and acyl-CoA: cholesterol transferase (ACAT), the cholesterol-esterifying enzyme in tissues, and by increasing the acidic sterol excretion [71].
More over, FA is a potent antioxidant and prevents low density lipoprotein (LDL) oxidation induced by copper ions and facilitates the uptake and degradation of cholesterol by the liver. FA was also found to be effective in treating ischemic stroke in China. Moreover, reports have shown that γ-oryzanol, a mixture of FA, can lower the cholesterol level in blood and lower the incidence of coronary heart disease [72]. Pre-cotreatment with FA and ascorbic acid significantly reduces the levels of triglycerides, total cholesterol, free fatty acids free and ester cholesterol in serum and cardiac tissues. It was also significantly decrease the levels of phosphlipids, lipid peroxides, low density lipoprotein and very low density lipoprotein-cholesterol was also observed in the serum of isoproterenol intoxicated rats [73]. Hiramatsu et al. [74], reported that FA and its steryl esters, γ-oryzanol, exhibit strong hypocholesterolemic and antiatherogenic properties.
Studies on the lipid peroxidation and antioxidant changes and their significance during myocardial injury have provided a new insight into the pathogensis of heart disease. The heart failure subsequent to myocardial infarction may be associated with an antioxidant deficit as well as increased myocardial oxidative stress [73]. Prospective studies have found that habitual intake of whole grain food is associated with reduced coronary heart diseases, total cancer mortality, incidence of diabetes and coronary heart disease mortality [75]. A review of ancient Chinese herbal drugs revealed FA to be one of the active constituents for the treatment of cardiovascular disease [76]. Studies have shown that pre co-treatment with FA and ascorbic acid significantly counteracted the pronounced oxidative stress effect of isoprotorenol by the inhibition of lipid peroxidation, restoration of antioxidant status, and myocardial marker enzyme activities [73]. FA produces a pronounced protective antistressor effect and reduces both the stomach mucous membrane damage and the myocardial injury caused by painful immobilizing stress. This might be because FA inhibits the process of lipid peroxidation and increases the antioxidant activity in myocardium [77].
Conclusion
In conclusion, FA exhibits a wide range of therapeutic properties like anti-inflammatory, antiatherogenic, antidiabetic, antiageing, neuroprotective, radioprotective and hepatoprotective effects. Many of these activities can be attributed to its potent antioxidant capacity because of its phenolic nucleus and extended side chain conjugation. It readily forms a resonance stabilized phenoxyl radical which accounts for its potent antioxidant potential. FA works well in all herbal antioxidant formula, vitamin and herbal health supplements. Thus our body’s immune system can be benefited from FA. These reports heavily favor the idea that regular ingestion of FA may provide substantial protection against various oxidative stress related diseases.
References
- 1.Soobrattee M.A., Neergheen V.S., Luximon-Ramma A., Aruoma O.I., Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Luximon-Ramma A., Bahorun T., Crozier A., Zbarsky V., Datla K.K., Dexter D.T., Aruoma O.I. Characteristion of the antioxidant functions of flavonoids and proanthocyanidins in mauritian black teas. Food Res. Int. 2005;38:357–367. [Google Scholar]
- 3.Hollman P.C., Katan M.B. Bioavailability and health effects of dietary flavonols in man. Arch. Toxicol. Suppl. 1998;20:237–248. doi: 10.1007/978-3-642-46856-8_21. [DOI] [PubMed] [Google Scholar]
- 4.Middleton E., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol. Rev. 2000;52:673–839. [PubMed] [Google Scholar]
- 5.Nardini M., Ghiselli A. Determination of free and bound phenolic acids in beer. Anal. Nutr. Clin. Methods. 2004;84:137–143. [Google Scholar]
- 6.Fulcher R.G. American Association of cereal chemists, editor. Fluorescence microscopy of cereals in, New frontiers in food microstructure. Ottawa, Canada: 1983. pp. 167–175. [Google Scholar]
- 7.Balasubashini M.S., Rukkumani R., Menon V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003;40:118–122. doi: 10.1007/s00592-003-0099-6. [DOI] [PubMed] [Google Scholar]
- 8.Kanaski J., Aksenova M., Stoyanova A., Butter field D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure activity studies. J. Nutr. Biochem. 2002;13:273–281. doi: 10.1016/s0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 9.Graf E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 2000;28:1249–1256. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- 10.Deeds F., Booth A.N., Jones F.T. Methylation and dehydroxylation of phenolic compounds by rats and rabbits. J. Biol. Chem. 1957;225:615–621. [PubMed] [Google Scholar]
- 11.Booth A.N., Emmeron O.H., Jones F.T., Deeds F. Urinary metabolites of coumarin and omicron-comaric acid. J. Biol. Chem. 1959;234:946–948. [PubMed] [Google Scholar]
- 12.Scheline R.R. The metabolism of drugs and other compounds by the intestinal microflora. Acta. Pharmacol. Toxicol. 1968;26:332–342. doi: 10.1111/j.1600-0773.1968.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 13.Beecher G.R. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 1998;218:98–100. doi: 10.3181/00379727-218-44282a. [DOI] [PubMed] [Google Scholar]
- 14.Adam A., Crespy V., Levrat-Verny M.A., Leenhardt F., Leuillet M., Demigne C., Remesy C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002;132:1962–1968. doi: 10.1093/jn/132.7.1962. [DOI] [PubMed] [Google Scholar]
- 15.Walsh L.J. Mast cells and oral inflammation. Crit. Rev. Oral. Biol. Med. 2003;14:188–198. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 16.Ou L., Kong L.Y., Zhang X.M., Niwa M. Oxidation of ferulic acid by momordica charantia peroxidase and related anti-inflammation activity changes. Biol. Pharm. Bull. 2003;26:1511–1516. doi: 10.1248/bpb.26.1511. [DOI] [PubMed] [Google Scholar]
- 17.Tetsuka T., Baier L.D., Morrison A.R. Antioxidants inhibit interleukin-1 induced cyclooxygenase and nitric oxide synthase expression in rat mesangial cells. Evidence for post-transcriptional regulation. J. Biol. Chem. 1996;271:1168–1169. doi: 10.1074/jbc.271.20.11689. [DOI] [PubMed] [Google Scholar]
- 18.Murakami A., Nakamura Y., Koshimizu K., Takahashi D., Matsumata K., Hagihara K., Taniguchi H., Noumura E., Hosoda A., Tsuno T., Maruta Y., Kim H.W., Kawabata K., Ohigashi H. FA 15, a hydrophobic derivative of ferulic acid, suppresses inflammatory responses and skin tumor promotion: comparison with ferulic acid. Cancer Lett. 2002;180:121–129. doi: 10.1016/s0304-3835(01)00858-8. [DOI] [PubMed] [Google Scholar]
- 19.Hosoda A., Ozaki Y., Kashiwada A., Mutoh M., Wakabayashi K., Mizuno K., Nomura E., Taniguchi H. Synthesis of ferulic acid derivatives and their Suppressive effects on cyclooxygenase-2 promoter activity. Bioorg. Med. Chem. 2002;10:1189–1196. doi: 10.1016/s0968-0896(01)00386-8. [DOI] [PubMed] [Google Scholar]
- 20.Sakai S., Ochiai H., Nakajima K., Terasawa K. Inhibitory effect of ferulic acid on macrophage inflammatory protein-2 production in a murine macrophage cell line, Raw 264.7. cytokine. 1997;9:242–248. doi: 10.1006/cyto.1996.0160. [DOI] [PubMed] [Google Scholar]
- 21.Imaida K., Hirose M., Yamaguchi S., Takahashi S., Ito N. . Effects of naturally occurring antioxidants on combined 1,2-dimethylhydrazine and 1-methyl-nitrosourea- initiated carcinogenesis in F344 male rats. Cancer Lett. 1990;55:53–59. doi: 10.1016/0304-3835(90)90065-6. [DOI] [PubMed] [Google Scholar]
- 22.Huang M.T., Smart R.C., Wong C.Q., Conney A.H. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid and ferulic acid on tumor promotion in mouse skin by 12-o-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–5946. [PubMed] [Google Scholar]
- 23.Aragno M., Parola S., Tamagno E. Oxidative derangement in rat synaptosomes induced by hyperglycemia: restorative effect of dehydroepiandosterone treatment. Biochem. Pharmacol. 2000;60:389–395. doi: 10.1016/s0006-2952(00)00327-0. [DOI] [PubMed] [Google Scholar]
- 24.Villa-caballero C., Nava-Ocampo A.A., Frati-Munari A.C., Poncemonter H. Oxidative stress, should it be measured in diabetic patient. Gac-Med-Max. 2000;136:249–256. [PubMed] [Google Scholar]
- 25.Balasubashini M.S., Rukkumani R., Viswanathan P., Menon V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004;18:310–314. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
- 26.Noumura E., Kashiwada A., Hosoda A., Nakamura K., Morishita H., Tsuno T., Taniguchi H. Synthesis of amide compounds of ferulic acid and their stimulatory effects on insulin secretion in vitro. Bioorg. Med. Chem. 2003;11:3807–3813. doi: 10.1016/s0968-0896(03)00280-3. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi M., Matuo T., Tsuno T., Hosoda A., Nomura E., Taniguchi H., Susaki H ., Morishita H. Antioxidant activity and hypoglycemic effect of ferulic acid in streptozotocin induced diabetic mice and KK-AY mice. Biofactors. 2004;21:315–319. doi: 10.1002/biof.552210161. [DOI] [PubMed] [Google Scholar]
- 28.Dedoussis G.V.Z., Kaliora A.C., Andrikopoulos N.K. Effect of phenols on natural killer (NK) cell-mediated death in the K562 human leukemic cell line. Cell Biol. Int. 2005;29:884–889. doi: 10.1016/j.cellbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Roy M., Chakrabarty S., Sinha D., Bhattacharya R.K., Siddiqi M. Anticlastogenic, antigenotoxic and apoptotic activity of epigallocatechin gallate: a green tea polyphenol. Mutat. Res. 2003;523:33–41. doi: 10.1016/s0027-5107(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 30.Loo G. Redox-sensitive mechanisms of phytochemical mediated inhibition of cancer cell proliferation. J. Nutr. Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 31.Vermeulen K., Van Bockstaele D.R., Berneman Z.N. The cell cycle: a review of regulation deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawabata K., Yamamoto T., Hara A., Shimizu M., Yamada Y., Matsunaga K., Tanaka T., Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 33.Asanoma M., Takahashi K., Miyabe M., Yamamoto K., Yoshimi N., Mori H., Kawazoe Y. Inhibitory effect of topical application of polymerized ferulic acid, a synthetic lignin, on tumor promotion in mouse skin two stage tumorigenesis. Carcinogenesis. 1993;14:1321–1325. doi: 10.1093/carcin/15.9.2069. [DOI] [PubMed] [Google Scholar]
- 34.Lesca P. Protective effects of ellagic acid and other plant phenols on benzo[a] pyrene-induced neoplasia in mice. Carcinogenesis. 1983;4:1651–1653. doi: 10.1093/carcin/4.12.1651. [DOI] [PubMed] [Google Scholar]
- 35.Stich H.F., Ohshima H., Pignatelli B., Michelon J., Bartisch H. Inhibitory effect of betel nut extracts on endogenous nitrosation in humans. J. Natl. Cancer Inst. 1983;70:1047–1050. [PubMed] [Google Scholar]
- 36.Newmark H. Plant phenolics as inhibitors of mutational and precarcinogeneic events. Can. J. Physiol. Pharmacol. 1987;65:461–466. doi: 10.1139/y87-079. [DOI] [PubMed] [Google Scholar]
- 37.Newmark H. A hypothesis for dietary compounds as blocking agents of chemical carcinogenesis: plant phenolics and pyrrole pigments. Nutr. Cancer. 1984;6:58–70. doi: 10.1080/01635588509513807. [DOI] [PubMed] [Google Scholar]
- 38.Kuenzing W., Chau J., Norkus E., Holowaschenko H., Newmark H., Mergens W., Conney A.H. Caffeic and ferulic acid as blockers of nitrosamine formation. Carcinogenesis. 1984;5:309–313. doi: 10.1093/carcin/5.3.309. [DOI] [PubMed] [Google Scholar]
- 39.Jacobson M.D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 40.Taraphder A.K., Roy M., Bhattacharya R.K. Natural products as inducers of apoptosis: implication for cancer therapy and prevention. Curr. Sci. 2001;80:1387–1396. [Google Scholar]
- 41.Inoue M., Suzuki R., Koide T., Sakaguchi N., Ogihara Y., Yabu Y. Antioxidant, gallic acid, induces apoptosis in HL-60 RG cells. Biochem. Biophys. Res. Commun. 1994;204:898–904. doi: 10.1006/bbrc.1994.2544. [DOI] [PubMed] [Google Scholar]
- 42.Martin S.J., Reutelingsperger C.P., McGahon A.J., Radar J.A., Van Schie R.C., La Face D.M., Green D.R. Early redistribution of plasma membrane phosphatidyl serine is a general feature of apoptosis regardless of initiating stimulus: inhibition by overexpression of Bcl-2 and AbI. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khanduja K.L., Avti P.K., Kumar S., Mittal N., Sohi K.K., Pathak C.M. Anti-apopototic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. Biochim. Biophys. Acta. 2006;1760:283–289. doi: 10.1016/j.bbagen.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Anselmi C., Centini M., Andreassi M., Buonocore A., Rosa C.L., Facino R.M., Sega A., Tsuno F. Conformational analysis: a tool for the elucidation of the antioxidant properties of ferulic acid derivatives in membrane models. J. Pharm. Biomed. Anal. 2004;35:1241–1249. doi: 10.1016/j.jpba.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Black H.S., De Gruijl F.R., Forbes P.D., Cleaver J.E., Anathaswamy H.N., De Fabo E.C., Vlrich S.E., Tyrrell R.M. Photocarcinogenesis: an overview. J. Photochem. Photobiol. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 46.Lindi C., Montorfano G., Marciani P. Rat erythrocyte susceptibility to lipid peroxidation after chronic ethanol intake. Alc. 1998;16:311–316. doi: 10.1016/s0741-8329(98)00020-2. [DOI] [PubMed] [Google Scholar]
- 47.Rouach H., Fataccioli V., Gentil M., French S., Morimoto M., Nordman R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 48.Rukkumani R., Aruna K., Varma P.S., Menon V.P. Influence of ferulic acid on circulatory prooxidant antioxidant status during alcohol and PUFA induced toxicity. J. Physiol. Pharmacol. 2004;55:551–561. [PubMed] [Google Scholar]
- 49.Gil M.I., Francisco A., Barberan T., Pierce B.H., Holcroft D.M., Kader A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 50.Pan G.X., Spencer L., Leary G.J. Reactivity of ferulic acid and its derivative towards hydrogen peroxide and peracetic acid. J. Agric. Food Chem. 1999;47:3325–3331. doi: 10.1021/jf9902494. [DOI] [PubMed] [Google Scholar]
- 51.Srinivasan M., Rukkumani R., Ram Sudheer A., Menon V.P. Ferulic acid, a natural protector against carbon tetrachloride induced toxicity. Fundam. Clin. Pharmacol. 2005;19:491–496. doi: 10.1111/j.1472-8206.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 52.Butterfield D., Castergra A., Pocernich C., Drake J., Scapagini G., Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. . J. Nutr. Biochem. 2002;13:444. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 53.Joshi G., Perluigi M., Sultana R., Agrippino R., Calabrese V., Butterfield D.A. In vivo protection of synaptosomes by FA ethylester from oxidative stress mediated by 2,2-azo bis (2-amido-propane) dihydrochloride (AAPH) or Fe2+/H2O2: Insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem. Int. 2006;48:318–327. doi: 10.1016/j.neuint.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Kim H.S., Cho J.Y., Kim D.H., Yan J.J., Lee H.K., Suh H.W., Song D.K. Inhibitory effects of long term administration of ferulic acid on microgilal activation induced by intercerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol. Pharm. Bull. 2004;27:120–121. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- 55.Sultana R., Ravagna A., Mohmmad-Abdul H., Calabrese V., Butterfield D.A. Ferulic acid ethyl ester protects neurons against amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J. Neurochem. 2005;92:749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 56.Scapagini G., Butterfield D.A., Colombrita C., Sultana R., Pascale A., Calabrese V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid. Redox Signal. 2004;6:811–818. doi: 10.1089/ars.2004.6.811. [DOI] [PubMed] [Google Scholar]
- 57.Grdina D.J., Murley J.S., Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63:2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- 58.Von Sonntag C. Radiobiology: Chemistry, in The chemical basis of radiation biology. Taylor and Francis; London: 1987. pp. 31–36. [Google Scholar]
- 59.Nair C.K.K., Parida D.K., Nomura T. Radioprotectors in radiotherapy. J. Radiat. Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 60.Prasad N.R., Srinivasan M., Pugalendi K.V., Menon V.P. Protective effect of Ferulic acid on γ-radiation induced micronuclei, dicentric aberration and lipid peroxidation in human lymphocytes. Mutat. Res. 2006;603:129–134. doi: 10.1016/j.mrgentox.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Toda S., Kumura M., Ohnishi M. Effects of phenolic carboxylic acids on superoxide anion and lipid peroxidation induced by superoxide anion. Plant Med. 1991;57:8–10. doi: 10.1055/s-2006-960005. [DOI] [PubMed] [Google Scholar]
- 62.Dean J.V., Devarenne T.P., Iks L.L., Orlofsky E. Properties of a maize glutathione S-transferase that conjugates coumaric acid and other phenyl propanoids. Plant Physiol. 1995;108:985–994. doi: 10.1104/pp.108.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han B.S., Park C.B., Takasuka N., Naito A., Sekine E., Nomura H., Taniguchi T., Tsuno H., Tsuda A. Ferulic acid derivative ethyl 3-(4' genranyloxy-3-methoxy phenyl)-2-proleonyl as a new candidate chemopreventive agent for colon carcinogen in rat. Jpn. J. Cancer Res. 2001;92:404–409. doi: 10.1111/j.1349-7006.2001.tb01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yildiz D., Ercal N., Armstrong D.W. Nicotine enantiomers and oxidative stress. Toxicology. 1998;130:155–165. doi: 10.1016/s0300-483x(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 65.Sudheer A.R., Kalpana C., Srinivasan M., Menon V.P. Ferulic acid modulates lipid profiles and prooxidant/antioxidant status in circulation during nicotine-induced toxicity: A dose dependent study. Toxicol. Mech. Methods. 2005;15:375–381. doi: 10.1080/15376520500194783. [DOI] [PubMed] [Google Scholar]
- 66.Lassegue B., Griendling K.K. Reactive oxygen species in hypertension. Am. J. Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Dominiczak A.F., Bohr D.F. Nitric oxide and its putative role in hypertension. Hypertension. 1995;25:1202–1211. doi: 10.1161/01.hyp.25.6.1202. [DOI] [PubMed] [Google Scholar]
- 68.Tschudi M.R., Mesaros S., Luscher T.F., Malinski T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide anion in hypertension. Hypertension. 1996;27:32–35. doi: 10.1161/01.hyp.27.1.32. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki A., Kagawa D., Fujii A., Ochiai R., Tokimitsu I., Sato I. Short and long term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am. J. Hypertens. 2002;15:351–357. doi: 10.1016/s0895-7061(01)02337-8. [DOI] [PubMed] [Google Scholar]
- 70.Yokoyama M. Oxidative stress and atherosclerosis. Curr. Opin. Pharmacol. 2004;4:110–115. doi: 10.1016/j.coph.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Kim H.K., Jenog T.S., Lee M.K., Park B.Y., Choi M.S. Lipid lowering efficacy of hesperidine metabolities in high-cholesterol fed rats. Clin. Chim. Acta. 2003;327:129–137. doi: 10.1016/s0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 72.Rukkumani R., Aruna K., Varma P.S., Menon V.P. Ferulic acid, a natural phenolic antioxidant modulates altered lipid profiles during alcohol and thermally oxidized sunflower oil induced toxicity. J. Nutra. Func. Med. Foods. 2004;4:119–132. [Google Scholar]
- 73.Yogeeta S.K., Hanumantra R.B., Gnanapragasam A., Subramanian S., Rajakannu S., Devaki T. Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: beneficial effects of ferulic acid and ascorbic acid. Basic Clin. Pharmacol. Toxicol. 2006;98:467–472. doi: 10.1111/j.1742-7843.2006.pto_335.x. [DOI] [PubMed] [Google Scholar]
- 74.Hiramatsu K., Tani T., Kimura Y., Izumi S.I., Nakane P.I. Effect of γ-Oryzanol on atheroma formation in hyper-cholesterolemic rabbits. Tokai. J. Exp. Clin. Med. 1990;15:299–306. [PubMed] [Google Scholar]
- 75.Jacobs D.R., Pereira M.A., Meyer K.A., Kushi L.H. Fiber from whole grains, but not refined grains, is inversely associated with all cause mortality in older women: the lowa women’s health study. J. Am. Coll. Nutr. 2000;19:326–330. doi: 10.1080/07315724.2000.10718968. [DOI] [PubMed] [Google Scholar]
- 76.Li L., Sun H. Active constituents of Chinese herbal drugs for the treatment of cardiovascular diseases. Drugs Today. 1991;27:243–249. [Google Scholar]
- 77.Perfilova V.N., D’iakova A.V., Tiurenkov I.N. Cardioprotective action of ferulic acid upon heart under stressor damage condition. Eksp. Klin. Farmakol. 2005;68:19–22. [PubMed] [Google Scholar]