Abstract
Targeting and innervation of the cerebral cortex by thalamic afferents is a key event in the specification of cortical areas. The molecular targets of thalamic regulation, however, have remained elusive. We now demonstrate that thalamic afferents regulate the expression of γ-aminobutyric acid type A (GABAA) receptors in developing rat neocortex, leading to the area-specific expression of receptor subtypes in the primary visual (V1) and somatosensory (S1) areas. Most strikingly, the α1- and α5-GABAA receptors exhibited a reciprocal expression pattern, which precisely reflected the distribution of thalamocortical afferents at postnatal day 7. Following unilateral lesions at the birth of the thalamic nuclei innervating V1 and S1 (lateral geniculate nucleus and ventrobasal complex, respectively), profound changes in subunit expression were detected 1 week later in the deprived cortical territories (layers III–IV of V1 and S1). The expression of the α1 subunit was strongly down-regulated in these layers to a level comparable to that in neighboring areas. Conversely, the α5 subunit was up-regulated and areal boundaries were no longer discernible in the lesioned hemisphere. Changes similar to the α5 subunit were also seen for the α2 and α3 subunits. These results indicate that the differential expression of GABAA receptor subtypes in developing neocortex is dependent on thalamic innervation, contributing to the emergence of functionally distinct areas.
Targeting and innervation of the cerebral cortex by thalamic axons is a key event in the demarcation of the cortical anlage into a tangential map of individual areas (1–4). The specificity in targeting by thalamic axons is thought to be achieved through dynamic interactions with specific spatial or temporal cues at the appropriate cortical locus (5–9). However, the molecular postsynaptic targets regulated by thalamic afferents have remained elusive so far.
Neurotransmitter receptors play a central role in shaping the functional properties of neuronal circuits. Among excitatory amino acid receptors, the N-methyl-d-aspartate (NMDA) receptors are involved in synaptic plasticity and in the refinement of synaptic connections (10–13). However, the area-specific demarcation of the neocortex during development does not appear to involve NMDA receptor-mediated activity. The level of expression of NMDA receptors does not vary between distinct areas of developing neocortex, irrespective of thalamic innervation (14, 15). Furthermore, in primary somatosensory cortex (S1), the formation of barrels (the neuronal aggregates representing individual whiskers of the rodent snout) is not affected by chronic NMDA receptor blockade (16).
In contrast to NMDA receptors, γ-aminobutyric acid type A (GABAA) receptors exhibit a prominent area-specific expression pattern in developing neocortex. The α1 subunit, which represents the vast majority of GABAA receptors in adult cortex, is highly expressed in a selective manner in layers III–IV of primary visual cortex (V1) and of S1 (17, 18). The area- and layer-specific distribution of the α1 subunit coincides with the innervation of V1 and S1 by fibers originating from the lateral geniculate nucleus (LGN) and from the ventrobasal complex of the thalamus (VB), respectively. This suggests that thalamic innervation influences the level of expression of GABAA receptors in these areas. Moreover, in view of the extensive subunit repertoire of GABAA receptors that is based on a family of at least 17 subunits (α1–6, β1–3, γ1–3, δ, ɛ, ρ1–3) (19–23), thalamic afferents might differentially affect the expression of distinct GABAA receptor subtypes, thereby contributing to the emergence of functionally distinct cortical areas.
To assess the potential role of thalamic input on the expression of GABAA receptors in primary sensory areas, unilateral electrolytic lesions of the rat LGN and VB were performed at birth postnatal day 0 (P0). One week later, the laminar and regional distribution of GABAA receptor subunits was investigated in the neocortex, using the nonoperated side as control. The four main α subunit variants (α1, α2, α3, and α5), representing the major GABAA receptor subtypes in the neocortex, were analyzed immunohistochemically. The results point to a program of GABAA receptor gene regulation that is intrinsic to the entire developing neocortex. However, in V1 and S1, extrinsic thalamic cues strongly modulate the expression of distinct GABAA receptor subtypes.
MATERIALS AND METHODS
The immunohistochemical analysis of the GABAA receptor subunits α1, α2, α3, and α5 was performed with guinea pig subunit-specific antibodies (24) between embryonic day 19 (E19) and P28. Fetuses were removed from timed-pregnant Sprague–Dawley rats anesthetized with ether. They were placed on ice for hypothermia-induced anesthesia and perfused transcardially with a mixture of 4% paraformaldehyde and 15% picric acid in phosphate buffer (pH 7.4) (see ref. 17 for details). Neonates were anesthetized and perfused as described for the fetuses, whereas animals older than P5 were anesthetized with pentobarbital (50 mg/kg, i.p.) prior to the perfusion. Brains were postfixed for 48–72 hr and cryoprotected with 10% dimethyl sulfoxide in phosphate buffered saline. Transverse sections cut with a freezing microtome were processed free-floating for immunoperoxidase staining, as described in ref. 18. Sections were mounted on gelatin-coated slides, dehydrated, cleared in xylene, and coverslipped.
Lesions of the thalamus centered on the LGN or on VB were performed unilaterally on the day of birth (P0) in rat pups anesthetized by hypothermia. Insulated, sharpened tungsten electrodes were introduced stereotaxically in the dorsal thalamus (25), and electrolytic lesions were placed upon application of 500 μA current pulses (5 times for 5 sec). The pups were then allowed to recover and returned to their nest. The increase in body weight and the behavior of the lesioned animals were indistinguishable from the nonoperated littermates. At P7, the animals were anesthetized, perfusion-fixed, and their brains processed for immunohistochemistry as described above.
The distribution of GABAA receptor subunits was analyzed by light microscopy and photographed with Kodak T-Max 100 film. In addition, sections were digitized using a computer-based image analysis system (MCID 2, Imaging Research, St. Catherine’s, ON, Canada) for visualizing the regional variations in staining intensity in color-coded video images. Since the thalamocortical projection is strictly ipsilateral in the rat, the nonlesioned hemisphere served as a control. Nine of 14 operated animals were included for analysis in this study, based on the morphological analysis of the size and location of the lesions.
RESULTS
GABAA Receptors as Early Area Markers in Developing Neocortex.
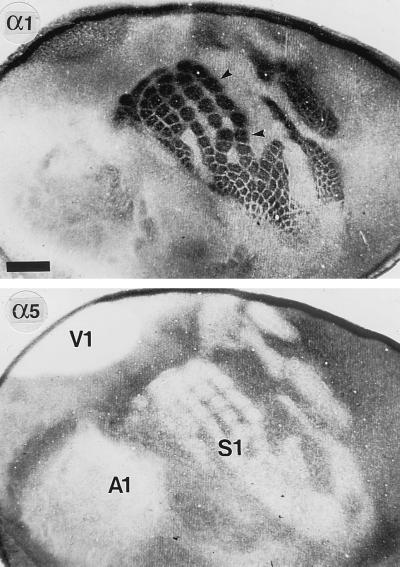
GABAA receptors containing the α1 and α5 subunits displayed a reciprocal area-specific distribution in developing neocortex. This pattern of immunoreactivity (IR) was most apparent in layers III–IV of V1 and S1 and was best visualized at P7 in flattened tangential sections through these layers of the neocortex (Fig. 1). The somatotopic organization was strikingly revealed in S1, where distinct patches of intense α1 subunit IR corresponded to the barrels, the cortical representations of individual whiskers of the rat snout. The large barrels represent the five rows of whiskers, smaller barrels represent sinus hairs of the rat snout. Other parts of the body (e.g., hind paw, forepaw) were revealed by additional patches (Fig. 1). Thus, the pattern of α1 subunit IR in layers III–IV precisely reflected the histochemical distribution of thalamocortical afferents in V1 and S1 at P7 (26, 27). This view is supported by the virtual absence of α1 subunit staining in the intervening association areas, which receive only sparse and diffuse thalamic inputs at this age. In striking contrast, the α5 subunit IR displayed a reciprocal pattern of distribution, being almost completely suppressed in the regions of high α1 subunit expression (layers III–IV of S1 and V1) but enriched in association areas lacking the α1 subunit, as shown for P7 (Fig. 1). This complementary expression pattern, which emerged from very low levels of both the α1 and α5 subunit IR at birth in the neocortex, suggests a reciprocal regulation of α1 and α5 subunit expression by thalamocortical afferents in V1 and S1 during the first postnatal week.
Figure 1.
Complementary distribution of the α1 and α5 subunits in the neocortex at P7, as seen in adjacent tangential sections through layers III–IV processed for immunoperoxidase staining with subunit-specific antibodies. To prepare these sections, the cortex was dissected free of subcortical tissue, flattened between two glass coverslips, and postfixed for 48 hr at 4°C. Sections were then cut parallel to the pial surface and processed for immunoperoxidase staining (intense signals appear black). The localization of the three major primary sensory areas S1, V1, and A1 (the primary auditory area) is revealed by the complete lack of α5 subunit staining in layer IV. Intervening association areas are moderately stained for the α5 subunit. By contrast, the α1 subunit IR is particularly prominent in S1 and V1 and reveals in great detail the somatotopic organization of the barrel field (arrowheads). In the remaining neocortex, including A1, the α1 subunit is weak to moderate. The reciprocal expression pattern of the α1 and α5 subunits is best seen in S1, where patches of intense α1 subunit staining are matched by corresponding patches devoid of α5 subunit IR. (Scale bar = 1 mm.)
Distinct areal distributions were also apparent for the α2 and α3 subunit IR at P7, as shown in transverse sections (Fig. 2). Like the α5 subunit, the α2 and α3 subunits were more abundant in layers III–IV of association areas than of S1 and V1, demarcating these areas by sharp boundaries. This pattern emerged postnatally due to a more rapid decrease in α2 and α3 subunit IR in layers III–IV of V1 and S1 compared with adjacent areas in the first weeks of life (17). Thus, all four α subunit variants displayed a level of expression that differed between primary sensory areas and association areas of the developing neocortex.
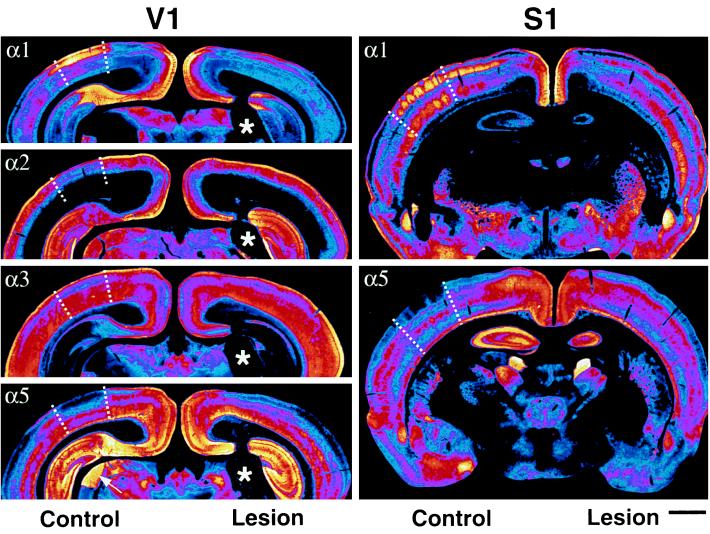
Figure 2.
Changes in GABAA receptor subunit expression at P7 in V1 (Left) and S1 (Right) induced by a neonatal lesion of the LGN and of VB, respectively. Transverse sections from two representative cases were processed for immunoperoxidase staining, digitized with a high resolution camera, and color-coded according to the relative intensity of the immunoreactivity (from black and dark blue for background to blue, pink, red, orange, yellow, and white for maximal intensity). On the left, the boundaries of V1 (dotted lines) are clearly visible in the control hemisphere, owing to the increased (α1) or decreased (α2, α3, and α5) staining in the superficial layers relative to adjacent association areas. In the lesioned hemisphere, the increase in α2, α3, and α5 subunit expression in layers I–IV of V1 results in a uniform distribution across the hemisphere and therefore in the disappearance of areal boundaries. By contrast, the α1 subunit IR is reduced, but remains slightly more intense in layer III of V1 than in association areas. Notice the lack of change in the expression of GABAA receptor subunits in cingulate cortex, hippocampus, and thalamus between the control and lesioned hemisphere. The lesion (five-point star) can be seen best with the α2 and α5 subunit IR, which label the LGN intensely at this age. On the right, the reciprocal regulation of the α1 and α5 subunit IR in S1 induced by a thalamic lesion involving VB is depicted. In the control hemisphere, barrels are clearly outlined by the intense α1 subunit IR in layers III–IV (each of the five patches visible between the dotted lines represents a barrel), whereas the α5 subunit is lacking in the corresponding locations. In the lesioned hemisphere, the α1 subunit staining is reduced and barrels cannot be seen any longer. Like in V1, the α5 subunit staining in the deprived S1 is increased to a level similar to adjacent areas, resulting in the disappearance of areal boundaries. (Scale bar = 1 mm.)
Effect of LGN Lesions on GABAA Receptor Expression in V1.
To directly determine the role of thalamic input on GABAA receptor subunit expression in V1, unilateral electrolytic ablations of the LGN were performed in neonates. The size and position of the lesion were assessed in sections stained for the GABAA receptor subunits, in which the nuclear subdivisions of the thalamus were revealed in detail. In the case illustrated in Fig. 2 (Left) and Fig. 3, the lesion encompassed the entire LGN in the right hemisphere and slightly affected the posterior cap of VB, but spared the posterior thalamic complex and the reticular nucleus. Lesions that extended into the lateral or medial poles of the thalamus, or into the internal capsule, were not included in these experiments.
Figure 3.
Effects of an ablation of the LGN performed at P0 on the laminar distribution of the GABAA receptor subunits α1, α2, α3, and α5 in V1 at P7. Each pair of photomicrographs depicts a field from the control (Left) and lesioned (Right) hemispheres taken from the same transverse section. A loss of α1 subunit IR is evident in layer IV, which appears almost unstained on the lesioned side (small arrowheads). In addition, the α1 subunit IR is decreased in layers I, II–III, Va, Vc, and VI and increased in layer Vb (open arrow). The α5 subunit staining, which is almost completely lacking in layers III–IV of the control V1, is increased in layer IV and in the deeper part of layer III (arrow) and in layer I on the lesioned side. The α5 subunit IR decreases in the upper portion of layer VI and remains practically unchanged in layers V and VIb. Lesion-induced changes are also seen for the α2 and α3 subunit IR in the deprived hemisphere. An overall increase in α2 subunit IR is seen in layers I, II–III, and V, masking the relatively higher staining of layer IV seen on the control side (triangle). For the α3 subunit, a moderate increase in staining is evident in layers II–III on the lesioned side (arrowheads), whereas layers IV–VI remain unchanged. (Scale bar = 200 μm.)
The pattern of α subunit staining in V1 was analyzed immunohistochemically at P7 in five LGN-lesioned animals, with the contralateral side serving as control. Ablation of the LGN resulted in a nearly complete loss of α1 subunit staining in layer IV, the main normal target layer of thalamic neurons. A pronounced, though partial decrease of α1 subunit staining was also apparent in layers I and III (Fig. 3). Thus, the α1 subunit was down-regulated in all layers of V1, except for a narrow band in the middle of layer V (layer Vb) (Fig. 3; Table 1). For the α5 subunit, changes opposite to those occurring for the α1 subunit were observed in V1 (Fig. 3; Table 1). Thus, following LGN lesions, the α5 subunit IR was increased substantially in layers III–IV of V1 (Fig. 3). This is all the more remarkable since these layers are normally devoid of α5 subunit staining. The lesion-induced up-regulation of α5 subunit expression resulted in a laminar distribution in V1 that was similar to that in the adjacent association areas receiving only sparse thalamic innervation. At P7, the areal boundaries of V1 normally formed by the α5 subunit IR were no longer apparent on the lesioned side (Fig. 2). Thus, thalamic input plays a significant role in the area-specific distribution of the α5 subunit in occipital cortex.
Table 1.
Reciprocal changes in GABAA receptor subunit IR in V1 following a unilateral ablation of the LGN
| Subunit | Layers
|
|||||||
|---|---|---|---|---|---|---|---|---|
| I | II–III | IV | Va | Vb | Vc | VI | VIb | |
| α1 | ⇓⇓ | ⇓⇓ | ⇓⇓⇓ | ⇓ | ⇑ | ⇓ | ⇓ | ⇔ |
| α5 | ⇑ | ⇑ | ⇑⇑ | ⇑ | ⇑ | ⇑ | ⇓⇓ | ⇔ |
The number of arrows denotes weak, moderate, and strong changes in staining intensity in the deprived hemisphere relative to the control side; ⇔ indicates no change.
Similar to the regulation of the α5 subunit, staining for the α2- and α3 subunits was increased on the lesioned side of V1 (layers I–III and V for α2, layers II–III for α3; Fig. 3) to an extent that the areal boundaries between V1 and neighboring association areas were likewise no longer discernible (Fig. 2).
Effect of VB Lesions on GABAA Receptor Expression in S1.
To test whether the thalamic influence on GABAA receptor expression in neocortex also holds for receptors in S1, thalamic lesions that included major parts of VB were analyzed in four animals. These lesions drastically altered α subunit expression not only in V1 but also in S1 in a manner very similar to that described above for V1 following lesions restricted to the LGN. In particular, the loss of α1 subunit staining in layers III–IV and the corresponding increase of α5 subunit IR were preeminent (Fig. 2). In addition, as expected for a thalamic lesion involving VB (28, 29), the barrels failed to develop on the deprived side (Fig. 2, Right). The effect is specific, since the barrel-like pattern of the α1 subunit persisted on the control side, as visualized by the five distinct patches of staining in layers III–IV (Fig. 2, Right, control hemisphere). Thus, similar to the observations in V1, GABAA receptor expression in S1 appears to be patterned by thalamic innervation.
Specificity of Lesion-Induced Alterations in GABAA Receptor Expression.
The relative thickness of individual cortical layers was comparable between the two hemispheres. The alterations in GABAA receptor subunit expression are therefore unlikely to reflect an abnormal lamination of the neocortex or cell loss in the deprived layers (30). Furthermore, the striking areal and laminar specificity of the lesion-induced changes and the distinction in up- or down-regulation of the expression of the four subunits argue against the involvement of nonspecific factors related to tissue injury.
Receptor Expression Independent of Thalamic Innervation.
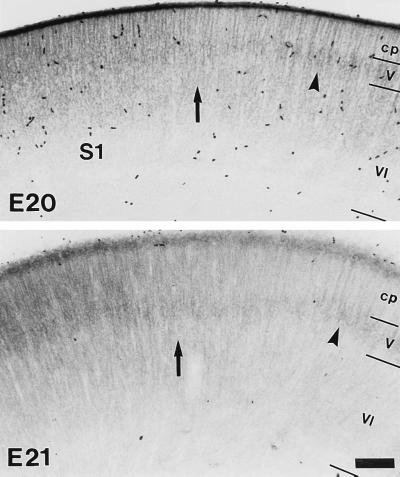
To determine whether thalamic afferents are a necessary prerequisite for the expression pattern of GABAA receptor subtypes in V1 and S1, the distribution of the α subunits was analyzed at a time that clearly preceded the establishment of functional thalamocortical connections. Starting at E20, i.e., 36–48 hr prior to birth, a distinct area-specific distribution of the α1 subunit IR was observed in the cortical anlage (Fig. 4). In the territories corresponding to the future V1 and S1 (26), a uniform, though moderate α1 subunit staining was found in the cortical plate. In contrast, the cortical plate was unstained in neighboring territories in which a weak α1 subunit IR was detected only in the developing layer V (Fig. 4). This pattern was more pronounced at E21, because the deep cortical layers had developed further and the α1 subunit staining had increased in the cortical plate of V1 and S1 (Fig. 4). Thus, a region-specific expression pattern, apparently innate to the neocortex, is operative prior to functional thalamic innervation. This intrinsically specified program of GABAA receptor expression shows, however, a high degree of plasticity, as demonstrated by the dramatic alteration of subunit expression following ablation of specific thalamic nuclei.
Figure 4.
Area-specific distribution of the α1 subunit IR in fetal neocortex. The photomicrographs illustrate the lateral boundary of S1 (arrows) as seen in transverse sections at E20 and E21 in sections processed for immunoperoxidase staining. In the territory corresponding to the future area S1, the α1 subunit IR is initially diffuse and uniform across the cortical plate (cp), whereas in the adjacent cortex, it is confined to the developing layer V (arrowhead), with a narrow transition zone in between. These areal boundaries are more prominent at E21, because the deeper cortical layers appear more differentiated and the α1 subunit IR increases in S1. (Scale bar = 100 μm.)
DISCUSSION
GABAA receptors containing the α1 subunit are expressed in an area-specific manner in prenatal neocortex, as early as E20 (Fig. 4), i.e., 2 days before functional thalamocortical connections are established (31, 32). The α1 subunit thus represents the earliest known postsynaptic marker to reveal areal boundaries in the cortical plate. These observations suggest the existence of an endogenous program regulating GABAA receptor expression in developing neocortex. Furthermore, mechanisms independent of thalamic innervation appear to initiate the formation of primary sensory areas. Additional evidence supports the view that intrinsic cortical mechanisms are operative at early stages of area specification: the cytoarchitectural differentiation of the primary visual cortex of primates (area 17), as well as the characteristic laminar pattern of monoamine receptors in this area, emerge in the absence of cues from the retina (33, 34). Transplantation experiments in transgenic mice also suggest that layer IV neurons of S1 are molecularly committed as early as E14 to become a distinct subpopulation (35). Finally, the laminar specificity of V1 is retained in cortical slices growing in the absence of thalamic input (36–38).
The innate program regulating GABAA receptor expression in developing neocortex appears to persist even at P7. It emerges in the deprived hemisphere following thalamic lesions, as shown by the disappearance of the areal boundaries formed by each of the four α subunit variants on the control side (Fig. 2). After ablation of the thalamic input, the laminar distribution and level of expression of each of the α subunits in the deprived V1 and S1 are very similar to those seen in adjacent association areas, which are apparently unaffected by the lesion. Thus, when the expression of GABAA receptor subtypes in V1 and S1 is freed from the modulatory influence of thalamic afferents, a nearly uniform expression pattern of GABAA receptor subunits becomes apparent throughout the neocortex.
The profound influences of the thalamocortical projection on GABAA receptor expression strongly suggest that GABAA receptors, not unlike α7 nicotinic acetylcholine receptors (27), represent a major target regulated by ingrowing thalamic afferents. This conclusion is based on the following two findings: (i) The regulation of the α1 and α5 subunits in layers III–IV of the primary sensory areas V1 and S1 matches precisely the distribution of thalamocortical afferents (26, 27); the high degree of specificity of the thalamic regulation is demonstrated, for instance, by the reciprocal expression of the α1 and α5 subunits in the barrels and in the intervening barrel septa (Fig. 1). (ii) The regulation of α subunits depends on the integrity of thalamocortical projection, as shown by the profound decrease of α1-GABAA receptors and the up-regulation of α5-GABAA receptors in V1 and S1 following lesions of the LGN and VB, respectively. Thus, thalamic afferents provide neurons in the primary sensory areas V1 and S1 with receptor subtypes distinct from those in neighboring association areas. The differential expression of GABAA receptor subtypes at an early stage of cortical specification may represent a mechanism contributing to the emergence of functionally distinct areas. Indeed, the type of α subunit is a major determinant of affinity, efficacy, and kinetic properties of recombinant GABAA receptors (39–42). The particular receptor subtypes expressed in layers III–IV of S1 and V1 (which contain notably the α1, but not the α5 subunit) might be required specifically for the processing of sensory information, or for preventing the emergence of excessive cortical activity induced by the ingrowing excitatory thalamic input.
Thalamocortical input regulates both barrel formation in S1 (Fig. 2; refs. 28 and 29) and the pattern of α1 and α5 subunit expression in V1 and S1. A reduction in neuronal activity might represent one of the major effects of cortical deafferentation after thalamic lesion contributing to the changes in GABAA receptor distribution observed in the lesioned hemisphere. However, additional factors are likely to be involved in the patterning of S1 and V1. Barrels are formed even following chronic neuronal blockade with either tetrodotoxin or the NMDA-receptor antagonist 2-amino-5-phosphopentanoic acid (13, 16, 43). Likewise, the expression of the α1 and α5 subunits in S1 is not affected by chronic local application of MK-801.** For GABAA receptor regulation, activity-independent mechanisms can be considered, such as Ca2+-dependent signal transduction and neurotrophins (44, 45). Several neurotrophic factors are able to modulate ion channel activity, including GABAergic synaptic transmission (46, 47), and to promote selectively the maturation of GABAergic neurons in vitro (48, 49). The future identification of factors regulating GABAA receptor gene expression may be of relevance also for disease states such as epilepsy or Huntington disease.
Acknowledgments
This study was supported in part by Swiss National Science Foundation Grant 31-32624.91.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- IR
immunoreactivity
- LGN
lateral geniculate nucleus
- S1
primary somatosensory cortex
- V1
primary visual cortex
- VB
ventrobasal complex of the thalamus
- NMDA
N-methyl-d-aspartate
- P
postnatal day
- E
embryonic day
Footnotes
Penschuck, S., Mohler, H. & Fritschy, J. M., Proceedings of the 24th Göttingen Neurobiology Conference, May 31–June 2, 1996, Göttingen, Germany, p. 630.
References
- 1.Rakic P. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 2.O’Leary D D M. Trends Neurosci. 1989;12:400–406. doi: 10.1016/0166-2236(89)90080-5. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh A, Shatz C J. J Neurosci. 1992;12:39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary D D M, Schlaggar B L, Tuttle R. Annu Rev Neurosci. 1994;17:419–440. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- 5.Bolz J, Gotz M, Hubener M, Novak N. Trends Neurosci. 1993;16:310–316. doi: 10.1016/0166-2236(93)90107-w. [DOI] [PubMed] [Google Scholar]
- 6.O’Leary D D M, Koester S E. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 7.Hata Y, Stryker M P. Science. 1994;265:1732–1735. doi: 10.1126/science.8085163. [DOI] [PubMed] [Google Scholar]
- 8.Yuasa S, Kitoh J, Kawamura K. Anat Embryol. 1994;190:137–154. doi: 10.1007/BF00193411. [DOI] [PubMed] [Google Scholar]
- 9.Crair M C, Malenka R C. Nature (London) 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 10.Goodman, C. S. & Shatz, C. J. (1993) Neuron 10, Suppl., 77–98.
- 11.O’Leary D D M, Ruff N L, Dyck R H. Curr Opin Neurobiol. 1994;4:535–544. doi: 10.1016/0959-4388(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 12.Scheetz A J, Constantine-Paton M. FASEB J. 1994;8:745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- 13.Fox K, Schlaggar B L, Glazewski S, O’Leary D D M. Proc Natl Acad Sci USA. 1996;93:5584–5589. doi: 10.1073/pnas.93.11.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blue M E, Johnston M V. Brain Res. 1995;84:11–25. doi: 10.1016/0165-3806(94)00147-r. [DOI] [PubMed] [Google Scholar]
- 15.Rema V, Ebner F F. J Comp Neurol. 1996;368:165–184. doi: 10.1002/(SICI)1096-9861(19960429)368:2<165::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Schlaggar B L, Fox K, O’Leary D D M. Nature (London) 1993;364:623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- 17.Fritschy J M, Paysan J, Enna A, Mohler H. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paysan J, Bolz J, Mohler H, Fritschy J M. J Comp Neurol. 1994;350:133–149. doi: 10.1002/cne.903500110. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald R L, Olsen R W. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 20.Barnard E A. Adv Biochem Psychopharmacol. 1995;48:1–16. [PubMed] [Google Scholar]
- 21.Sieghart W. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 22.Mohler H, Fritschy J M, Luscher B, Rudolph U, Benson J, Benke D. In: Ion channels. Narahashi T, editor. Vol. 4. New York: Plenum; 1996. pp. 89–113. [PubMed] [Google Scholar]
- 23.Davies P A, Hanna M C, Hales T G, Kirkness E F. Nature (London) 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 24.Fritschy J M, Mohler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Tork I, Tecott L H, Valentino K L, editors. Atlas of the Developing Rat Brain. San Diego: Academic; 1991. [Google Scholar]
- 26.Schlaggar B L, O’Leary D D M. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- 27.Broide R S, Robertson R T, Leslie F M. J Neurosci. 1996;16:2956–2971. doi: 10.1523/JNEUROSCI.16-09-02956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise S P, Jones E G. J Comp Neurol. 1978;178:187–208. doi: 10.1002/cne.901780202. [DOI] [PubMed] [Google Scholar]
- 29.Bennett-Clarke C A, Hankin M H, Leslie M J, Chiaia N L, Rhoades R W. J Comp Neurol. 1994;348:277–290. doi: 10.1002/cne.903480209. [DOI] [PubMed] [Google Scholar]
- 30.Windrem M S, Finlay B L. Cereb Cortex. 1991;1:230–240. doi: 10.1093/cercor/1.3.230. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama G, Robertson R T. J Comp Neurol. 1993;335:123–148. doi: 10.1002/cne.903350109. [DOI] [PubMed] [Google Scholar]
- 32.Agmon A, Hollrigel G, O’Dowd D K. J Neurosci. 1996;16:4684–4695. doi: 10.1523/JNEUROSCI.16-15-04684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakic P, Lidow M S. J Neurosci. 1995;15:2561–2574. doi: 10.1523/JNEUROSCI.15-03-02561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehay C, Giroud P, Berland M, Killackey H, Kennedy H. J Comp Neurol. 1996;367:70–89. doi: 10.1002/(SICI)1096-9861(19960325)367:1<70::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Tannoudji M, Babinet C, Wassef M. Nature (London) 1994;368:460–463. doi: 10.1038/368460a0. [DOI] [PubMed] [Google Scholar]
- 36.Bolz J, Novak N, Gotz M, Bonhoeffer T. Nature (London) 1990;346:359–362. doi: 10.1038/346359a0. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto N, Yamada K, Kurotani T, Toyama K. Neuron. 1992;9:217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- 38.Bolz J, Novak N, Staiger V. J Neurosci. 1992;12:3054–3070. doi: 10.1523/JNEUROSCI.12-08-03054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebert B, Wafford K A, Whiting P J, Krogsgaard-Larsen P, Kemp J A. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- 40.Ducic I, Caruncho H J, Zhu W J, Vicini S, Costa E. J Pharmacol Exp Ther. 1995;272:438–445. [PubMed] [Google Scholar]
- 41.Gingrich K J, Roberts W A, Kass R S. J Physiol (London) 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgard E C, Tietz E I, Neelands T R, Macdonald R L. Mol Pharmacol. 1996;50:119–127. [PubMed] [Google Scholar]
- 43.Chiaia N L, Fish S E, Bauer W R, Figley B A, Eck M, Bennett-Clarke C A, Rhoades R W. Dev Brain Res. 1994;79:301–306. doi: 10.1016/0165-3806(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 44.Lin M H, Takahashi M P, Takahashi Y, Tsumoto T. Neurosci Res. 1994;20:85–94. doi: 10.1016/0168-0102(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 45.Yuste R, Katz L C. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- 46.Kim H G, Wang T, Olafsson P, Lu B. Proc Natl Acad Sci USA. 1994;91:12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berninger B, Poo M M. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 48.Berninger B, Marty S, Zafra F, Berzaghi M P, Thoenen H, Lindholm D. Development (Cambridge, UK) 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- 49.Marty S, Carroll P, Cellerino A, Castren E, Staiger V, Thoenen H, Lindholm D. J Neurosci. 1996;16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]