Abstract
All living organisms alter their physiology in response to changes in oxygen tension. The photosynthetic bacterium uses the RegB–RegA signal transduction cascade to control a wide variety of oxygen-responding processes such as respiration, photosynthesis, carbon fixation and nitrogen fixation. We demonstrate that a highly conserved cysteine has a role in controlling the activity of the sensor kinase, RegB. In vitro studies indicate that exposure of RegB to oxidizing conditions results in the formation of an intermolecular disulfide bond and that disulfide bond formation is metal-dependent, with the metal fulfilling a structural role. Formation of a disulfide bond in vitro is also shown to convert the kinase from an active dimer into an inactive tetramer state. Mutational analysis indicates that a cysteine residue flanked by cationic amino acids is involved in redox sensing in vitro and in vivo. These residues appear to constitute a novel ‘redox-box’ that is present in sensor kinases from diverse species of bacteria.
Keywords: histidine kinase/photosystem gene expression/redox regulation/Rhodobacter capsulatus
Introduction
Oxygen is a highly reactive oxidant used by a majority of living organisms as a terminal electron acceptor. As respiratory-driven electron transport generates ATP by oxidative phosphorylation, it is extremely beneficial for cells to closely monitor environmental oxygen levels and to quickly respond to changes in oxygen tension. In a wide range of proteobacteria, the sensor kinase RegB plays a major role in the redox control of many diverse cellular processes. Under anaerobic conditions, RegB autophosphorylates at a conserved histidine residue and subsequently transduces a signal to its cognate response regulator, RegA by phosphoryl group transfer to an aspartate residue (Inoue et al., 1995; Bird et al., 1999). RegA∼P then regulates transcription of genes whose products are directly involved in photosynthesis (Sganga and Bauer, 1992; Mosley et al., 1995), respiration (Swem et al., 2001), carbon fixation (Vichivanives et al., 2000), nitrogen fixation (Elsen et al., 2000), hydrogen utilization (Elsen et al., 2000), cytochrome biosynthesis (Swem et al., 2001) and aerotaxis (Romagnoli et al., 2002).
There are numerous histidine kinase/aspartate response regulator systems present in bacteria (West and Stock, 2001), with a few also observed in eukaryotic cells (Saito, 2001). However, mechanisms by which sensor kinases perceive the environment and control kinase activity are widely unknown. In fact, a detailed understanding of a mechanism for sensing oxygen is known for only one other histidine sensor kinase, FixL, which can directly sense oxygen using a bound heme (Miyatake et al., 2000). There is also a report that the activity of the redox-responding global histidine kinase ArcB is affected by the oxidation state of quinones (Georgellis et al., 2001). However, it remains to be determined how oxidized quinone inhibits ArcB activity. In this paper, we describe the mechanism by which a truncated version of RegB (RegBs) from Rhodobacter capsulatus can sense environmental redox potential by demonstrating that RegBs is capable of forming a metal-dependent, intermolecular disulfide bond that acts as a molecular switch for controlling kinase activity in vitro. Therefore, RegB may be mechanistically similar to OxyR, CrtJ and RsrA that contain redox-reactive cysteines (Aslund et al., 1999; Paget et al., 2001; Kim et al., 2002; Masuda et al., 2002). Genome analysis indicates that the redox-reactive cysteine residue is highly conserved in histidine kinases from a broad distribution of eubacteria.
Results
Metal is a required cofactor for redox control in vitro
Like many other sensor kinases, RegB is a membrane spanning protein. However, RegB is atypical in that it contains very small periplasmic and cytoplasmic loops between its transmembrane regions (Ouchane and Kaplan, 1999; Chen et al., 2000). This led us to inquire whether a soluble truncated version of RegB that encodes the conserved cytosolic histidine phosphorylation and kinase domains may contain redox-sensing capabilities. For this analysis, a truncated form of RegB (RegBs) that lacks all membrane spanning domains was overexpressed in Escherichia coli and purified by anion exchange chromatography. Our initial kinase assays indicated that purified RegBs was capable of autophosphorylating at a rate that was indistinguishable from that of full-length purified RegB (Potter et al., 2002). Furthermore, RegBs exhibited a slight increase in autophosphorylation under reduced conditions (data not shown). This latter result suggested that overexpressed RegBs could be largely depleted of a cofactor needed for redox-dependent autophosphorylation.
One hint as to the nature of a required redox cofactor was obtained from the highly conserved regB-senC-regA genome organization that is found in six different genera of α-proteobacteria (Masuda et al., 1999). In these species, the regB gene is divergently transcribed from the senC gene (Buggy and Bauer, 1995), with transcription of both genes co-regulated by the phosphorylation state of RegA (Du et al., 1999). Previous in vivo mutational analysis indicates that RegB is unable to properly sense oxygen when senC is deleted from the chromosome, suggesting an involvement of SenC in the redox-sensing capabilities of RegB (Eraso and Kaplan, 2000). Interestingly, SenC exhibits significant sequence similarity to the mitochondrial inner membrane protein Sco1, which is thought to function as a mitochondrial copper chaperone (Nittis et al., 2001). Both SenC and Sco1 have been shown to bind stoichiometric amounts of copper in vitro, indicating that they may have analogous roles (Beers et al., 2002; McEwan et al., 2002). This suggests that the redox-sensing activity of RegB may be dependent on the putative copper chaperone activity of SenC, raising the possibility that RegB may bind copper.
Copper-binding proteins that are highly overexpressed in E.coli are devoid of significant amounts of copper, owing to the fact that E.coli cells contain little or no free copper ions (Silver, 1996) and that E.coli actively inhibits transport of excess copper into the cytoplasm from the growth medium (Cervantes and Gutierrez-Corona, 1994). However, isolated copper-binding apoproteins can be reconstituted by the addition of exogenous CuCl2, followed by extensive dialysis (Lamb et al., 2000). To test whether RegBs is able to bind copper, 300 µM CuCl2 was slowly added to 100 µM RegBs and then dialyzed extensively. Three independent preparations yielded bound copper-to-RegBs molar ratios of 0.9, 0.9 and 0.8 (Brenner and Harris, 1995). As a control, similar treatment of lysozyme with CuCl2, followed by dialysis, yielded no detectable bound Cu2+. These results indicate that RegBs contains a single copper-binding site per monomer.
The electronic absorption spectrum of copper-replete RegBs exhibits two features not observed in copper-depleted protein: a broad peak at 615 nm (Figure 1A, inset) and a shoulder at 315 nm (data not shown). The broad feature centered at 615 nm can be attributed to ligand field transitions within the d-orbitals of Cu2+ (Figure 1A, inset). For typical nitrogen/oxygen coordination, the dd-absorption band energy is too high to be assigned to a trigonal bipyramidial geometry, and too low to be consistent with a square coplanar structure (Lever, 1984; Hathaway, 1987). However, the energy of the absorption band does fall within the energy range typically observed for either a five-coordinate square-based pyramid or a six-coordinate tetragonally elongated octahedron with mixed nitrogen/oxygen ligands (Lever, 1984; Hathaway, 1987; Astley et al., 1995). The higher energy shoulder observed at 315 nm has a broad tail extending into the visible region of the spectrum. Given the absence of other chromophores associated with RegBs, these high energy features are consistent with the energies of copper-His ligand-to-metal charge transfer transitions (Fawcett et al., 1980; Bernarducci et al., 1981; Innes et al., 1988).
Fig. 1. Electron paramagnetic resonance (EPR) analysis of the RegBs copper center. (A) X-band EPR spectra of wild-type RegBs and (B) C265A RegBs collected at 77 K in 10 mM Tris pH 8.0, 300 mM NaCl and 50% glycerol. Top spectrum (solid line) is experimental data and the bottom trace (dotted) is simulated data for both (A) and (B). Insets: electronic absorption spectra of the visible region for wild-type RegBs and C265A RegBs collected at 298 K in buffer mentioned in Materials and methods.
Analysis of the electron paramagnetic resonance (EPR) spectrum of oxidized RegBs revealed the presence of two distinct d9 Cu2+ centers in approximately equimolar amounts (Figure 1A). Since RegBs binds copper with a 1:1 stoichiometry, it appears that ∼50% of RegB sites contain a type a copper center (Cua) and ∼50% contain a type b copper center (Cub). The EPR copper g-values indicate that there are two independent, low symmetry Cu2+ sites, with both sites comprised of mixed nitrogen and oxygen coordination (Peisach and Blumberg, 1974; Hathaway, 1987; McLachlan et al., 1995; Hemmert et al., 1999; Huang et al., 2000). The A-values suggest that these centers are likely six-coordinate (Aza) in the Cua site and five-coordinate or weakly six-coordinate (Azb) at the Cub site (Peisach and Blumberg, 1974; Hathaway, 1987). Nitrogen-only coordination is precluded by the amino acid composition, as there are only three completely conserved histidine residues in the truncated form of RegB.
To determine whether the presence of copper influences kinase activity, autophosphorylation assays (Bird et al., 1999) were performed in the presence and absence of a reducing agent with aliquots removed at 1, 3, 5 and 10 min. Remarkably, there is barely detectable autophosphorylation of copper-replete RegBs under air-oxidizing conditions, whereas under reducing (2 mM dithiothreitol, DTT) conditions there is a high level of autophosphorylation (Figure 2A). Measurement of 32P incorporation indicates that there is a 104-fold increase in autophosphorylation when copper-replete RegBs is converted from an air-oxidized to a reduced form using DTT. Redox regulation is a reversible process, since reduced RegBs can be oxidized with potassium permanganate to turn off kinase activity and then again treated with a reductant to regain activity (data not shown).
Fig. 2. Involvement of copper and Cys265 in redox-regulated activity of RegBs. (A) Autophosphorylation of oxidized (open circles) and reduced (filled circles) copper-reconstituted RegBs for defined periods after addition of [γ-32P]ATP. (B) Autophosphorylation of oxidized (open circles) and reduced (closed circles) EDTA-treated RegBs. (C) Kinase activity redox titration where RegBs samples were placed at defined ambient redox potentials for 2 h before autophosphorylation assays were initiated with the addition of [γ-32P]ATP. Each kinase assay was allowed to proceed for 10 min, quenched by the addition of a reducing 0.1% SDS dye solution and then separated by gel electrophoresis. The level of autophosphorylation was visually assayed for phosphate incorporation by exposure to film, and was also quantitated using a phosphorImager. Percent phosphorylation was plotted against potential, with the data fitted to a two-electron Nernst equation. (D) Autophosphorylation of oxidized (open circles) and reduced (closed circles) copper-reconstituted C265A mutant RegBs under oxidizing (open circles) and reducing (closed circles) conditions. Each lane contains 10 µmol of RegBs.
To further show that metal is needed for redox control, copper-replete RegBs protein was treated with the metal-chelating agent EDTA (ethylenediaminetetraacetic acid), dialyzed and then assayed for activity. After EDTA treatment, which removed assayable copper, the RegBs preparation no longer responded to redox equivalents. Instead, both oxidized and reduced copper-depleted RegBs phosphorylated to levels that are very similar to those observed with reduced copper-replete RegBs (Figure 2B), indicating that copper is crucial for oxidation-dependent inhibition of kinase activity.
Kinase activity of copper-replete RegBs was measured at defined ambient redox potentials to determine the oxidation-reduction midpoint potential of the ‘redox switch’ in RegBs. Individual autophosphorylation assays were performed using samples equilibrated at different defined ambient redox potentials ranging from –230 to –410 mV, in 10 mV increments, prior to kinase activity measurements (Hirasawa et al., 1998; Krimm et al., 1998; Setterdahl et al., 2000). As shown in the plot of percentage activity against ambient redox potential (Figure 2C), the data give an excellent fit to the Nernst equation for a two-electron process. The calculated average midpoint potential (Em) was –300 mV (±10 mV based on six replicates) at pH 8.0. The Em value was independent of incubation time (2, 4 or 18 h) and the concentration of the reducing agent in the buffer, as is expected for true equilibrium titrations.
Metal reduction does not stimulate kinase activity
Given that reduction of copper from Cu2+ to Cu+ would only require one electron, a ‘fit’ of the activity redox plot to a two-electron event indicates that reduction of copper may not be involved in converting RegBs into an active state. To further clarify this point, we assayed the effect of adding ascorbate to RegBs, which is a poor reductant for disulfide bonds but is known to reduce nitrogen/oxygen-coordinated Cu2+. Electronic absorption and EPR analysis of ascorbate-treated RegBs revealed a loss of the absorption feature at 615 nm and the EPR signal in the g∼2 region, indicating the reduction of the RegBs copper center (data not shown). However, despite the reduction of copper, ascorbate-treated RegBs exhibited no significant autophosphorylation (Figure 3A). The fact that ascorbate with an Em value of 58 mV can reduce the copper center, but is not capable of stimulating kinase activity which has a measured Em value of –300 mV (Figure 2C), clearly reveals that reduction of copper is independent of the stimulation of autophosphorylation. This suggests that the copper ion has another role, such as placing RegBs into a conformation that allows the true redox sensor to function properly. As a control, we observed that kinase activity was restored when ascorbate-treated RegBs was subsequently treated with 10 mM DTT (Em = –330 mV) (Figure 3A), indicating that ascorbate is not simply inactivating or denaturing the protein, but is instead failing to reduce the actual redox sensor that is needed to stimulate kinase activity.
Fig. 3. A structural role for the metal center. (A) Copper-replete RegBs was pre-reduced with ascorbate at pH 8.0 for 1 h, or first with ascorbate for 30 min and then with dithiothreitol (DTT) for 30 min at pH 8.0, before the kinase assay was initiated with the addition of [γ-32P]ATP. After 10 min incubation, the samples were quenched with the addition of a reducing 0.1% SDS dye solution and then separated by gel electrophoresis. (B) RegBs apoprotein was reconstituted with different metals as indicated. Kinase assays were performed under air-oxidizing (no DTT) or reducing conditions (+10 mM DTT). The reactions were initiated by the addition of [γ-32P]ATP and quenched after 10 min incubation followed by gel electrophoresis.
To test for metal specificity, we first treated RegBs with EDTA to remove copper and then reconstituted the metal-depleted apoprotein with the divalent metals, nickel, iron, cobalt, zinc, cadmium or manganese. After metal reconstitution, kinase assays were performed on each sample under both air-oxidizing and reducing (2 mM DTT) conditions. This analysis demonstrated that redox-responsive autophosphorylation of RegBs occurs with each of the tested metals (Figure 3B). The fact that Zn2+ does not undergo oxidation/reduction at the tested potentials, coupled with the observation that zinc-reconstituted RegBs shows excellent redox control, clearly demonstrates that the metal plays a structural rather than a redox role.
RegB kinase activity is regulated by intermolecular disulfide bond formation in vitro
Since the metal center in RegBs does not play a redox role, we used the fluorescent probe monobromobimane (mBBr) that forms a covalent adduct with the free sulfhydryl group of reduced cysteine to determine whether a redox-active disulfide existed in RegBs. Although mBBr is itself weakly fluorescent, a covalent adduct of mBBr with cysteine thiols shows significant fluorescence. Thus, disulfide bond breakage/formation can be directly assayed by measuring the amount of mBBr that covalently binds to RegBs under defined redox conditions (Hirasawa et al., 1998; Krimm et al., 1998; Setterdahl et al., 2000). To measure free thiols in copper-replete RegBs, aliquots of the protein were first incubated for 2 h at potentials ranging from –230 to –400 mV, in 10 mV increments, and then allowed to react with mBBr. A plot of the fluorescence of the mBBr-protein adduct against ambient redox potential (Figure 4) demonstrates that a significant increase in mBBr-protein fluorescence occurs as the ambient redox potential becomes more reducing. As is the case for the activity-based redox titrations, the mBBr data fit to a Nernst two-electron equation. The observed Em value for the mBBr fluorescence titration was –294 mV (±10 mV) at pH 8.0 (based on four replicate titrations), which is within experimental error of the Em value derived from activity-based redox titrations (–300 mV). These data suggest that a reversible two-electron disulfide/dithiol redox couple may be the actual redox-active site that affects RegBs kinase activity.
Fig. 4. Monobromobimane (mBBr) titration of RegBs disulfide bond. RegBs samples were placed at given potentials as indicated for 2 h at room temperature using ratios of oxidized and reduced dithiothreitol as the redox mediator before mBBr was incubated with each sample. The fluorescence of each sample was then converted to percent fluorescence and plotted against redox potential. The data were fitted to a two- electron Nernst equation yielding a midpoint potential of –294 mV.
Analysis of the amino acid sequence of RegBs reveals only a single cysteine residue (Cys265) in the overexpressed cytosolic domain of RegB. To test whether Cys265 is indeed involved in redox sensing, a cysteine-to-alanine mutation was constructed (C265As) as described in Materials and methods, with mutant C265As protein purified, reconstituted with copper and assayed for kinase activity. The results of this analysis (Figure 2D) indicate that C265As exhibits constitutively high activity under both oxidizing and reducing conditions. Indeed, the level of phosphorylation exhibited by reduced and oxidized C265As protein is very similar to that observed in reduced wild-type RegBs. An mBBr-based redox titration of C265As protein also demonstrated constitutively low fluorescence levels that were independent of the ambient potential imposed on the samples (data not shown), indicating an absence of redox-active thiols in the mutant RegBs.
As C265As and copper-deplete wild-type RegBs were both constitutively active under both oxidizing and reducing conditions, there is a possibility that C265As is incapable of binding copper. However, metal analysis on copper-reconstituted C265As protein shows that the mutant protein bound stoichiometric amounts of copper (1:1). EPR analysis of oxidized C265As also revealed the presence of a strong Cu2+ EPR signal deriving from two distinct Cu2+ centers that are comparable to those observed with wild-type RegBs (Figure 1B), thereby indicating that Cys265 does not directly bind copper. However, the Cua and Cub stoichiometry exist in a ∼5:1 ratio in the mutant rather than the ∼1:1 stoichiometry observed with wild-type RegBs. The fact that the mutant and wild-type proteins both contain Cua and Cub EPR features also indicates that the Cys265 sulfhydryl is not providing a coordinating ligand to Cu2+. This correlates well with the g-values, which indicate that both Cu2+ sites contain primarily mixed nitrogen/oxygen coordination precluding a sulfur ligand.
In addition to alterations in the Cua:Cub ratio, the C264A RegBs protein exhibits a 20 nm blue-shift in the Cu2+ ligand field absorption band, from 615 nm in wild-type RegBs to 595 nm in C265As (Figure 1B, inset). In the light of the 5:1 Cua:Cub ratio, and the EPR parameter for Cua, this spectral shift indicates that the mixed nitrogen/oxygen tetragonal Cua center contains more tightly coordinated nitrogen-donor ligands than does the Cub site. These results also indicate that the inability of the C265As mutant to form a disulfide bridge does not significantly affect the geometry of the copper coordinating ligands but does affect the structure of the copper centers.
Disulfide bond formation affects oligomerization of RegB in vitro
To obtain a better understanding of the role of disulfide bond formation on controlling RegBs activity, we performed size-exclusion chromatography of copper-replete RegBs under oxidizing and reducing conditions. The estimated molecular mass of copper-replete RegBs was determined to be ∼70 kDa under reducing conditions (Figure 5A and C). Since RegBs has a calculated mass of 36 kDa, this indicates that reduced wild-type RegBs exists as a dimer in solution, which is in good agreement with numerous studies that have shown that sensor kinases autophosphorylate in vivo as dimers (West and Stock, 2001). In contrast, oxidized wild-type RegBs, which is inactive as a kinase, elutes with a molecular mass of 140 kDa, which corresponds closely to the calculated mass for a tetramer of RegBs (Figure 5A and C). Interestingly, constitutively active C265As predominately exists as a dimer under both oxidizing and reducing conditions (Figure 5B and C). Interconversion of RegBS between dimer and tetrameric states appears to be an inherent property of the protein, given that oxidized C265As, reduced C265As and reduced wild-type RegBs all contain some tetrameric form of the protein (∼15–20%). Conversely, oxidized wild-type RegBs exists primarily as a tetramer, but also contains some dimer form (∼15%) (Figure 5). Thus, the role of the disulfide appears to be to affect the equilibrium between these states in favor of the tetrameric form.
Fig. 5. Size-exclusion chromatography of RegBs and C265As. (A) Elution profile of Superose 12 eluted wild-type RegBs and (B) C265A mutant RegBs under oxidizing (solid line) and reducing (dashed line) conditions. (C) Standard curve drawn according to the peak elution volumes (Ve, elution volume; V0, void volume) of the molecular weight standards: 158, 44, 17 and 1.35 kDa (green circles) as detected by absorption at 280 nm. The elution position of oxidized and reduced wild-type RegBs (blue diamonds) and C265A (red open squares) were then applied to the plot based upon their elution position. (D) SDS–PAGE of RegBs after equilibration at defined redox potentials.
We also performed SDS–polyacrylamide gel electrophoresis (PAGE) of RegBs that was pre-equilibrated in buffers designed to poise the protein at different defined ambient redox potentials. In this assay, we observed that RegBs formed a tetramer that was stable in the presence of SDS under oxidizing conditions (Eh ≥ –220 mV) (Figure 5D). In contrast, under highly reducing conditions (Eh = –380 mV), RegBs migrated as a monomer during electrophoresis, which we attribute to SDS-mediated dissociation of the dimer complex. The amount of tetrameric RegBs was decreased by half when the protein was incubated at an ambient potential of ∼–310 mV (i.e. at an Eh value were the disulfide would be expected to be ∼50% converted to the dithiol form) for 2 h before being subjected to electrophoresis. These oligomerization-based redox titration data are in good agreement with midpoint potentials that were calculated for kinase activity and mBBr labeling. Gel electrophoresis also indicted that the C265As protein was also largely deplete of tetramers under oxidizing and reducing conditions, thereby confirming that Cys265 is crucial for oligomerization (data not shown). From these data, we conclude that the off state of oxidized RegBs corresponds to a tetramer, which is promoted by the formation of an intermolecular disulfide bond between Cys265 residues.
Cys265 also regulates RegB kinase activity in vivo
To ascertain the involvement of Cys265 in vivo, we placed the C265A regB point mutation into the chromosome of R.capsulatus using allelic replacement. Inspection of colony pigmentation indicated that the C265A regB mutant exhibited a significantly darker pigmentation than that of the wild-type strain when grown under aerobic conditions. Since the RegB–RegA signal transduction system activates photosynthesis gene expression, one would expect that a constitutively active RegB protein would lead to an increase in photosystem pigments under aerobic growth conditions. To quantitatively assess this phenotype, the wild-type and the regB mutant strains were grown semi-aerobically to the same cell density, lysed by sonication and spectrally scanned from 400 to 900 nm. The results of this analysis demonstrate that the regB mutant strain made 73% more photopigments than wild-type cells grown under the same conditions (Figure 6A). A similar increase in pigment production was also observed during aerobic and photosynthetic growth conditions (data not shown).
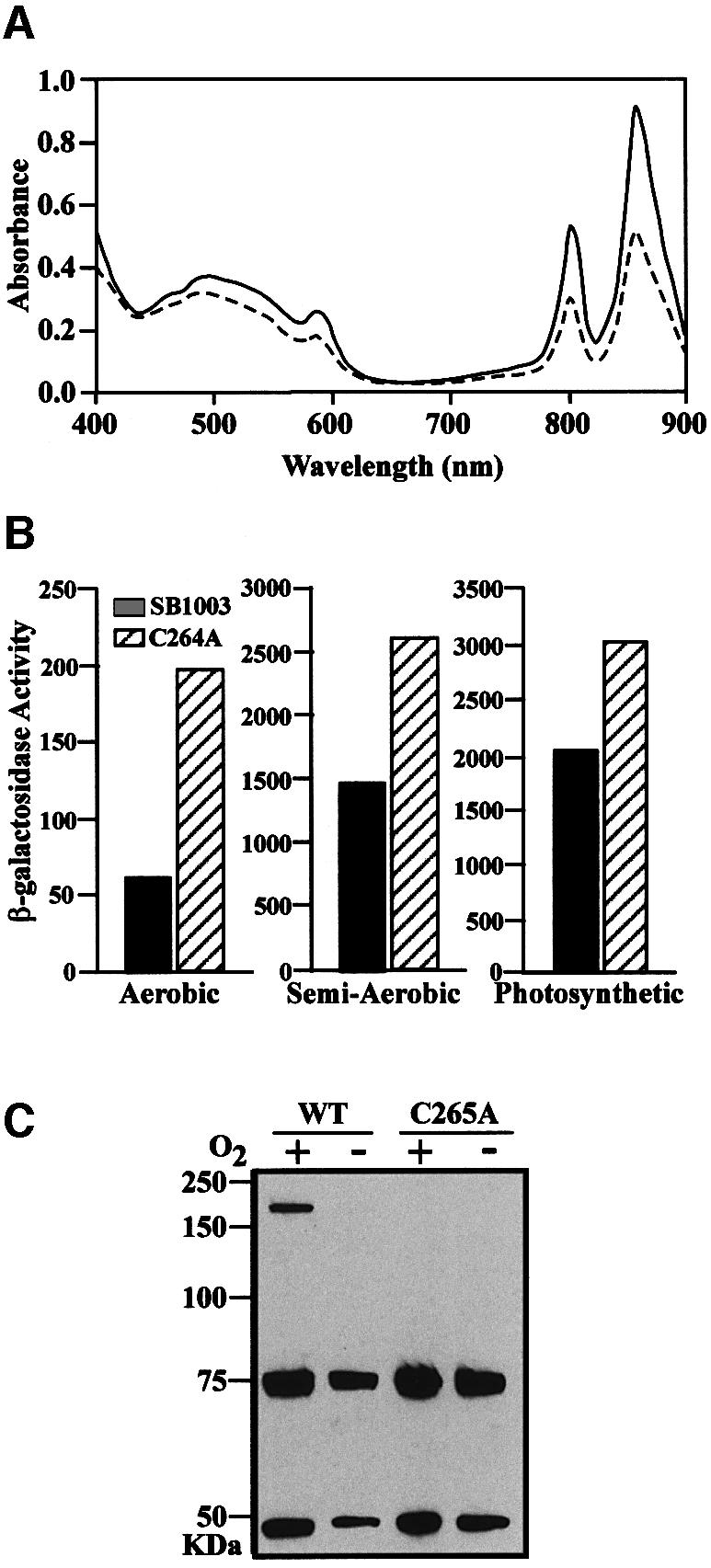
Fig. 6. In vivo importance of Cys265 in RegBs. (A) A spectral scan of crude cell extracts from semi-aerobically grown wild-type (dashed line) and C264A mutant (solid line) RegBs strains. (B) β-Galactosidase activity of puf promoter in wild-type and C264A mutant RegB strains grown aerobically, semi-aerobically and photosynthetically. Activity units represent micromoles of ONPG hydrolyzed/min/mg protein. (C) Western blot analysis of wild-type and C265A RegB-flag tagged strains of R.capsulatus grown aerobically or anaerobically. The 75 kDa band is a cross-reacting protein used as a loading standard, whereas the 47 kDa band is the RegB monomer and the 200 kDa band in the wild-type oxidized lane is the RegB tetramer.
To measure the in vivo effect of the C265A regB mutation on photosynthesis gene expression, we performed β-galactosidase assays on strains containing the puf promoter plasmid pCB532Ω (Bauer et al., 1988). The puf operon, which encodes structural components for light harvesting and reaction center complexes, is anaerobically activated by the RegB–RegA signal transduction cascade, as well as aerobically repressed by the transcription factor AerR (Dong et al., 2002). The results of this analysis indicate that expression of the puf operon is elevated 3-fold in C265A regB mutant strain under aerobic conditions and 1.7- and 1.5-fold under semi-aerobic and anaerobic conditions, respectively, relative to wild-type R.capsulatus (Figure 6B).
To investigate whether full-length RegB is capable of undergoing changes in oligomeric state in vivo, we constructed chromosomally encoded C-terminal flag-tag variants of wild-type and C265A regB that allowed detection of full-length RegB protein by non-denaturing western blot analysis. Western blot analysis of aerobically and anaerobically grown wild-type R.capsulatus cell extracts that do not contain a flag-tagged RegB indicated that there is a 75 kDa protein that strongly interacts with the anti-flag antibody (data not shown). Because this cross-hybridizing protein was constitutively present, it was used as a loading control. Extracts from anaerobically grown wild-type RegB-flag cells yielded a single 47 kDa band that corresponds to the size of full-length RegB monomer. In contrast, when the cells were grown aerobically, a significant portion of RegB exists as a 250 kDa tetramer (Figure 6C). Also consistent with in vitro observations, the C265A-flag regB mutant strain did not contain any tetramer when grown aerobically or anaerobically, indicating that the cysteine residue is indeed needed for tetramerization both in vitro and in vivo (Figure 6C). RegB dimer was not observed on the gel because the SDS disrupts the hydrophobic interactions that hold the dimer together. However, when the disulfide bond is formed between dimer pairs, they become resistant to SDS degradation and run as a tetramer. These data indicate that Cys265 is not only crucial for the in vitro redox sensing but is also functional in vivo.
Discussion
Our data indicate that the redox-regulated activity of the truncated cytosolic portion of RegB (RegBs) is mediated in vitro by a redox-reactive cysteine that is capable of undergoing metal-dependent formation of a disulfide bond. It is evident from our redox and metal substitution studies that the metal in RegBs plays a structural role, as oxidation or reduction of the metal does not affect disulfide bond formation. Presumably, the metal is needed to place RegBs in a conformation that will allow disulfide bond formation to occur under oxidizing conditions. Given that a mutation in the putative copper chaperone SenC renders RegB constitutively active (Eraso and Kaplan, 2000), we suspect that the metal associated with RegB in vivo is copper, although purification of RegB from R.capsulatus will ultimately be needed to address this question. This conclusion is supported by the finding that mutations in the putative copper transport proteins RdxI, RdxH and RdxS result in derepression of the RegB–RegA pathway in Rhodobacter sphaeroides (Roh and Kaplan, 2000). However, our in vitro results clearly indicate that other metals can fulfill this role.
Although the in vitro data suggest that disulfide bond formation may be responsible for the redox-sensing capability of RegB, it cannot be discounted that other oxidized cysteine derivatives may be occurring in vivo that affect the autophosphorylation activity of full-length RegB. Indeed, it has been recently discovered that the redox-responsive transcriptional regulator OxyR can alter DNA-binding ability in response to different modifications of a highly conserved cysteine residue (Kim et al., 2002). Specifically, a highly reactive cysteine residue in OxyR can undergo sulfur-hydroxylation, sulfur-nitrosylation and sulfur-glutathionylation in vivo, with each modification differently affecting the DNA-binding activity of OxyR. While these modifications may facilitate eventual disulfide bond formation, they are all stable intermediates in vivo. A similar scenario may apply to Cys265 of RegB where modifications of the cysteine may modulate kinase activity. Cysteine residues residing within a basic pocket will have a significantly lower pKa value, resulting in deprotonation of the sulfhydryl group at physiological pH. Interestingly, all RegB homologs contain fully conserved basic residues that flank Cys265, which may decrease Cys265 pKa, causing deprotonation. Deprotonated cysteine is a highly reactive species that can react spontaneously with H2O2 (a byproduct of respiration) to form SOH (sulfenic acid). Sulfenic acid is also a known intermediate step toward disulfide bond formation, and since <20% of RegB forms a disulfide bond in vivo when grown under aerobic conditions (Figure 6C), it is quite possible that the remainder of air-oxidized RegB may exist as an SOH. This conclusion is supported by our analysis that shows that Cys265 of RegB has the capability to undergo disulfide bond formation in vivo and in vitro, classifying it as a truly redox-active residue. Our western blot data also demonstrate that mutational disruption of Cys265 abolishes all oligomerization (Figure 6C), in contrast to a Cys68 mutation that is still capable of forming oligomers (data not shown). Thus, only Cys265 appears to have a role in the redox-sensing mechanism of RegB in vivo and in vitro.
Although it is generally assumed that most sulfhydryls remain in a reduced state in the cytosol of prokaryotes, the presence of a redox-active cysteine in the cytosolic domain of RegB is not unprecedented. We recently demonstrated that the DNA-binding activity of the aerobic repressor CrtJ in R.capsulatus is enhanced by oxygen-mediated formation of an intramolecular disulfide bond (Masuda et al., 2002). Thus, RegB constitutes a second R.capsulatus transcription factor that utilizes a redox-active cysteine as a mechanism of controlling gene expression in response to alteration in oxygen tension.
It is also apparent that many bacterial species are frequently faced with the need to respond to sudden changes in ambient oxygen tension. Inspection of available genome databases indicates that the highly conserved RegB–RegA regulon is present in a diverse number of α-proteobacterial species. An alignment of various RegB homologs indicates that they each contain a highly conserved H-box with a histidine residue that undergoes phosphorylation. In addition, there is an area of conservation centered around Cys265, which we have designated the ‘redox domain’, that exists in a linker region between the H-box and the downstream ATP binding kinase domain (Figure 7). On both sides of Cys265 there are highly conserved cationic amino acids such as Arg and Lys that are thought to stimulate reactive formation of disulfide bonds or sulfenic acid derivatives (Jones, 2002). In addition to the α-proteobacterial RegB homologs, there are a number of other additional HPK 3e type sensor kinases (Grebe and Stock, 1999) that also contain the same redox-box (Figure 7). This includes histidine kinases from such diverse species as Myxococcus xhanthus (δ-proteobacteria), Psuedomonas aeruginosa (γ-proteobacteria), Bacillus halodurans (gram positive) and Aquifex aeolicus (aquificales). Although it remains to be established that these sensor kinases autophosphorylate in a redox-dependent manner, it is quite possible that this region constitutes a domain that controls a redox-responsive sensory transduction cascade in these diverse bacterial species.
Fig. 7. Sequence alignment of several RegB homologs. Alignment of sensor kinases that exhibit sequence similarity to the region of RegBs from the site of phosphorylation through Cys265. The H-box represents the site of phosphorylation, and the redox-box represents the reactive cysteine residue flanked by cationic amino acids.
Materials and methods
Strains, media and growth conditions
R.capsulatus strain SB1003 was used as the parent strain for all RegB mutational analysis. BL21(DE3) was utilized for the expression of RegBs and C265As. R.capsulatus conjugation was performed using S17-1 λpir. Terrific broth/Luria broth and PY medium were used for agar solidified plates and liquid cultures of E.coli and R.capsulatus, respectively. Kanamycin and gentamycin was used at a concentration of 50 and 10 µg/ml for E.coli and 10 and 1.5 µg/ml for R.capsulatus, respectively.
Generating RegB point mutation
Exchanging the RegBs region with a KmR cartridge interrupted the R.capsulatus regB gene, and the mutant strain was named SM01 (S.Masuda and C.E.Bauer, unpublished strain construction). The cysteine residue at position 265 in RegB was changed to alanine by PCR mutagenesis using Pfu DNA polymerase as described previously (Wang and Malcolm, 1999), and the fragment was cloned into the GmR-suicide vector pZJD29A (J.Jiang and C.E.Bauer, unpublished plasmid construction) that has a sacB gene encoding the lavansucrase of Bacillus subtilis. The resulting plasmid was then transferred to the R.capsulatus strain SM01 by S17-1 λpir based conjugation. GmR cells were selected for and subsequently grown in the presence of 5% sucrose to select double-crossover candidates. The resulting colonies were checked for Km and Gm sensitivity, before confirming chromosomal replacement of the C265A point mutation in regB, by PCR and sequence analysis.
Construction of expression vectors
A truncated version of RegB that starts at amino acid M196 (RegBs) was constructed by PCR amplifying the C-terminal region of regB with primers 5′-CCCATGGCGGATGCGCTTTTCGC and 5′-CCTCGAGAA CGATTGTGATCATCAGGC. The C265As (RegBs mutant) was first generated in the chromosome of R.capsulatus and then PCR amplified from the chromosome with the primers stated above. The DNA segment was cloned into NcoI and XhoI sites of pET29(+) (Novagen) to construct an in-frame fusion of regB at M196 with an N-terminus S-tag that is present in the overexpression vector.
Protein overexpression and purification
The expression plasmids pET29RegBs and pET29C265As were transformed into BL21 (DE3) and selected with kanamycin. RegBs and C265As was overexpresssed in the E.coli strain BL21(DE3) by growing to an OD of 0.5 and then inducing expression with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h at 37°C. The cells were harvested by centrifugation and lysed in 10 mM Tris–HCl pH 8.0, 150 mM NaCl by three passes through a French press cell. After removal of cell debris by centrifugation at 27 000 g for 30 min, and the supernatant was then passed through a Hitrap Q (HP) column (Pharmacia) and washed with 10 mM Tris–HCl pH 8.0. A salt gradient from 0–500 mM NaCl in 10 mM Tris–HCl pH 8.0 was passed through the column, with RegBs eluting at ∼300 mM NaCl. The resulting RegBs was treated with 300 µM divalent cation and allowed to incubate on ice for 30 min before extensive dialysis against 10 mM Tris pH 8.0 and 300 mM NaCl. The RegBs was ultimately dialyzed against 10 mM Tris pH 8.0, 300 mM NaCl and 50% glycerol for storage at –20°C.
Construction of the R.capsulatus strain carrying flag-tagged RegB
The C-terminus of regB was PCR amplified from R.capsulatus genomic DNA with primers RegBflagHindIII 5′-CCAAGCTTATCCTGCGG TCGATGGGGC and RegBflagPstI 5′-TTCTGCAGGGCGGTGATCG GAACATTC. The PCR product was then force cloned into the HindIII, PstI site of pJM21, placing regB in frame with the flag M2 epitope sequence. The regB-flag tag sequence was then subcloned into the suicide vector pZJD3 using the enzymes SpeI and HindIII and mated into wild-type and C265A R.capsulatus strains using S17-1 λpir. Single recombination events were selected by gentamycin resistance encoded by the pZJD3 vector.
Autophosphorylation assays
Phosphorylation assays were in 1× kinase buffer (20 mM Tris pH 8.0, 10 mM MgCl2, 1 mM CaCl2, 100 mM KCl and 300 mM NaCl) and either no DTT for oxidized or 10 mM DTT for reduced conditions. The reactions were started at t = 0 by adding a one-tenth volume of ATP mix (0.5 µCi [γ-32P]ATP, 10 mM cold ATP) and then quenched by removing aliquots and mixing with SDS–PAGE loading dye containing 25 mM DTT. The samples were then separated by SDS–PAGE, visualized with autoradiographic film and quantitated with a phosphorImager.
Autophosphorylation redox potential assays
Different redox potentials were generated using ratios of oxidized and reduced DTT. Aliquots of copper-replete RegBs were equilibrated to a given potential by incubation in 1× kinase buffer at specific potentials for 2, 4 or 18 h before phosphorylation assays were started as described previously. Samples were allowed to phosphorylate 10 min before being quenched with SDS–PAGE loading dye and separated by electrophoresis. The level of autophosphorylation was assayed by exposure to autoradiographic film as well as quantified for percent activity using a phosphorImager. The percent activity was plotted against the redox potential of each assay, with data points best fit by a Nernst two-electron line equation.
mBBr redox titration
The fluorescent probe mBBr (Calbiochem) was used to determine whether a redox-active disulfide existed in RegBs. To measure free sulfhydryls in copper-replete RegBs, aliquots of the protein were first incubated for 2 h at potentials ranging from –230 mV to –400 mV, in 10 mV increments, before being allowed to react with 300 mM mBBr for 20 min. The protein was then precipitated by the addition of 100 µl of 20% trichloroacetic acid, incubated on ice for 30 min, pelleted by centrifugation at 12 000 g and resuspended in 100 mM Tris pH 8.0, 1% SDS, before scanning for fluorescence on an Aminco-Bowman Series 2 Luminescence spectrometer with excitation at 380 nm and emission at 480 nm. The percent fluorescence was plotted against redox potential, and then best fit by a Nernst two-electron line equation.
EPR analysis
The RegBs and C265As proteins were concentrated to 1.5 mM and then dialyzed against 10 mM Tris pH 8.0, 300 mM NaCl and 50% glycerol. All EPR spectra were recorded at 77 K at X-band (9.5 GHz) on an ESP 300 Bruker instrument. Typical EPR conditions: microwave power, 10 mW; modulation amplitude, 2–20 G; modulation frequency, 100 kHz; receiver gain; (2–5) × 104. EPR spectra were simulated using a Monte Carlo method for the copper signals (Gaffney and Silverstone, 1993; Neese, 1995).
Spectroscopy
All UV/Vis spectroscopy was performed on a Beckman BU 640 spectrophotometer. Extinction coefficients were determined for the copper dd-bands using the copper concentration within the RegBs samples and the following equation: Abs = ε cell path (cm) concentration (M). For R.capsulatus spectral scans, R.capsulatus wild-type and C265A strains were grown aerobically, semi-aerobically and photosynthetically to 50, 90 and 80 Klett units, respectively. Then, 10 ml of each culture was pelleted by centrifugation at 6000 g for 10 min and resuspended in 1 ml of 10 mM Tris pH 8.0. The samples were sonicated to lyse the cells and then centrifuged at 12 000 g to remove cell debris before being spectrally scanned from 900 to 400 nm.
Gel filtration chromatography
RegBs and C265As were fractionated on a Superose 12 XK 16 column (Pharmacia Biotech) equilibrated with 10 mM Tris pH 8.0, 300 mM NaCl and either no DTT for oxidizing or 10 mM DTT for reducing conditions. The column was size calibrated with commercial gel filtration standards (Bio-Rad).
Western blot analysis
R.capsulatus RegB flag-tag strains were grown aerobically in 1 l baffled flasks shaking at 400 r.p.m to a density of 35 Klett units and pelletted. Pellets were resuspended in 1 ml of cold aerated 10 mM Tris pH 8.0 and sonicated. Sample (15 µl) was added to non-reducing loading dye just prior to heating to 55°C. Anaerobic cultures were grown in screw top tubes in a jar with anaerobic mix to 85 Klett units, and then 10 ml was transferred to ice cold tubes and centrifuged at 6000 g for 5 min. Pellets were resuspended in 1 ml of degassed cold 10 mM Tris pH 8.0 and sonicated in an anaerobic chamber. Samples (10.5 µl) were added to non-reducing loading dye and then heated to 55°C before being separated by non-reducing SDS–PAGE electrophoresis. Western blot analysis was achieved using a 1:750 dilution mouse anti-flag M2 HRP conjugate antibody (US Biological) in 8 ml of TBST and 2 ml of 5% milk for 45–60 min. Membrane was washed with 200 ml of 1× TBST and visualized with 1.5 ml of Super Signal West Dura Extended Duration Substrate illuminator (Pierce), prior to exposure to autoradiographic film for 2–10 min.
β-galactosidase activity assays
R.capsulatus wild-type and C265A strains harboring the puf::lacZ fusion plasmid (pCB532ΩSpec) (Bauer and Marrs, 1988) were grown aerobically, semi-aerobically and photosynthetically to 50, 90 and 80 Klett units, respectively. The β-galactosidase assays were performed as described by Swem et al. (2001).
Acknowledgments
Acknowledgements
We also acknowledge an initial observation by Dr Terry Bird for showing that early preparations of RegB exhibited slightly higher phosphorylation levels under reducing conditions than under oxidizing conditions. This study is supported by an NIH grant GM53940 to C.E.B. and a Robert A.Welch Foundation grant D-0710 to D.B.K.
References
- Aslund F., Zheng,M., Beckwith,J. and Storz,G. (1999) Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl Acad. Sci. USA, 96, 6161–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley T., Ellis,P.J., Freeman,H.C., Hitchman,M.A., Keene,F.R. and Tiekink,E.R.T. (1995) Extended x-ray absorption fine structure, crystal structures at 295 and 173 K and electron paramagnetic resonance and electronic spectra of bis[tris(2-pyridyl)methane]copper(II) dinitrate. J. Chem. Soc. Dalton Trans., 595–601. [Google Scholar]
- Bauer C.E., Young,D.A. and Marrs,B.L. (1988) Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J. Biol. Chem., 263, 4820–4827. [PubMed] [Google Scholar]
- Beers J., Glerum,D.M. and Tzagoloff,A. (2002) Purification and characterization of yeast Sco1p, a mitochondrial copper protein. J. Biol. Chem., 277, 22185–22190. [DOI] [PubMed] [Google Scholar]
- Bernarducci E., Schwindinger,W.F., Hughey,J.L. IV, Krogh-Jespersen,K. and Schugar,H.J. (1981) Electronic spectra of copper(II)-imidazole and copper(II)-pyrazole chromophores. J. Am. Chem. Soc., 103, 1686–1691. [Google Scholar]
- Bird T.H., Du,S. and Bauer,C.E. (1999) Autophosphorylation, phosphotransfer and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J. Biol. Chem., 274, 16343–16348. [DOI] [PubMed] [Google Scholar]
- Brenner A.J. and Harris,E.D. (1995) A quantitative test for copper using bicinchoninic acid. Anal. Biochem., 226, 80–84. [DOI] [PubMed] [Google Scholar]
- Buggy J. and Bauer,C.E. (1995) Cloning and characterization of senC, a gene involved in both aerobic respiration and photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol., 177, 6958–6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes C. and Gutierrez-Corona,F. (1994) Copper resistance mechanisms in bacteria and fungi. FEMS Microbiol. Rev., 14, 121–137. [DOI] [PubMed] [Google Scholar]
- Chen W., Jager,A. and Klug,G. (2000) Correction of the DNA sequence of the regB gene of Rhodobacter capsulatus with implications for the membrane topology of the sensor kinase regB. J. Bacteriol., 182, 818–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Elsen,S., Swem,L.R. and Bauer,C.E. (2002) AerR, a second aerobic repressor of photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol., 184, 2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Kouadio,J.L. and Bauer,C.E. (1999) Regulated expression of a highly conserved regulatory gene cluster is necessary for controlling photosynthesis gene expression in response to anaerobiosis in Rhodobacter capsulatus. J. Bacteriol., 181, 4334–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen S., Dischert,W., Colbeau,A. and Bauer,C.E. (2000) Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J. Bacteriol., 182, 2831–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraso J.M. and Kaplan,S. (2000) From redox flow to gene regulation: role of the PrrC protein of Rhodobacter sphaeroides 2.4.1. Biochemistry, 39, 2052–2062. [DOI] [PubMed] [Google Scholar]
- Fawcett T.G., Bernarducci,E.E., Krogh-Jespersen,K. and Schugar,H.J. (1980) Charge-transfer absorptions of copper(II)-imidazole and copper(II)-imidazolate chromophores. J. Am. Chem. Soc., 102, 2598–2604. [Google Scholar]
- Gaffney B.J. and Silverstone,H.J. (1993) Biological magnetic resonance. In Reuben,J. and Berliner,J. (eds), NMR of Paramagnetic Molecules, Vol. 13. Plenum Press, New York, NY, p. 1. [Google Scholar]
- Georgellis D., Kwon,O. and Lin,E.C. (2001) Quinones as the redox signal for the arc two-component system of bacteria. Science, 292, 2314–2316. [DOI] [PubMed] [Google Scholar]
- Grebe T.W. and Stock,J.B. (1999) The histidine protein kinase superfamily. Adv. Microb. Physiol., 41, 139–227. [DOI] [PubMed] [Google Scholar]
- Hathaway B.J. (1987) Copper. In Wilkinson,G. (ed.), Comprehensive Coordination Chemistry. Pergamon Press, Oxford, UK, pp. 662–674. [Google Scholar]
- Hemmert C., Renz,M., Gornitzka,H. and Meunier,B. (1999) Synthesis, characterization and crystal structures of copper(II) complexes containing multidentate polypyridine ligands. J. Chem. Soc. Dalton Trans., 3989–3994. [Google Scholar]
- Hirasawa M., Hurley,J.K., Salamon,Z., Tollin,G., Markley,J.L., Cheng,H., Xia,B. and Knaff,D.B. (1998) The role of aromatic and acidic amino acids in the electron transfer reaction catalyzed by spinach ferredoxin-dependent glutamate synthase. Biochim. Biophys Acta, 1363, 134–146. [DOI] [PubMed] [Google Scholar]
- Huang G.-S., Lai,J.-K., Ueng,C.-H. and Su,C.-C. (2000) Molecular and electronic structures of bis(dipicolylamine)copper(II) perchlorate and bis[2-(2-pyridylethyl)picolylamine]copper(II) perchlorate. Transit. Met. Chem., 25, 84–92. [Google Scholar]
- Innes K.K., Ross,I.G. and Moomaw,W.R. (1988) Electronic states of azabenzenes and azanaphthalenes: a revised and extended critical review. J. Mol. Spectrosc., 132, 492–544. [Google Scholar]
- Inoue K., Mosley,C., Kouadio,J.-L. and Bauer,C.E. (1995) Isolation and in vitro phosphorylation of sensory transduction components controlling anaerobic induction of light harvesting and reaction center gene expression in R.capsulatus. Biochemistry, 34, 391–396. [DOI] [PubMed] [Google Scholar]
- Jones D.P. (2002) Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol., 348, 93–112. [DOI] [PubMed] [Google Scholar]
- Kim S.O., Merchant,K., Nudelman,R., Beyer,W.F.,Jr, Keng,T., DeAngelo,J., Hausladen,A. and Stamler,J.S. (2002) OxyR: a molecular code for redox-related signaling. Cell, 109, 383–396. [DOI] [PubMed] [Google Scholar]
- Krimm I., Lemaire,S., Ruelland,E., Miginiac-Maslow,M., Jaquot,J.P., Hirasawa,M., Knaff,D.B. and Lancelin,J.M. (1998) The single mutation Trp35→Ala in the 35–40 redox site of Chlamydomonas reinhardtii thioredoxin h affects its biochemical activity and the pH dependence of C36-C39 1H-13C NMR. Eur. J. Biochem., 255, 185–195. [DOI] [PubMed] [Google Scholar]
- Lamb A.L., Torres,A.S., O’Halloran,T.V. and Rosenzweig,A.C. (2000) Heterodimer formation between superoxide dismutase and its copper chaperone. Biochemistry, 39, 14720–14727. [DOI] [PubMed] [Google Scholar]
- Lever A.B.P. (1984) Studies in Physical and Theoretical Chemistry, Vol. 33: Inorganic Electronic Spectroscopy, 2nd edn. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- Masuda S., Matsumoto,Y., Nagashima,K.V., Shimada,K., Inoue,K., Bauer,C.E. and Matsuura,K. (1999) Structural and functional analyses of photosynthetic regulatory genes regA and regB from Rhodovulum sulfidophilum, Roseobacter denitrificans and Rhodobacter capsulatus. J. Bacteriol., 181, 4205–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S., Dong,C., Swem,D., Setterdahl,A.T., Knaff,D.B. and Bauer,C.E. (2002) Repression of photosynthesis gene expression by formation of a disulfide bond in CrtJ. Proc. Natl Acad. Sci.USA, 99, 7078–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan A.G., Lewin,A., Davy,S.L., Boetzel,R., Leech,A., Walker,D., Wood,T. and Moore,G.R. (2002) PrrC from Rhodobacter sphaeroides, a homologue of eukaryotic Sco proteins, is a copper-binding protein and may have a thiol-disulfide oxidoreductase activity. FEBS Lett., 518, 10–16. [DOI] [PubMed] [Google Scholar]
- McLachlan G.A., Fallon,G.D., Martin,R.L. and Spiccia,L. (1995) Synthesis, structure and properties of five-coordinate copper(II) complexes of entadentate ligands with pyridyl pendant arms. Inorg. Chem., 34, 254–261. [Google Scholar]
- Miyatake H. et al. (2000) Sensory mechanism of oxygen sensor FixL from Rhizobium meliloti: crystallographic, mutagenesis and resonance Raman spectroscopic studies. J. Mol. Biol., 301, 415–431. [DOI] [PubMed] [Google Scholar]
- Mosley C.S., Suzuki,J.Y. and Bauer,C.E. (1995) Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J. Bacteriol., 177, 3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese F. (1995) The EPR program. Quantum Chemistry Program Exchange, Bull., 15, 5. [Google Scholar]
- Nittis T., George,G.N. and Winge,D.R. (2001) Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J. Biol. Chem., 276, 42520–42526. [DOI] [PubMed] [Google Scholar]
- Ouchane S. and Kaplan,S. (1999) Topological analysis of the membrane-localized redox-responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J. Biol. Chem., 274, 17290–17296. [DOI] [PubMed] [Google Scholar]
- Paget M.S., Bae,J.B., Hahn,M.Y., Li,W., Kleanthous,C., Roe,J.H. and Buttner,M.J. (2001) Mutational analysis of RsrA, a zinc-binding anti-σ factor with a thiol-disulphide redox switch. Mol. Microbiol., 39, 1036–1047. [DOI] [PubMed] [Google Scholar]
- Peisach J. and Blumberg,W.E. (1974) Structural implications derived from the analysis of EPR spectra of natural and artificial copper proteins. Arch. Biochem. Biophys., 165, 691–708. [DOI] [PubMed] [Google Scholar]
- Potter C.A., Ward,A., Laguri,C., Williamson,M.P., Henderson,P.J. and Phillips-Jones,M.K. (2002) Expression, purification and characterization of full-length histidine protein kinase RegB from Rhodobacter sphaeroides. J. Mol. Biol., 320, 201–213. [DOI] [PubMed] [Google Scholar]
- Roh J.H. and Kaplan,S. (2000) Genetic and phenotypic analyses of the rdx locus of Rhodobacter sphaeroides 2.4.1. J. Bacteriol., 182, 3475–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli S., Packer,H.L. and Armitage,J.P. (2002) Tactic responses to oxygen in the phototrophic bacterium Rhodobacter sphaeroides WS8N. J. Bacteriol., 184, 5590–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H. (2001) Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem. Rev., 101, 2497–2509. [DOI] [PubMed] [Google Scholar]
- Setterdahl A. et al. (2000) Oxidation-reduction properties of two engineered redox-sensitive mutant Escherichia coli malate dehydrogenases. Arch. Biochem. Biophys., 382, 15–21. [DOI] [PubMed] [Google Scholar]
- Sganga M.W. and Bauer,C.E. (1992) Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell, 68, 945–954. [DOI] [PubMed] [Google Scholar]
- Silver S. (1996) Transport of inorganic cations. In Neidhardt,F.C. (ed.), Escherichia coli and Salmonella Cellular and Molecular Biology. ASM Press, Washington DC, pp. 1091–1102. [Google Scholar]
- Swem L.R., Elsen,S., Bird,T.H., Swem,D.L., Koch,H.G., Myllykallio,H., Daldal,F. and Bauer,C.E. (2001) The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J. Mol. Biol., 309, 121–138. [DOI] [PubMed] [Google Scholar]
- Vichivanives P., Bird,T.H., Bauer,C.E. and Robert Tabita,F. (2000) Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J. Mol. Biol., 300, 1079–1099. [DOI] [PubMed] [Google Scholar]
- Wang W. and Malcolm,B.A. (1999) Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. Biotechniques, 26, 680–682. [DOI] [PubMed] [Google Scholar]
- West A.H. and Stock,A.M. (2001) Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci., 26, 369–376. [DOI] [PubMed] [Google Scholar]








