Abstract
We employed Cre/loxP technology to generate mPDK1–/– mice, which lack PDK1 in cardiac muscle. Insulin did not activate PKB and S6K, nor did it stimulate 6-phosphofructo-2-kinase and production of fructose 2,6-bisphosphate, in the hearts of mPDK1–/– mice, consistent with PDK1 mediating these processes. All mPDK1–/– mice died suddenly between 5 and 11 weeks of age. The mPDK1–/– animals had thinner ventricular walls, enlarged atria and right ventricles. Moreover, mPDK1–/– muscle mass was markedly reduced due to a reduction in cardiomyocyte volume rather than cardiomyocyte cell number, and markers of heart failure were elevated. These results suggested mPDK1–/– mice died of heart failure, a conclusion supported by echocardiographic analysis. By employing a single-cell assay we found that cardiomyocytes from mPDK1–/– mice are markedly more sensitive to hypoxia. These results establish that the PDK1 signalling network plays an important role in regulating cardiac viability and preventing heart failure. They also suggest that a deficiency of the PDK1 pathway might contribute to development of cardiac disease in humans.
Keywords: cardiac muscle/heart failure/hypoxia/PDK1/PI 3-kinase/PKB/Akt
Introduction
Hormones and growth factors trigger the activation of members of a group of protein kinases including protein kinase B (PKB) and p70 ribosomal S6K (S6K), which belong to the AGC family of protein kinases (Brazil and Hemmings, 2001; Lawlor and Alessi, 2001; Newton, 2002). The 3-phosphoinositide-dependent protein kinase-1 (PDK1) plays a central role in activating these AGC kinase members by phosphorylating these enzymes at their activation loop (Toker and Newton, 2000; Alessi, 2001). Much research has shown that the PDK1/AGC kinase-signalling pathway regulates diverse cellular processes, such as those relevant to cell survival, proliferation and metabolic responses to insulin. Misregulation of AGC kinase members is thought to contribute to many diseases. For example, hyperactivation of this pathway is implicated in inducing cardiac hypertrophy (Sugden, 2001) and promoting the survival and proliferation of a significant number of cancers (Simpson and Parsons, 2001). A deficiency in the activation of AGC kinases may be a primary cause of the insulin-resistant form of diabetes (Saltiel and Kahn, 2001), as well as neuronal cell death following a stroke (Wick et al., 2002).
The activation of PKB and S6K isoforms by insulin and growth factors, as well as being dependent on PDK1, requires the prior activation of the phosphoinositide 3-kinase (PI 3-kinase) (Vanhaesebroeck et al., 2001). This produces the second messenger, phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3], which binds to the pleckstrin homology domains of PKB and PDK1, recruiting these enzymes to the plasma membrane where PKB is activated by phosphorylation of its activation-loop residue (Thr308 in PKBα) by PDK1 (Brazil and Hemmings, 2001; Scheid and Woodgett, 2001). PtdIns(3,4,5)P3 also stimulates the phosphorylation of PKB at its hydrophobic motif residue (Ser473 in PKBα), which is required for maximal activation of PKB (Alessi et al., 1996; Yang et al., 2002), by an as yet unknown mechanism. In contrast, S6K does not interact with PtdIns(3,4,5)P3 but, instead, PtdIns(3,4,5)P3 stimulates, by a not completely characterized mechanism, the phosphorylation of the hydrophobic motif residue (Thr389 in S6K1) (Avruch et al., 2001; Volarevic and Thomas, 2001). This generates a binding site for PDK1 to interact with, phosphorylate and activate S6K (Biondi et al., 2001, 2002; Frodin et al., 2002; Collins et al., 2003).
The key role that PDK1 plays in activating certain AGC kinase members in mammalian cells was established by the finding that in mouse embryonic stem (ES) cells lacking PDK1, PKB and S6K could not be activated (Williams et al., 2000). Moreover, genetic analysis in Saccharomyces cerevisiae (Casamayor et al., 1999; Inagaki et al., 1999), Saccharomyces pombe (Niederberger and Schweingruber, 1999), Drosophila (Cho et al., 2001c; Rintelen et al., 2001) and Caenorhabditis elegans (Paradis et al., 1999) supports the conclusion that PDK1 is required for the activation of PKB and S6K homologues, as well as being necessary for the viability and development of these model organisms. We have also recently provided evidence that PDK1 is essential for mammalian development, as mice lacking PDK1 die at day E9.5 of embryogenesis due to multiple abnormalities (Lawlor et al., 2002). In this study, to learn more about the roles that PDK1 plays after birth/development, we have generated and analysed the cardiac phenotype of mice that lack PDK1 in skeletal and heart muscle.
Results
Generation of mice lacking PDK1 in heart
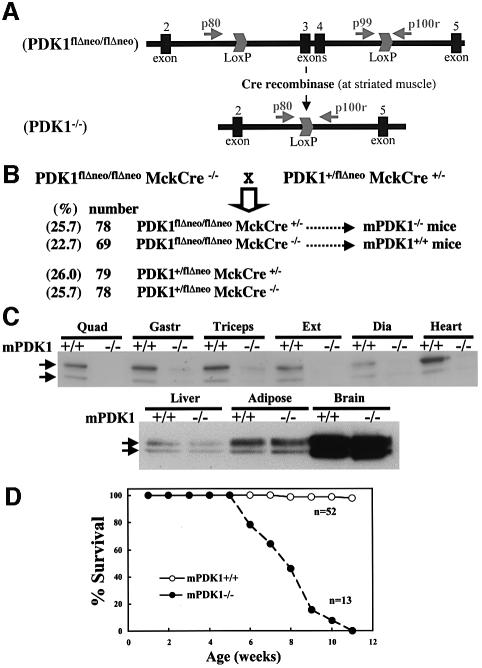
We previously described (Lawlor et al., 2002) the generation of PDK1flΔneo/flΔneo mice in which exons 3 and 4 of the PDK1 gene were flanked with the loxP CRE excision sequence (Figure 1A). These mice, in which PDK1 was expressed in all tissues at the same level as in wild-type mice, were crossed with transgenic mice expressing the CRE recombinase under the muscular creatine kinase promoter which induces expression of this enzyme specifically in skeletal muscle and heart just prior to birth (Bruning et al., 1998). In the resulting PDK1flΔneo/flΔneo mice that express the CRE recombinase (termed mPDK1–/– mice), exons 3 and 4 of the PDK1 would be specifically excised in skeletal and cardiac muscle, thereby ablating functional PDK1 expression, as this truncation prevents translation of the entire kinase and pleckstrin homology domains (Williams et al., 2000). mPDK1–/– mice were born at the expected Mendelian frequency (Figure 1B), and the littermate PDK1flΔneo/flΔneo mice not expressing CRE (termed mPDK1+/+) were utilized as the control animals throughout this study.
Fig. 1. Generation and survival of mice lacking PDK1 in skeletal muscle and heart. (A) Diagram illustrating the positions of exons 2–5 and the loxP Cre excision sites of the floxed PDK1 gene. The positions of the PCR primers used to genotype mice described in the Materials and methods are indicated with arrows. (PDK1flΔneo/flΔneo), allele with the loxP sites flanking exons 3 and 4 without the neomycin resistance cassette; (PDK1–/–), the allele in which exon 3 and 4 have been removed by Cre recombinase resulting in the ablation of the expression of PDK1 beyond exon 2, which includes the kinase and pleckstrin homology domain (Williams et al., 2000). (B) Breeding strategy used for the generation of mice lacking PDK1 in skeletal muscle and heart, where MckCre denotes transgenic mice expressing the Cre recombinase under the muscular creatine kinase promoter. Note that throughout this study, PDK1flΔneo/flΔneoMckCre+/– mice are termed mPDK1–/– and PDK1flΔneo/flΔneoMckCre–/– are termed mPDK1+/+. (C) PDK1 was affinity purified from the indicated tissues using PIF–Sepharose (as described in the Materials and methods), electrophoresed on a 10% SDS–polyacrylamide gel and immunoblotted with anti-PDK1 antibody. It should be noted that as observed in previous studies (Lawlor et al., 2002; Collins et al., 2003), PDK1 migrates as a doublet, although the reason for this is not known. Abbreviations used are (Qua), quadriceps; (Gastr), gastrocnemius; (Ext), extensor digitalis; and (Dia), diaphragm. Similar results were obtained in three separate experiments using different mice of 5–6 weeks of age. Direct immunoblotting of cell lysates with PDK1 antibody revealed identical findings except that PDK1 immuno-reactive bands were of lower intensity (data not shown). (D) The indicated number of male and female mice were maintained under standard husbandry conditions and the percentage of surviving mice of each age is indicated. (n) denotes the number of each genotype.
As expected, the mPDK1–/– mice displayed no significant expression of the PDK1 protein in six different muscles studied, including heart (Figure 1C). In contrast, expression of PDK1 in liver, adipose and brain was identical to the level found in the control mPDK1+/+ mice.
Survival of mPDK1–/– mice
Up to the age of 5 weeks, the mPDK1–/– mice survived normally (Figure 1D), were apparently healthy, displaying no abnormal phenotype, and their growth, as assessed by body weight, was indistinguishable from that of mPDK1+/+ littermates (Supplementary figure 1, available at The EMBO Journal Online). The mPDK1–/– mice breath, eat normally and display the same physical activity as mPDK1+/+ mice. We also measured fasting blood glucose levels in mice up to 8 weeks of age and observed no significant difference between mPDK1+/+ and mPDK1–/– animals (data not shown), indicating that these mice were not markedly diabetic. However, between the ages of 5 and 11 weeks of age, 100% of the mPDK1–/– mice died, whereas the mPDK1+/+ littermates survived as expected (Figure 1D). In all cases, the mice were noticeably less active 2–3 days before they died, and a significant reduction in body weight was observed (Supplementary figure 1). Before this, there were no noticeable differences between mPDK1+/+ and mPDK1–/– mice. There was also no significant difference in the ages at which male and female mice died (data not shown).
Evidence that mPDK1–/– mice develop heart failure
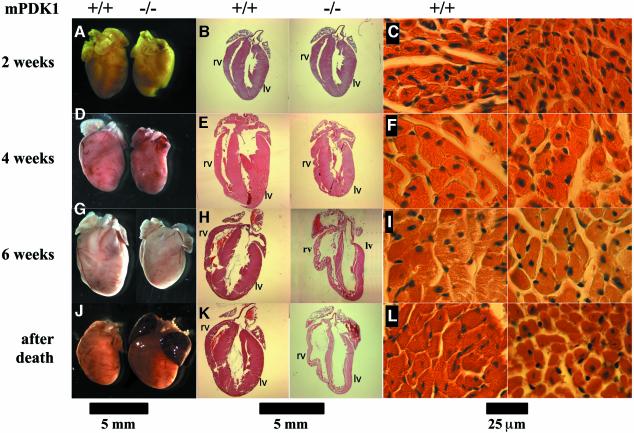
Post-mortem analysis of the mPDK1–/– mice revealed that the hearts of these animals were significantly enlarged, with blood clots found in both atria, which also appeared massively inflated. Morphological analysis of other organs (data not shown) of the mPDK1–/– mice, including skeletal muscle (Supplementary figure 2), presented no evidence of any abnormality. These observations suggested that a lack of PDK1 in cardiac muscle could be inducing heart failure leading to death of the animals. To investigate this in more detail, we performed histological analysis on hearts derived from mPDK1–/– and mPDK1+/+ mice of 2, 4 and 6 weeks of age, as well as on hearts from mPDK1–/– animals after death. This analysis revealed that the mPDK1–/– hearts were slightly smaller at 2 weeks of age (Figure 2A), the thickness of the ventricular walls was normal and the atria of normal size (Figure 2B). Moreover, analysis of cardiac tissue of 2-week-old mice, revealed no evident abnormalities or significant differences in cardiomyocyte size (Figure 2C). By 4 weeks of age, the difference in size between the mPDK1–/– and mPDK1+/+ hearts was more obvious (Figure 2D), but no major abnormality was noticeable in the ventricular walls (Figure 2E) or cardiac tissue (Figure 2F). By 6 weeks of age, however, the mPDK1–/– hearts were markedly smaller (Figure 2G), the ventricle walls were significantly thinner (Figures 2H and 3A) and the atria enlarged compared with control mPDK1+/+ heart (Figure 2H). Strikingly, the right ventricle was significantly enlarged in mPDK1–/– hearts, while the overall size of the left ventricle was similar but the muscle walls were much thinner (Figures 2H and 3A). Additionally, in the cardiac tissue, the cardiomyocytes appeared more separated from each other and possibly slightly smaller (Figure 2I). After death, the mPDK1–/– hearts were enlarged and appeared bigger than control mPDK1+/+ hearts (Figure 2J). Moreover, at this stage, the ventricular walls of the mPDK1–/– hearts were vastly thinner (Figure 2K). Post-mortem analysis of mPDK1–/– hearts confirmed that cardiac tissue had significantly expanded, resulting in marked separation of myocytes (Figure 2L). We were unable to detect myocyte disarray in mPDK1–/– hearts at any age, including after death (Supplementary figure 3).
Fig. 2. Histological analysis of hearts from mPDK1+/+ and mPDK1+/+ mice. At the indicated times the hearts were fixed in 10% formalin, embedded in wax and stained with haematoxilin and eosin. (A, D, G and J) Comparison of a representative image of heart of littermate mPDK1–/– and mPDK1+/+ mice of the same sex, before the fixation and staining. (B, E, H and K) Representative longitudinal sections after fixation and staining. (C, F, I and L) Representative micrographs of transversal sections of the muscular fibres. Scale bar is shown at the bottom of each set of panels.
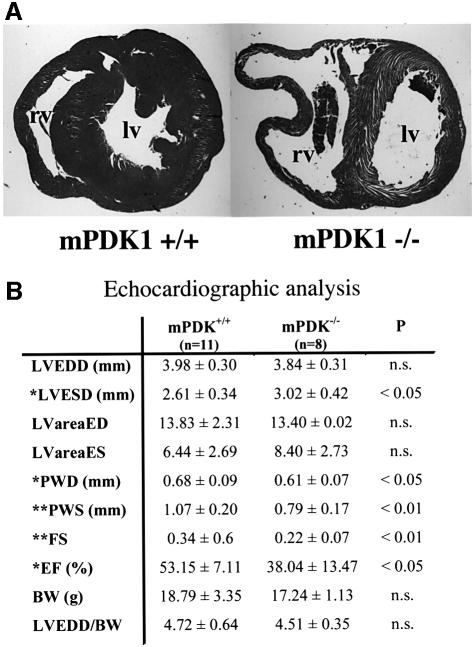
Fig. 3. Echocardiographic analysis of mPDK1–/– hearts. (A) Repre sentative transversal section of 6-week-old mPDK1–/– and mPDK1+/+ mouse heart fixed in 10% formalin, embedded in wax and stained with haematoxylin and eosin. rv, right ventricle; lv, left ventricle. (B) Echocardiographic analysis of 5- to 6-week-old mPDK1+/+ and mPDK1–/– mice. LVEDD, left ventricle end diastolic dimensions; LVESD, left ventricle end systolic dimensions; LVareaED, left ventricle area end diastolic; LvareaSD, left ventricle area end systolic; PWD, posterior wall diastolic thickness; PWS, posterior wall systolic thickness; FS, fractional shortening; EF, ejection fraction; BW, body weight; LVEDD/BW, left ventricular end-diastolic dimension corrected for body weight. Data are presented as the mean ± SEM and were compared by Student’s t-test, n = 11 for mPDK+/+ and 9 for mPDK–/–.
In vivo echocardiographic analysis in mPDK1–/– mice
Echocardiographic analysis of 5- to 6-week-old mPDK1–/– mice revealed the development of heart failure, reflected in a reduction in both fractional shortening and ejection fraction (Figure 3B). Thinning of the left ventricle was also confirmed by a significant reduction in the posterior wall thickness measured during diastole (Figure 3B). At this stage, there was no evidence for an increase in left ventricular dimension, although an enlarged right ventricle was visible in some of the echocardiographic images.
Quantitative analysis of muscle mass, cardiomyocyte volume and number
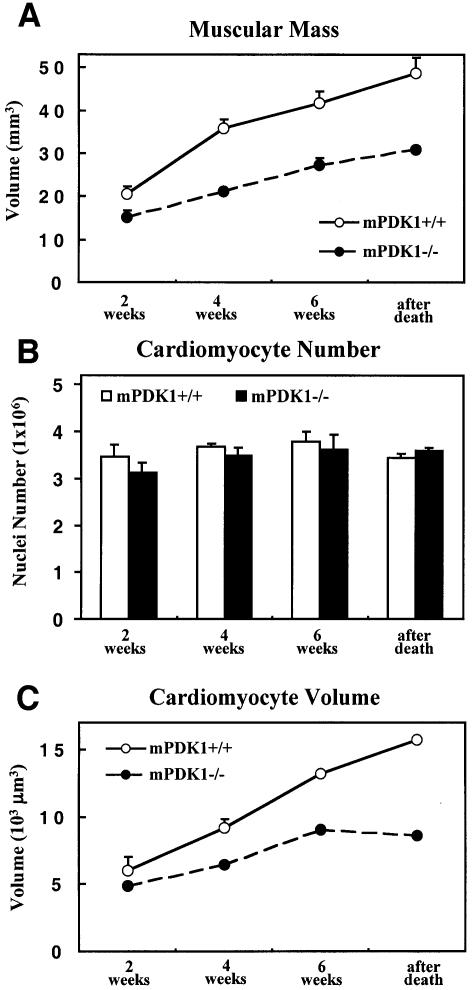
In order to further characterize the cardiac phenotype of mPDK1–/– mice, we employed rigorous unbiased methods for determining total muscle mass, average cardiomyocyte volume and total cardiomyocyte number. The muscle mass was calculated using the Cavalieri method, whereas the cardiomyocyte volume and cardiomyocyte number in the heart tissue were determined using a stereological approach called the dissector principle (Sterio, 1984; Gunderson, 1986). The muscle mass of mPDK1–/– hearts was significantly reduced and the difference in muscle mass between mPDK1–/– and mPDK1+/+ hearts increased with age (Figure 4A). The number of cardiomyocytes in the mPDK1–/– and mPDK1+/+ hearts was similar at all ages (Figure 4B). The average estimated volume of mPDK1–/– cardiomyocytes was significantly lower and the difference in volume between mPDK1–/– and mPDK1+/+ myocytes increased with age (Figure 4C). These findings show that a reduced cardiomyocyte volume rather than a decrease in cell number caused the reduction in muscle mass of the mPDK1–/– hearts.
Fig. 4. Quantitative analysis of muscle mass, cardiomyocyte volume and number. (A) The muscle mass of mPDK1+/+ and mPDK1–/– hearts of the indicated ages or after death. The results shown were determined by the Cavalieri method and presented as the average volume ± SD of the results obtained from analysing three separate hearts of each age and genotype. The cardiomyocyte volume (B) and cardiomyocyte number (C) were determined using the dissector principle. The cardiomyocyte number was determined by calculating the nuclei number, assuming one nuclei per cardiomyocyte. The data are presented as the mean ± SD of three separate hearts of each age and genotype.
Interestingly, histological analysis of the quadriceps of skeletal muscle of 6-weeks-old mPDK1–/– and mPDK1+/+ mice revealed no significant reduction in muscle fibre diameter (Supplementary figure 2). As muscle fibres are formed from fused cells, it is difficult to assess whether the fibres are formed from a larger number of smaller cells or whether limb skeletal muscle cells lacking PDK1 are not reduced in cell size.
Further evidence of cardiomyopathy in mPDK1–/– mice
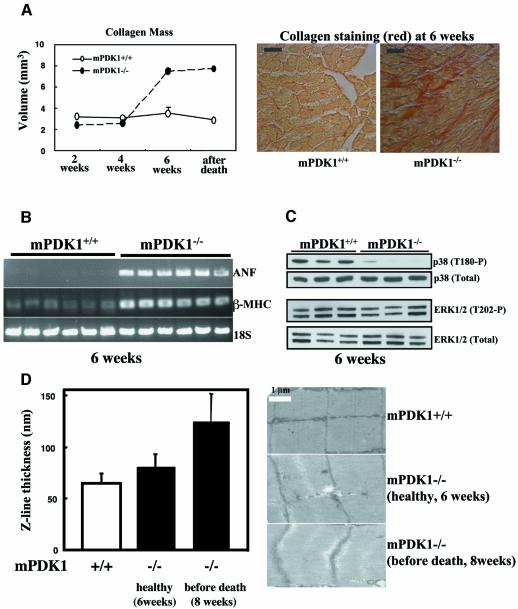
Disease progression in cardiomyopathy in humans can result primarily from myocardial injury and hypertension, as well as genetic factors, causing a marked increase in cardiac chamber volume and separation of cardiomyocytes. This leads to the accumulation of collagen in the intracellular space (Crackower et al., 2002b). We therefore measured the collagen content of the mPDK1–/– and mPDK1+/+ hearts using the quantitative Cavalieri method and a collagen specific stain. At weeks 2 and 4, mPDK1–/– and mPDK1+/+ hearts contained similar levels of collagen (Figure 5A). However, by 6 weeks of age and after death, the collagen content in the mPDK1–/– hearts was markedly elevated compared with control mPDK1+/+ hearts. Another key feature of heart failure is the reactivation of an embryonic gene programme (Hunter and Chien, 1999). The mRNA levels of both the atrial natriuretic factor and β-myosin heavy chain, two embryonic genes used as molecular markers of heart failure (Crackower et al., 2002b), were found to be significantly increased in the hearts derived from 6-week-old mPDK1–/– mice (Figure 5B).
Fig. 5. Analysis of markers of heart failure. (A) Histological sections of mPDK1+/+ and mPDK1–/– hearts of the indicated ages or after death were stained with Picric-Sirius Red dye, which stains collagen in red. The amount of collagen present in these sections was quantified using the Cavalieri method. The data are presented as the mean ± SD of three separate hearts of each age and genotype. Representative micrographs of Picric-Sirius Red-stained 6-week-old mPDK1–/– and mPDK1+/+ heart sections are shown. (B) RNA was isolated from mPDK1–/– and mPDK1+/+ hearts of 6 weeks of age, and RT–PCR analysis, described in the materials and methods, was employed to assess the mRNA expression levels of atrial natriuretic factor (ANF), β-myosin heavy chain (β-MHC) and 18S ribosomal RNA (18S) as a control. Each lane on the agarose gel represents a different mouse. (C) Cell extracts were prepared from mPDK1–/– and mPDK1+/+ hearts of 6 weeks of age and immunoblotted with the indicated antibodies. Each lane on the immunoblot represents a different mouse. (D) Electron microscope sections of mPDK1+/+ and mPDK1–/– hearts of the indicated ages. The Z-line thickness was quantitated by counting 135 randomly derived Z-lines from three hearts of each genotype and age. The data are presented as average thickness ± SD.
The p38 mitogen activated protein kinase has been reported to play a role in regulating myocardial cell growth. p38 is partially activated in normal hearts and is further stimulated by diverse hypertrophic insults (Behr et al., 2001). Moreover, it has also been reported that p38 is inactivated in failing human heart (Communal et al., 2002). We therefore assessed p38 activity using a phosphospecific antibody and found that in 6-week-old mPDK1–/– hearts, p38 phosphorylation was markedly reduced compared with control mPDK1+/+ hearts (Figure 5C, upper panel), consistent with the notion that p38 activity might be down regulated in the failing heart. The phosphorylation of other MAP kinase members, ERK1/ERK2 (Figure 5C, lower panel) and JNK1/JNK2 (data not shown) was similar in 6-week-old mPDK1–/– and mPDK1+/+ hearts. A characteristic feature of cardiomyopathy, termed dilated cardiomyopathy, is an increase in the Z-line thickness in sarcomeres resulting from over-stretching of cardiac muscle (Knoll et al., 2002). We therefore measured Z-line thickness, using electron microscopy, of mPDK1–/– mice, normal-weight mice (∼6 weeks old) and mice that had started to lose weight (∼8 weeks old) and were predicted to die soon. This analysis revealed that at 6 weeks of age there was no significant difference in Z-line thickness but by 8 weeks of age the Z-line was found to be significantly thicker in the mPDK1–/– mice (Figure 5D). The structure, length and organization of the mPDK1–/– and mPDK1+/+ sacromeres was found to be similar in mice of up to 6 weeks of age.
Analysis of PKB and S6K activation in mPDK1+/+ and mPDK1–/– hearts
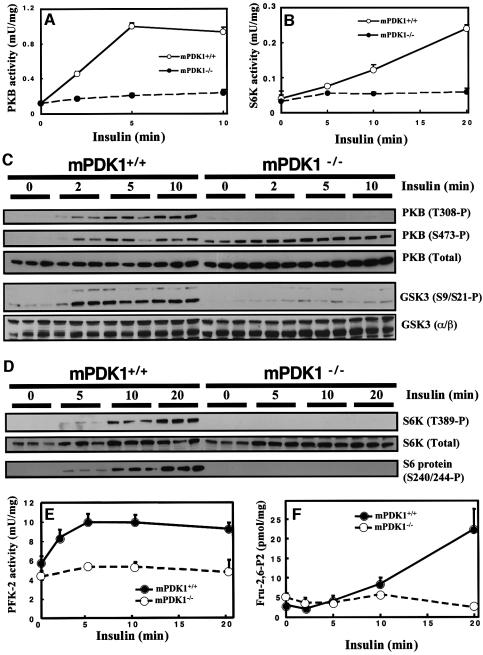
To determine whether lack of expression of PDK1 in heart impaired activation of characterized PDK1 substrates, mPDK1–/– and control mPDK1+/+ mice were injected with insulin for varying times and heart extracts generated. Activity of PKB (Figure 6A) and S6K (Figure 6B) was measured by a quantitative immunoprecipitation kinase assay. In hearts derived from mPDK1+/+ mice, insulin induced a 10-fold activation of PKB within 5 min and a 6-fold activation of S6K within 20 min. The low basal activity of PKB and S6K in unstimulated mPDK1–/– hearts was similar to that observed in mPDK1+/+ mice, but was not further activated by insulin.
Fig. 6. Activation of PKB, S6K and PFK2 in heart by insulin. Mice were fasted overnight and injected with either saline for 10–20 min (for the 0 time point control) or 1 mU/g of insulin for the indicated times. The heart was then rapidly extracted and frozen in liquid nitrogen. PKB (A) or S6K (B) were immunoprecipitated from cardiac extract and the activity determined using a quantitative peptide phosphorylation assay. Each point represents the mean activity ± SD of three different hearts with each assayed in triplicate. (C and D) As above except that cell lysates form the indicated hearts were immunoblotted with the indicated antibodies. Each lane represents a different mouse PFK-2 activity (E) and fructose-2,6-bisphosphate (Fru-2,6-P2) levels (F) were measured as described in the Materials and methods. Each point represents the mean activity ± SD of three to five different hearts with each assayed in duplicate.
In Figure 6C, the activation of PKB was also assessed using phosphospecific antibodies raised against the site of PDK1 phosphorylation (Thr308) as well as the hydrophobic motif phosphorylation site (Ser473), which is thought to be phosphorylated independently of PDK1. Insulin induced Thr308 phosphorylation of PKB in mPDK1+/+ hearts within 2 min, but as expected, no phosphorylation of Thr308 was detected in insulin-treated mPDK1–/– hearts at any time point tested. In unstimulated mPDK1+/+ hearts, PKB is not phosphorylated at Ser473 and insulin induced a marked phosphorylation of this residue (Figure 6C). In contrast, in unstimulated mPDK1–/– hearts, PKB was markedly phosphorylated at Ser473, to a level similar to that observed in insulin-stimulated mPDK1+/+ hearts. Stimulation of mPDK1–/– mice with insulin only induced a small additional increase in phosphorylation of PKB at Ser473. Similar findings have been made in mouse ES cells lacking PDK1 (Williams et al., 2000) and suggest that there may be some cross-talk between the regulation of the as yet uncharacterized PKB Ser473 kinase and PDK1.
The hydrophobic motif phosphorylation site on S6K (Thr389) plays a key role in regulating the activation of this kinase (Pearson et al., 1995). Recent work by several groups suggested that phosphorylation of Thr389 is regulated by PKB-catalysed phosphorylation of the tuberin tumour suppressor protein, and also involves mTOR (reviewed in McManus and Alessi, 2002). Consistent with this notion and the lack of PKB activity in mPDK1–/– hearts, insulin failed to stimulate S6K phosphorylation at Thr389 in mPDK1–/– hearts (Figure 6D). In contrast, in mPDK1+/+ hearts, insulin stimulated a marked phosphorylation of Thr389 within 10–20 min. Moreover, insulin also induced the phosphorylation of the PKB substrates GSK3α and GSK3β (Figure 6C) and the S6K substrate ribosomal S6 protein (Figure 6D), in mPDK1+/+ hearts but not in mPDK1–/– hearts.
Genetic evidence that PDK1 mediates insulin activation of 6-phosphofructo-2-kinase
The heart adapts its energy consumption to the availability of substrates and hormones. Under normal conditions, fatty acids are preferred to glucose. Hormones like insulin modify this hierarchy. Insulin inhibits fatty acid oxidation and stimulates glucose consumption (Hue et al., 2002). Stimulation of heart glycolysis by insulin is thought to be mediated through glucose transporter translocation and activation of 6-phosphofructo-2-kinase (PFK-2), which generates fructose 2,6-bisphosphate, the allosteric activator of 6-phosphofructo-1-kinase, a key enzyme of glycolysis (Depre et al., 1998; Hue et al., 2002). PFK-2 is activated in vivo by phosphorylation of Ser466 and Ser483 lying in an AGC kinase phosphorylation motif (Bertrand et al., 1999). In vitro Ser466 and Ser483 are phosphorylated by several AGC kinases including PKB (Deprez et al., 1997). In vivo phosphorylation of these sites is induced by insulin and this phosphorylation is prevented by treatment of cells with PI 3-kinase inhibitors, but it is not yet clear whether PKB or another insulin-stimulated wortmannin-sensitive kinase mediates this phosphorylation in vivo (Deprez et al., 2000). In order to assess the role of PDK1 in regulating activation of PFK-2, mPDK1+/+ and mPDK1–/– mice were injected with insulin and at varying times hearts extracted and PFK-2 activity (Figure 6E) and fructose 2,6-bisphosphate levels (Figure 6F) measured. In mPDK1+/+ mice, PFK-2 activity was stimulated nearly 2-fold within 5 min of insulin injection and accumulation of fructose 2,6-bisphosphate was detected after 10 min, reaching ∼5-fold higher levels than in unstimulated mPDK1+/+ hearts after 20 min. In the mPDK1–/– hearts, no significant increase in PFK-2 activity or fructose 2,6-bisphosphate levels was detected after insulin injection.
Evidence that mPDK1–/– cardiomyocytes are more sensitive to hypoxia
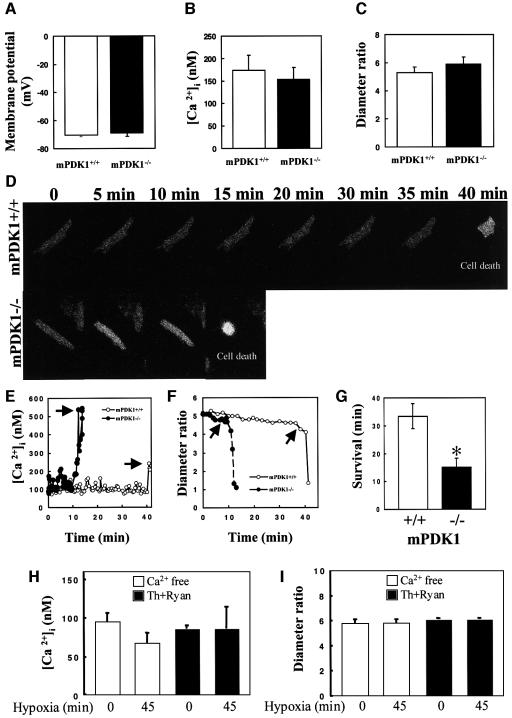
Metabolic stresses such as hypoxia leading to myocardial injury are one of the known causes of heart failure (Hirota et al., 1999; Cesselli et al., 2001). We therefore decided to test whether PDK1–/– cardiomyocytes are more sensitive to hypoxia compared with control animals. Cardiomyocytes were isolated from mPDK1–/– and mPDK1+/+ hearts of 4-week-old mice, when significant heart disease had not yet developed (Figures 2–5). The isolated mPDK1–/– cardiomyocytes were 40% smaller as estimated by laser confocal microscopy (Supplementary figure 4), similar to the reduction observed for cardiomyocytes in cardiac tissue (Figure 4C). Parameters of cell viability of mPDK1–/– cardiomyocytes including membrane potential (Figure 7A), resting intracellular calcium concentration ([Ca2+]i) (Figure 7B) and diameter ratio (Figure 7C) were similar to those observed for mPDK1+/+ cardiomyocytes. Isolated mPDK1–/– and mPDK1+/+ field-stimulated beating cardiomyocytes were subjected to hypoxic conditions and monitored continuously for [Ca2+]i and cell morphology (Figure 7D–F). In mPDK1+/+ cardiomyocytes, hypoxia induced intracellular Ca2+ overload associated with an irreversible cell collapse of the rod-shape structure (hypercontracture, leading to decrease in diameter ratio; indicative of cell death) (Jovanović et al., 1998) after ∼35 min (Figure 7D–F). The beating mPDK1–/– cardiomyocytes were markedly more sensitive to hypoxia than mPDK1+/+ cardiomyocytes, surviving for an average of only ∼15 min (Figure 7D–G). Hypoxia did not induce intracellular Ca2+ loading or cellular hypercontracture when extracellular Ca2+ was removed from the extracellular medium (Figure 7H) or intracellular Ca2+ stores were blocked with thapsigargin plus ryanodine (Figure 7I). We also performed measurements of Ca2+ transients in mPDK1–/– and mPDK1+/+ mice in field-stimulated cardiomyocytes using laser confocal microscopy and Fluo-3 Ca2+-sensitive dye, and no differences in properties of Ca2+ transients or cell shortening between different cell types were observed (data not shown), suggesting that PDK1 may not act as a negative regulator of cardiac contractility.
Fig. 7. Increased sensitivity of isolated mPDK1–/– cardiomyocytes to hypoxia. Cardiomyocytes were isolated from hearts of 4-week-old mice and processed as described in the materials and methods (A–C) Average values of membrane potential (A), ([Ca2+]i) (B) and diameter ratio (C) of mPDK1–/– and mPDK1+/+ cardiomyocytes. The results shown represent mean ± SEM with measurements performed with three to eight cells of each genotype. (D) Field stimulated beating mPDK1–/– and mPDK1+/+ cardiomyocytes were loaded with Fura 2-dye and imaged using a epifluorescent camera. At time zero, to induce hypoxia, the cells were subjected to an environment of pO2 20 mmHg. Images of the cardiomyocytes were taken at the indicated time points, and cell death is observed as a rounding up of the cardiomyocyte. (E and F) Depict time-courses of ([Ca2+]i (E) and diameter ratio (F) from cells in (D). Arrows indicate time of cardiomyocyte death. (G) The average survival time ± SEM of five to seven different mPDK1–/– and mPDK1+/+ cardiomyocytes subjected to hypoxic conditions was quantitated. *P < 0.05. (H and I) Average values of [Ca2+]i (H) and diameter ratio (I) of mPDK1+/+ cardiomyocytes in extracellular solution without Ca2+ (Ca2+ free) or pretreated with thapsigargin plus ryanodine (Th+Ryan) prior (0 min) and after 45 min-long exposure to hypoxia. The results shown represent mean ± SEM with measurements performed with four cells under each condition.
To analyse whether the increased sensitivity of mPDK1–/– cardiomyocytes to hypoxia was due to apoptosis, we set up a whole-heart assay in which mPDK1–/– and mPDK1+/+ hearts were cannulated and perfused under normoxic or hypoxic conditions for 4 h. The activity of caspase 3, and cleavage of caspase 3, caspase 7 and PARP protein, general markers for apoptosis, were found to be similar in normoxic and hypoxic mPDK1–/– and mPDK1+/+ hearts (Supplementary figure 5), indicating that the mPDK1–/– hearts were not failing due to an increase in apoptosis. As a control, mPDK1+/+ hearts were treated with the apoptosis-inducing non-specific kinase inhibitor staurosporine, which markedly increased caspase-3 activity and the cleavage of caspase 3, caspase 7 and PARP (Supplementary figure 5).
Discussion
In this study, we provide the first genetic evidence that PDK1 plays multiple roles in regulating cardiac viability. Our analysis indicates that mPDK1–/– mice die suddenly at an early stage of adult life, possess the same number of smaller cardiomyocytes and develop heart failure, and that cardiomyocytes lacking PDK1 are significantly more sensitive to hypoxia.
Mice models have previously been used to explore the role of the PI 3-kinase pathway in heart. Mimicking the activation of the PI 3-kinase pathway by overexpression of active forms of PI 3-kinase-α (Shioi et al., 2000), knockout of PTEN (Crackower et al., 2002a) and overexpression of active PKB (Condorelli et al., 2002; Shioi et al., 2002), resulted in an increase in the size of the heart (hypertrophy) due to increased cell size. Conversely, knockout of the insulin receptor (Belke et al., 2002) or the overexpression of dominant-negative PI 3-kinase (Shioi et al., 2000), decreased heart size (hypotrophy) by reducing cardiomyocyte volume. A number of studies have established that in both Drosophila and mice the PI 3-kinase/PDK1/AGC kinase pathway plays an important role in regulating cell size in all tissues investigated (reviewed in Kozma and Thomas, 2002). Consistent with this notion, we have also shown that mouse embryonic endoderm cells lacking PDK1 have a 60% reduced cytoplasmic volume (Lawlor et al., 2002). Moreover, PDK1 hypomorphic mice that express only 10% of the normal level of PDK1 are ∼40% smaller, and our analysis indicated this was due to these animals possessing smaller cells (Lawlor et al., 2002). In agreement with the notion that PDK1 plays a major role in regulating cell volume, we show that mPDK1–/– hearts are smaller, as a consequence of decreased cardiomyocyte size (Figures 2 and 4; Supplementary figure 4). At present, it is not possible to conclude whether it is the lack of activation of PKB, S6K or another PDK1 substrate that mediates the reduction in cardiomyocyte volume in mPDK1–/– mice. The knockouts thus far described for PKBα (Chen et al., 2001b; Cho et al., 2001b), PKBβ (Cho et al., 2001a) and S6K1 (Shima et al., 1998) were not reported to reduced heart size or cardiac phenotype, but this could be due to the presence of other isoforms of these kinases. PKB was assessed to play a central role in regulating cardiac size based on the results obtained with mice overexpressing 10- to 30-fold higher PKB levels than found physiologically (Condorelli et al., 2002; Shioi et al., 2002). However, it should be pointed out that a decrease in cell size in the PDK1 hypomorphic mice occurred under conditions where insulin was able to trigger normal activation of PKB and S6K (Lawlor et al., 2002), suggesting that PDK1 may regulate cell size independently of PKB and S6K.
In contrast to other genetic models of the PI 3-kinase pathway generated thus far, the mPDK1–/– mice are the only ones that develop cardiomyopathy leading to death in early adulthood. One of the reasons for this could be that the smaller mPDK1–/– hearts are not able to properly fulfil necessary cardiac function in adult mice. The mPDK1–/– mice fulfil the main criteria to support the diagnosis of heart failure. This includes marked thinning of ventricular walls assessed by both histological and echocardiographic analysis, contractile dysfunction assessed by echocardiography, separation of cardiomyocytes within cardiac tissue, increased collagen content, abnormal expression of embryonic genes and an increase in Z-line thickness prior to death. The findings that the myofibrils are not disorganized in the mPDK1–/– mice (Supplementary figure 3), that the ventricle walls are thinner (Figures 2 and 3) and that p38 activation in hearts of mPDK1–/– mice is reduced (Fig 5C) argues against hypertrophy. Previously, cardiac hypotrophy was observed in mice lacking the insulin receptor (Belke et al., 2002) or overexpressing dominant-negative PI 3-kinase (Shioi et al., 2000), but the mice did not develop heart failure. The main difference between these models and the mPDK1–/– mice is that the activity of PKB, S6K and probably other PDK1-regulated AGC kinase substrates are totally ablated in mPDK1–/– hearts. PDK1 hypomorphic mice possess markedly hypotrophic hearts (A.Mora and A.Jovanović, unpublished data), but did not develop heart failure (Lawlor et al., 2002). A key role for the PKB pathway is to suppress apoptosis, and therefore the development of heart failure in mPDK1–/– mice could have resulted from increased apoptotic cell death. Our data would argue against this, as the number of cardiomyocytes in mPDK1+/+ and mPDK1–/– hearts is similar (Figure 4B), and by the finding that markers of apoptosis (caspase 3, cleavage of caspase 3,caspase 7 and PARP) are not elevated in mPDK1–/– hearts (Supplementary figure 5).
In humans, one of the major causes of heart failure is ischaemic heart disease associated with hypoxia (Hirota et al., 1999; Cesselli et al., 2001). Work carried out over the last 15 years has demonstrated that ischaemia/hypoxia activates signalling pathways that enable cardiomyocytes to survive under these adverse conditions (Sugden and Clerk, 1998; Marber, 2000). Previous studies employing inhibitors of PI 3-kinase and overexpression of constitutively active or dominant-negative mutants of PI 3-kinase and PKB, in general, support the conclusion that this pathway plays a role in promoting survival in hypoxic and other stressful conditions (Fujio et al., 2000; Chen et al., 2001a; Jonassen et al., 2001; Baines et al., 2002). In the present study, we have used field-stimulated beating cardiomyocytes to directly measure the effect that lack of PDK1 has on resistance to hypoxia. We employed this approach as single cardiomyocytes are free of hormonal, neuronal and vascular influences, and thus any difference between control and mPDK1–/– myocytes can be ascribed reliably to the absence of PDK1. Bearing in mind that the initial metabolic condition of a cardiomyocyte may determine the outcome of a stress (Jovanović et al., 1996), it is important to note that mPDK1–/– and mPDK1+/+ cardiomyocytes had similar parameters of cell viability at rest (Figure 7A–C). We found that mPDK1–/– cardiomyocytes were considerably more sensitive to hypoxia than control mPDK1+/+ cardiomyocytes (Figure 7). In previous studies, it has been shown that ‘chemical’ hypoxia induces necrotic cell death by increasing intracellular Ca2+ via mechanisms that involve both extracellular and intracellular Ca2+ pools (Jovanović and Jovanović, 2001). In addition to these studies, we now provide evidence that extracellular Ca2+ as well as Ca2+ stored in subcellular compartments contribute to Ca2+ overload under ‘genuine’ hypoxia. We show that removal of extracellular Ca2+ throughout hypoxia can abolish Ca2+ loading as well as treatment with agents that deplete or inhibit intracellular Ca2+ stores (Figure 7H and I). Thus, Ca2+ loading following hypoxia of cardiomyocytes may have occurred through influx and release of Ca2+ from intracellular stores. Whole-heart experiments suggest a necrotic nature of hypoxia-induced cell death rather than apoptotic (Supplementary figure 5).
Although we have provided evidence for a role of PDK1 in promoting cardiomyocyte survival and preventing heart failure, it should be noted that further research is required to establish whether the increased sensitivity of mPDK1–/– cardiomyocytes to metabolic stresses (Figure 7) contributes to the development of heart failure in mPDK1–/– mice. Moreover, we have shown that in mPDK1–/– hearts, PFK-2 cannot be activated by insulin (Figure 6E and F), which would prevent the stimulation of glycolysis by insulin. The loss of the stimulation of glycolysis by insulin could modify the regulation of the fuel hierarchy by this hormone and such disruption could contribute to onset of heart failure in mPDK1–/– mice. It has to be noted that insulin, in combination with glucose and potassium, exerts a protective effect on the ischaemic heart by reducing infarct size and improving post-ischaemic left ventricular function. Even if the exact role of insulin remains unclear, the cardiac protection conferred by the hormone appears when the coronary flow is restored and may be related to the metabolic effect favouring glucose utilization instead of fatty acid oxidation (Beauloye et al., 2001; Hue et al., 2002). The loss of this beneficial effect of insulin could also contribute to the heart phenotype of mPDK1–/– mice.
In conclusion, we have provided firm genetic evidence that PDK1 regulates heart growth and viability. Moreover, we also show that PDK1 is necessary for the activation of PFK-2 and production of fructose 2,6-bisphosphate induced by insulin, and provide evidence that PDK1 is implicated in cardioprotective signalling. Taken together, the data presented in this study suggest that cardiomyopathies resulting in ventricular thinning and dilatation and ischaemic heart disease in humans could perhaps be treated by strategies that trigger the activity of PDK1.
Materials and methods
Materials and methods are presented as Supplementary data available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Vicky Murray-Tait for help importing and rederiving the MckCre mice, Luke Newman and Sandy Harper for help in organizing mouse studies, Margaret Lawlor for discussion, Jane Leitch and Moustapha Aoubala for preparation of antibodies and Calum Thomson for technical help in the cell size studies. The work was supported by BBSRC (A.J.), British Heart Foundation (A.J., D.R.A. and G.A.G.), Diabetes UK (D.R.A.), Medical Research Council (D.R.A. and G.A.G.), Tenovus-Scotland (A.J.), the Wellcome Trust (A.J. and J.M.L.-059767/Z/99/Z), the ‘Actions de Recherche Concertées’ 98/03-216 from the French Community of Belgium (L.B.), Belgian F.R.I.A. (V.M.), the Belgian Fund for Medical Research and the Belgian Federal Government (IAP 5). A.M. was supported by a postdoctoral fellowship from the Spanish government. D.R.A. also acknowledges the support of the pharmaceutical companies backing the Division of Signal Transduction Therapy unit in Dundee (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novo-Nordisk and Pfizer).
References
- Alessi D.R. (2001) Discovery of PDK1, one of the missing links in insulin signal transduction. Biochem. Soc. Trans., 29, 1–14. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Andjelkovic,M., Caudwell,B., Cron,P., Morrice,N., Cohen,P. and Hemmings,B.A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J., 15, 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Belham,C., Weng,Q., Hara,K. and Yonezawa,K. (2001) The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog. Mol. Subcell. Biol., 26, 115–154. [DOI] [PubMed] [Google Scholar]
- Baines C.P., Zhang,J., Wang,G.W., Zheng,Y.T., Xiu,J.X., Cardwell,E.M., Bolli,R. and Ping,P. (2002) Mitochondrial PKCε and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCε-MAPK interactions and differential MAPK activation in PKCε-induced cardioprotection. Circ. Res., 90, 390–397. [DOI] [PubMed] [Google Scholar]
- Beauloye C., Bertrand,L., Krause,U., Marsin,A.S., Dresselaers,T., Vanstapel,F., Vanoverschelde,J.L. and Hue,L. (2001) No-flow ischemia inhibits insulin signaling in heart by decreasing intracellular pH. Circ. Res., 88, 513–519. [DOI] [PubMed] [Google Scholar]
- Behr T.M. et al. (2001) Hypertensive end-organ damage and premature mortality are p38 mitogen-activated protein kinase-dependent in a rat model of cardiac hypertrophy and dysfunction. Circulation, 104, 1292–1298. [DOI] [PubMed] [Google Scholar]
- Belke D.D. et al. (2002) Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J. Clin. Invest., 109, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand L., Alessi,D.R., Deprez,J., Deak,M., Viaene,E., Rider,M.H. and Hue,L. (1999) Heart 6-phosphofructo-2-kinase activation by insulin results from ser-466 and ser-483 phosphorylation and requires 3-phosphoinositide-dependent kinase-1, but not protein kinase B. J. Biol. Chem., 274, 30927–30933. [DOI] [PubMed] [Google Scholar]
- Biondi R.M., Kieloch,A., Currie,R.A., Deak,M. and Alessi,D.R. (2001) The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J., 20, 4380–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi R.M., Komander,D., Thomas,C.C., Lizcano,J.M., Deak,M., Alessi,D.R. and Van Aalten,D.M. (2002) High resolution crystal structure of the human PDK1 catalytic domain defines the regulatory phosphopeptide docking site. EMBO J., 21, 4219–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil D.P. and Hemmings,B.A. (2001) Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci., 26, 657–664. [DOI] [PubMed] [Google Scholar]
- Bruning J.C., Michael,M.D., Winnay,J.N., Hayashi,T., Horsch,D., Accili,D., Goodyear,L.J. and Kahn,C.R. (1998) A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell, 2, 559–569. [DOI] [PubMed] [Google Scholar]
- Casamayor A., Torrance,P.D., Kobayashi,T., Thorner,J. and Alessi,D.R. (1999) Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol., 9, 186–197. [DOI] [PubMed] [Google Scholar]
- Cesselli D., Jakoniuk,I., Barlucchi,L., Beltrami,A.P., Hintze,T.H., Nadal-Ginard,B., Kajstura,J., Leri,A. and Anversa,P. (2001) Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res., 89, 279–286. [DOI] [PubMed] [Google Scholar]
- Chen L. et al. (2001a) Opposing cardioprotective actions and parallel hypertrophic effects of δ PKC and ε PKC. Proc. Natl Acad. Sci. USA, 98, 11114–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.S. et al. (2001b) Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev., 15, 2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. et al. (2001a) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science, 292, 1728–1731. [DOI] [PubMed] [Google Scholar]
- Cho H., Thorvaldsen,J.L., Chu,Q., Feng,F. and Birnbaum,M.J. (2001b) Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem., 276, 38349–38352. [DOI] [PubMed] [Google Scholar]
- Cho K.S., Lee,J.H., Kim,S., Kim,D., Koh,H., Lee,J., Kim,C., Kim,J. and Chung,J. (2001c) Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc. Natl Acad. Sci. USA, 98, 6144–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B.J., Deak,M., Arthur,J.S., Armit,L.J. and Alessi,D.R. (2003) In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J., 22, 4202–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communal C., Colucci,W.S., Remondino,A., Sawyer,D.B., Port,J.D., Wichman,S.E., Bristow,M.R. and Singh,K. (2002) Reciprocal modulation of mitogen-activated protein kinases and mitogen-activated protein kinase phosphatase 1 and 2 in failing human myocardium. J. Card. Fail., 8, 86–92. [DOI] [PubMed] [Google Scholar]
- Condorelli G. et al. (2002) Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc. Natl Acad. Sci. USA, 99, 12333–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crackower M.A. et al. (2002a) Regulation of myocardial contractility and cell size by distinct PI3K–PTEN signaling pathways. Cell, 110, 737–749. [DOI] [PubMed] [Google Scholar]
- Crackower M.A. et al. (2002b) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature, 417, 822–828. [DOI] [PubMed] [Google Scholar]
- Depre C., Rider,M.H. and Hue,L. (1998) Mechanisms of control of heart glycolysis. Eur. J. Biochem., 258, 277–290. [DOI] [PubMed] [Google Scholar]
- Deprez J., Vertommen,D., Alessi,D.R., Hue,L. and Rider,M.H. (1997) Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J. Biol. Chem., 272, 17269–17275. [DOI] [PubMed] [Google Scholar]
- Deprez J., Bertrand,L., Alessi,D.R., Krause,U., Hue,L. and Rider,M.H. (2000) Partial purification and characterization of a wortmannin-sensitive and insulin-stimulated protein kinase that activates heart 6-phosphofructo-2-kinase. Biochem. J., 347, 305–312. [PMC free article] [PubMed] [Google Scholar]
- Frodin M., Antal,T.L., Dummler,B.A., Jensen,C.J., Deak,M., Gammeltoft,S. and Biondi,R.M. (2002) A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J., 21, 5396–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y., Nguyen,T., Wencker,D., Kitsis,R.N. and Walsh,K. (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation, 101, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson H.J. (1986) Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc., 143, 3–45. [PubMed] [Google Scholar]
- Hirota H., Chen,J., Betz,U.A., Rajewsky,K., Gu,Y., Ross,J.,Jr., Muller,W. and Chien,K.R. (1999) Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell, 97, 189–198. [DOI] [PubMed] [Google Scholar]
- Hue L., Beauloye,C., Marsin,A.S., Bertrand,L., Horman,S. and Rider,M.H. (2002) Insulin and ischemia stimulate glycolysis by acting on the same targets through different and opposing signaling pathways. J. Mol. Cell. Cardiol., 34, 1091–1097. [DOI] [PubMed] [Google Scholar]
- Hunter J.J. and Chien,K.R. (1999) Signaling pathways for cardiac hypertrophy and failure. N. Engl. J. Med., 341, 1276–1283. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Schmelzle,T., Yamaguchi,K., Irie,K., Hall,M.N. and Matsumoto,K. (1999) PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol., 19, 8344–8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen A.K., Sack,M.N., Mjos,O.D. and Yellon,D.M. (2001) Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ. Res., 89, 1191–1198. [DOI] [PubMed] [Google Scholar]
- Jovanović S. and Jovanovic,A. (2001) Pinacidil prevents membrane depolarisation and intracellular Ca2+ loading in single cardiomyocytes exposed to severe metabolic stress. Int. J. Mol. Med., 7, 639–643. [DOI] [PubMed] [Google Scholar]
- Jovanović A., Lopez,J.R. and Terzic,A. (1996) Cytosolic Ca2+ domain-dependent protective action of adenosine in cardiomyocytes. Eur. J. Pharmacol., 298, 63–69. [DOI] [PubMed] [Google Scholar]
- Jovanović A., Jovanovic,S., Lorenz,E. and Terzic,A. (1998) Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance to chemical hypoxia-reoxygenation injury. Circulation, 98, 1548–1555. [DOI] [PubMed] [Google Scholar]
- Knoll R. et al. (2002) The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell, 111, 943–955. [DOI] [PubMed] [Google Scholar]
- Kozma S.C. and Thomas,G. (2002) Regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. BioEssays, 24, 65–71. [DOI] [PubMed] [Google Scholar]
- Lawlor M.A. and Alessi,D.R. (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci., 114, 2903–2910. [DOI] [PubMed] [Google Scholar]
- Lawlor M.A., Mora,A., Ashby,P.R., Williams,M.R., Murray-Tait,V., Malone,L., Prescott,A.R., Lucocq,J.M. and Alessi,D.R. (2002) Essential role of PDK1 in regulating cell size and development in mice. EMBO J., 21, 3728–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marber M.S. (2000) Ischemic preconditioning in isolated cells. Circ. Res., 86, 926–931. [DOI] [PubMed] [Google Scholar]
- McManus E.J. and Alessi,D.R. (2002) TSC1–TSC2: a complex tale of PKB-mediated S6K regulation. Nat. Cell Biol., 4, E214–E216. [DOI] [PubMed] [Google Scholar]
- Newton A.C. (2003) Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J., 370, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger C. and Schweingruber,M.E. (1999) A Schizosaccharo myces pombe gene, ksg1, that shows structural homology to the human phosphoinositide-dependent protein kinase PDK1, is essential for growth, mating and sporulation. Mol. Gen. Genet., 261, 177–183. [DOI] [PubMed] [Google Scholar]
- Paradis S., Ailion,M., Toker,A., Thomas,J.H. and Ruvkun,G. (1999) A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev., 13, 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.B., Dennis,P.B., Han,J.W., Williamson,N.A., Kozma,S.C., Wettenhall,R.E. and Thomas,G. (1995) The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J., 14, 5279–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintelen F., Stocker,H., Thomas,G. and Hafen,E. (2001) PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl Acad. Sci. USA, 98, 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A.R. and Kahn,C.R. (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature, 414, 799–806. [DOI] [PubMed] [Google Scholar]
- Scheid M.P. and Woodgett,J.R. (2001) PKB/Akt: functional insights from genetic models. Nat. Rev. Mol. Cell Biol., 2, 760–768. [DOI] [PubMed] [Google Scholar]
- Shima H., Pende,M., Chen,Y., Fumagalli,S., Thomas,G. and Kozma,S.C. (1998) Disruption of the p70s6k)/p85s6k gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J., 17, 6649–6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T., Kang,P.M., Douglas,P.S., Hampe,J., Yballe,C.M., Lawitts,J., Cantley,L.C. and Izumo,S. (2000) The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J., 19, 2537–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T., McMullen,J.R., Kang,P.M., Douglas,P.S., Obata,T., Franke,T.F., Cantley,L.C. and Izumo,S. (2002) Akt/protein kinase B promotes organ growth in transgenic mice. Mol. Cell. Biol., 22, 2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. and Parsons,R. (2001) PTEN: life as a tumor suppressor. Exp. Cell Res., 264, 29–41. [DOI] [PubMed] [Google Scholar]
- Sterio D.C. (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc., 134, 127–136. [DOI] [PubMed] [Google Scholar]
- Sugden P.H. (2001) Signalling pathways in cardiac myocyte hypertrophy. Ann. Med., 33, 611–622. [PubMed] [Google Scholar]
- Sugden P.H. and Clerk,A. (1998) Cellular mechanisms of cardiac hypertrophy. J. Mol. Med., 76, 725–746. [DOI] [PubMed] [Google Scholar]
- Toker A. and Newton,A.C. (2000) Cellular signaling: pivoting around PDK1. Cell, 103, 185–188. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers,S.J., Ahmadi,K., Timms,J., Katso,R., Driscoll,P.C., Woscholski,R., Parker,P.J. and Waterfield,M.D. (2001) Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem., 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Volarevic S. and Thomas,G. (2001) Role of S6 phosphorylation and S6 kinase in cell growth. Prog. Nucleic Acid Res. Mol. Biol., 65, 101–127. [DOI] [PubMed] [Google Scholar]
- Wick A., Wick,W., Waltenberger,J., Weller,M., Dichgans,J. and Schulz,J.B. (2002) Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J. Neurosci., 22, 6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.R., Arthur,J.S., Balendran,A., van der Kaay,J., Poli,V., Cohen,P. and Alessi,D.R. (2000) The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol., 10, 439–448. [DOI] [PubMed] [Google Scholar]
- Yang J., Cron,P., Good,V.M., Thompson,V., Hemmings,B.A. and Barford,D. (2002) Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP–PNP. Nat. Struct. Biol., 9, 940–944. [DOI] [PubMed] [Google Scholar]