Abstract
Members of a family of intracellular molecular switches, the small GTPases, sense modifications of the extracellular environment and transduce them into a variety of homeostatic signals. Among small GTPases, Ras and the Rho family of proteins hierarchically and/or coordinately regulate signaling pathways leading to phenotypes as important as proliferation, differentiation and apoptosis. Ras and Rho-GTPases are organized in a complex network of functional interactions, whose molecular mechanisms are being elucidated. Starting from the simple concept of linear cascades of events (GTPase→activator→ GTPase), the work of several laboratories is uncovering an increasingly complex scenario in which upstream regulators of GTPases also function as downstream effectors and influence the precise biological outcome. Furthermore, small GTPases assemble into macromolecular machineries that include upstream activators, downstream effectors, regulators and perhaps even final biochemical targets. We are starting to understand how these macromolecular complexes work and how they are regulated and targeted to their proper subcellular localization. Ultimately, the acquisition of a cogent picture of the various levels of integration and regulation in small GTPase-mediated signaling should define the physiology of early signal transduction events and the pathological implication of its subversion.
Keywords: GEFs/Rac/Ras/Rho-GTPases/signal transduction
Introduction
By cycling between inactive GDP- and active GTP-bound states, small GTPases function as critical relays in the transduction of signals originating from membrane receptors. Their regulation is achieved through the opposing effects of guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs). The superfamily of small GTPases includes >80 mammalian members whose prototypes are the Ras proteins (H-Ras, K-Ras and N-Ras, henceforth globally referred to as Ras), which became the focus of intense scrutiny after the discovery of their frequent alterations in neoplasia (for reviews see Barbacid, 1990; Yuspa et al., 1994). Ras is activated upon ligand engagement of membrane receptors (most notably receptor tyrosine kinases, RTKs) and mediates differentiation, proliferation, senescence and apoptosis. Such pleiotropic effects depend on the ability of Ras-GTP to activate multiple signaling pathways through direct binding to effector proteins. Overwhelming evidence identified the Raf–MEK–MAPK pathway as a key effector in Ras signaling (for reviews see McCormick, 1994; Marshall, 1995). However, the recruitment of many other Ras targets is indispensable to elicit a full Ras biological response. Among these Ras-dependent, Raf-independent pathways are those connecting Ras to the Rho subfamily of small GTPases. Rho-GTPases are regulators of actin reorganization, gene expression and cell cycle progression, and are important effectors of the function(s) of Ras (for reviews see Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). In addition to being regulated by Ras, Rho-GTPases establish a network of stimulatory/inhibitory functional interactions among themselves. We will focus on the current understanding of the molecular machinery that connects Ras to Rho-GTPases and Rho-GTPases among themselves. While it is outside the scope of this review to provide a comprehensive account of all important discoveries, examples will be used that illustrate general mechanisms and the emerging paradigms.
Rho GTPases and their regulators
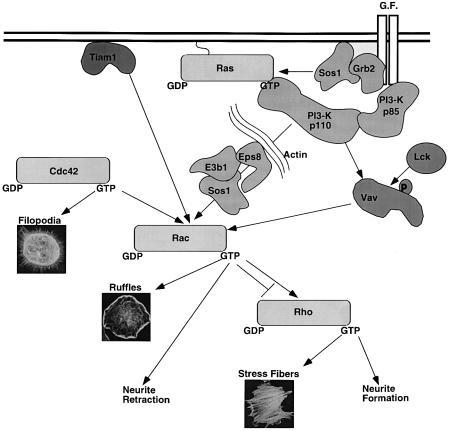
At least nine subfamilies make up the superfamily of small GTPases. The mammalian Rho subfamily comprises at least 14 members (RhoA, RhoB, Rhoc, RhoD, RhoE/rnd3, Rnd1/Rho6, rnd2/Rho7, Rhog, Rac1, Rac2, Rac3, Cdc42, TC10 and TTF). The best characterized function of Rho-GTPases is the regulation of the actin cytoskeleton (Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). Unique actin changes are associated with the activation of individual Rho-GTPases. Briefly, activated Cdc42 induces the formation of filopodia, thin finger-like extensions containing actin bundles and probably involved in the recognition of the extracellular environment. Rac regulates the formation of lamellipodia or ruffles, curtain-like extensions often formed along the edge of the cell. Rho mediates the formation of stress fibers, elongated actin bundles that traverse the cells and promote cell attachment to the extracellular matrix through focal adhesions. These specific actin changes have been widely used as markers of the activation of individual Rho-GTPases (Figure 1).
Fig. 1. Signaling from Ras to Rac and relationships among Rho-GTPases. Activation of Rho-GTPases by growth factors (G.F.) can be achieved by pathways requiring the activation of PI3-K, either directly through binding of its regulatory subunit, p85, to the activated receptor, or indirectly through activation of Ras and formation of a Ras-GTP–p110 complex. The phosphoinositides generated by PI3-K are thought to regulate the activity and/or the localization of Rac-specific GEFs (Vav, Sos-1 and Tiam-1). The formation of a multimolecular complex (Eps8–E3b1–Sos-1) in addition to PI3-K activation is required for proper signaling in the case of Sos-1-mediated Rac activation. Tyrosine phosphorylation of GEFs, as in the case of Vav, might also be required in synergy with PI3-K action. Linear cascades coordinate the activity of the Rho GTPases Cdc42, Rac and Rho leading to actin remodeling and other phenotypes. See the text for further details. [Insets showing Rho GTPase-mediated actin remodeling are reprinted with permission from Hall (1998). Copyright 1998 American Association for Advancement of Science. Figure courtesy of Kate Nobes).]
Similarly to all other small GTPases, GEFs, GAPs and GDIs regulate Rho-GTPases. A scenario is emerging in which GEFs, GAPs and GDIs do not regulate solely the GTP/GDP ratio of Rho-GTPases, but play additional roles in signal transduction pathways. GEFs catalyze the exchange of GDP for GTP, with ensuing activation of Rho-GTPases. More than 35 GEFs are known, which display activity on Rho-GTPases, at least in vitro. Rho-GEFs all contain a DH–PH signature composed of a Dbl homology domain (DH) in tandem with a pleckstrin homology (PH) domain (for reviews see Cerione and Zheng, 1996; Whitehead et al., 1997). The DH domain contains the catalytic core of the Rho-GEF enzymatic activity, whereas the PH domain is a lipid-binding surface and probably also a protein–protein interaction surface. The size of the Rho-GEF family, far exceeding that of Rho-GTPases, is compatible with redundancy of GEF function or anticipates discovery of more Rho-GTPases. In turn, redundancy of GEFs might be functional to an effector function of these molecules, a possibility that will be elaborated further below.
More than 16 different Rho-GAPs stimulate the intrinsic GTPase activity of Rho-GTPases, leading to attenuation of their signaling. They share a unique GAP domain and show a variable degree of specificity in vitro and in vivo (Ridley et al., 1993). Rho-GAPs might also mediate downstream functions, consistent with the observation that they are complex proteins displaying a variety of domains. For instance, p190Rho-Gap, specific for RhoA, is tyrosine phosphorylated and forms a complex with the SH2 domain of another GAP, p120Ras-GAP (Settleman et al., 1992). The association between p120Ras-GAP and p190Rho-GAP suggests that the former protein might act as a Ras effector and regulate the activity of Rho, potentially defining a cross-talk between Ras and Rho. This general concept is reinforced further by a number of studies on p120Ras-Gap demonstrating that mutants impaired in their GAP activity are still actively involved in signaling (Medema et al., 1992; McGlade et al., 1993; Xu et al., 1994).
Rho-GDI, the prototype of a class of regulators comprising three members so far, inhibits the dissociation of radiolabeled GDP from several Rho-GTPases (Fukumoto et al., 1990; Ueda et al., 1990). Rho-GDI associates equally well with GTP-bound Rho-GTPases, thereby interfering with GTP hydrolysis, and thus possesses characteristics of a regulator of both the exchange and the hydrolysis of guanine nucleotides (Hart et al., 1992; Chuang et al., 1993). Rho-GDI might also direct Rho-GTPases to proper sites on the plasma membrane (Takai et al., 1995), possibly via interaction with members of the ERM (ezrin, radixin, moesin) family of proteins, which are thought to act as general cross-linkers between the plasma membrane and actin filaments (Tsukita et al., 1994; Hirao et al., 1996; Takahashi et al., 1997). The scenario is complicated further by the facts that radixin inhibits Rho-GDI activity and that it binds to Dbl, a GEF for Rho-GTPases (Takahashi et al., 1998). All this evidence, while not providing a cogent picture yet, supports the notion that Rho-GDIs, like Rho-GEFs and Rho-GAPs, are not just regulators of Rho-GTPases, but active players in their transduction pathways.
Communication among small GTPases: learning from lower organisms
In understanding how small GTPases communicate with each other, important lessons were learned by turning to yeast. Two major messages (for a more extensive review see Chant and Stowers, 1995) emerged from these studies. The first is that GEFs represent a major functional link, working both as upstream activators and downstream effectors of small GTPases. In Schizosaccharomyces pombe, for example, Scd1/Ral1 is a GEF for Cdc42sp and also acts as a Ras1 effector in cytoskeleton organization, morphogenesis and mating (Zheng et al., 1995b).
A second take home message is that components of the GTPase-mediated pathways assemble into multimolecular complexes held together by scaffold proteins. A paradigmatic case is represented by Bem1p, a protein containing two SH3 domains and involved in cell polarization and in the pheromone response pathway. Bem1p scaffolds together Cdc24p (a Cdc42p-GEF), Rsr-1 (a GTPase acting upstream of Cdc42p), Ste20p (a serine/threonine kinase target of Cdc42p), Ste5p (a scaffold protein essential for the MAPK pathways utilized for the pheromone response) and actin (Leberer et al., 1997). Bem1p mutants, which are impaired in establishing some of these interactions, interfere with the formation of this complex and lead to various defects in the transmission of signals (Leeuw et al., 1995). Similar organizations might also exist in mammals (see below).
From Ras to Rac in mammals: GEFs and more
Much evidence (reviewed in Van Aelst and D’Souza-Schorey, 1997; Zohn et al., 1998) indicates that Rho-GTPases are key downstream targets in Ras-mediated signaling (Figure 1). In mammals, the cross-talk between Ras and Rac, probably the best characterized one, provides an exemplar case in which concepts derived from yeast studies found confirmation.
In the transduction of signals from Ras to Rac, a simple hypothetic linear pathway Ras→GEF→Rac is immediately challenged by the fact that no known Rho-GEF is able to bind directly to Ras-GTP, suggesting additional complexity. Insights into this issue derived from investigations of phosphatidylinositol 3-kinase (PI3-K), an enzyme that catalyzes the conversion of phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3) (Carpenter and Cantley, 1996; Tolias and Cantley, 1999). Initial studies, employing the PI3-K inhibitor wortmannin and activated mutants of Rac and PI3-K, indicated that PI3-K functions upstream of Rac in the sequence of events activated by RTKs (Kotani et al., 1994; Wennstrom et al., 1994; Hawkins et al., 1995; Nobes et al., 1995). Moreover, the catalytic subunit of PI3-K, p110, binds directly to Ras-GTP (Rodriguez-Viciana et al., 1996), implying downstream positioning of PI3-K with respect to Ras. Functional studies subsequently established PI3-K as a major effector of Ras and upstream modulator of Rac activity (Ras→PI3-K→Rac pathway, Figure 1) (Rodriguez-Viciana et al., 1997). It should be pointed out, however, that, at least in Swiss 3T3 cells, wortmannin does not interfere with Ras-mediated actin remodeling (Nobes and Hall, 1995). Thus, a second pathway (RTK→PI3-K→Rac) might exist, which is Ras independent. Mechanistically this pathway might rely on direct binding of PI3-K to RTKs, through its regulatory subunit p85, with ensuing activation of the enzyme (Figure 1).
The mechanisms whereby PI3-K activates Rac are being elucidated. As frequently happens, the first hint came from yeast studies showing that TOR2 (a yeast PI3-K homolog) was involved in the actin cytoskeleton through activation of the RHO1 GTPase, via a GEF, ROM2 (Schmidt et al., 1996). In mammals, PI3-K might regulate at least two Rac-GEFs, Vav and Sos-1 (Han et al., 1998; Nimnual et al., 1998). Activation of GEF activity by PI3-K is most probably achieved through binding of PIP3, the product of PI3-K, to the PH domain, which is part of the DH–PH tandem characteristic of all Rho-GEFs. The mechanism of activation could be either direct or indirect. A direct mechanism is suggested by structural studies of the DH–PH tandem of Sos-1. This duplex domain consists of an L-shaped structure in which two distinct modules are clearly delineated corresponding to the regulatory PH and catalytic DH domains (Soisson et al., 1998). The active site of the DH domain lies near the interface between the two modules, suggesting that binding of PIP3 to the PH domain might regulate the activity of the DH domain. Activation might also proceed through indirect mechanisms, as indicated by studies of Vav. In this case, PIP3 (in addition to modulating Vav activity by binding directly to the DH–PH domain) was shown to increase tyrosine phosphorylation of Vav, a post-translational modification that enhances its GEF activity (Han et al., 1998) (Figure 1).
Similarly to yeast, macromolecular complexes are also at play in mammals, as shown in the case of Sos-1. Despite the presence of the characteristic DH–PH signature, isolated Sos-1 (or its DH–PH domain) does not appear to be endowed with Rac-GEF activity. However, a fraction of Sos-1 is present in the cell as a ternary complex with Eps8 and E3b1, two signaling proteins (Scita et al., 1999). The ternary complex, immunoprecipitated from cells, shows specific Rac-GEF activity, which requires the presence of all three components. Thus, a macromolecular complex seems to be needed to unmask the Rac-specific GEF activity of Sos-1 (Figure 1). In addition, Eps8 binds to actin (Provenzano et al., 1998; G.Scita and P.Tenca, unpublished observation), thus providing a potential mechanism for proper localization of the signaling complex, a situation reminiscent of the Bem1p-mediated macromolecular complex in yeast. It remains to be established how PI3-K participates in the regulation of this complex.
Activation of Rac: too many GEFs doing too many things?
An apparent paradox is emerging from studies of the mechanisms of activation of Rac: several GEFs exert exchange activity both on Rac (and probably on other Rho-GTPases) and on Ras. It is puzzling that redundancy should be coupled to promiscuity.
The case of Sos-1 is paradigmatic. Sos-1 is the prototype of a family of GEFs endowed with dual specificity for Ras and Rac. Members of this bifunctional class of molecules are characterized by the simultaneous presence of a Cdc25-homology domain, which encodes the catalytic core of a Ras-GEF activity (Boriack-Sjodin et al., 1998), and of a tandem DH–PH domain. The family also includes Ras-GRF1 and Ras-GRF2 (Fan et al., 1998; Innocenti et al., 1999; Kiyono et al., 1999). Sos-1 and the other members of the family therefore appear to act both upstream and downstream of Ras. It is tempting to speculate that the GEF specificity is dictated by the different complexes in which Sos-1 is engaged: a receptor–Grb2–Sos–1 complex leads to Ras activation (Downward, 1994; Margolis and Skolnik, 1994), whereas an Eps8–E3b1–Sos-1 complex regulates Rac activation. If the formation of the different complexes were to be coordinated temporally or spatially, then a convenient mechanism of regulation of signals would ensue. Sos-1 would initially activate Ras and subsequently be shifted to Rac, thus determining the switching off of the upstream signal, while allowing its propagation downstream.
Redundancy of GEF functions is also puzzling. At least eight GEFs display activity on Rac, either in vitro or in vivo (Van Aelst and D’Souza-Schorey, 1997). Obvious explanations for redundancy include cell- and/or tissue-specific expression or the need for Rac to be activated by different upstream pathways (Ras versus Cdc42, for example, see below). An intriguing alternative originates from studies on Tiam-1, a potent Rac-GEF (Habets et al., 1994). Tiam-1 is a poor inducer of Jun N-terminal kinase (JNK) but strongly up-regulates Pak1 (Zhou et al., 1998). It is of note that both these kinases are strongly induced by activated Rac. Thus, the upstream machinery that regulates Rac might also participate in its signaling. One can envisage a scenario in which redundancy of Rac-GEFs might be part of a mechanism that concomitantly regulates the activity of the GTPase while directing its signals towards specific components of the downstream pathway.
Molecular networking among Rho-GTPases
Many excellent reviews are available concerning the biological and biochemical functions of Rho-GTPases (Van Aelst and D’Souza-Schorey, 1997; Hall, 1998; Zohn et al., 1998). We will concentrate on those functions pointing to molecular networking among members of the family.
Initial studies performed in Swiss 3T3 cells showed how Cdc42, Rac and Rho are organized in hierarchical cascades. Activated Cdc42 leads to activation of Rac, which, in turn, activates Rho. A Cdc42-independent cascade also exists that links activated Ras to Rac and subsequently to Rho (Figure 1). Similar hierarchical organizations are also a common theme in yeast, where they mediate bud formation and morphogenesis (Chant and Stowers, 1995; Zheng et al., 1995a). The simple epistatic arrangement (and the functional consequences) detected in Swiss 3T3 cells is challenged by observations in other systems. For example, Cdc42 inhibits, rather than induces, stress fibers in various cell types (Kozma et al., 1995; Qiu et al., 1997). Furthermore, in neuronal cells, the activities of Rac and Rho are coordinated in an opposing fashion, rather than in a linear unidirectional way (Jalink et al., 1994; Kozma et al., 1997; Leeuwen et al., 1997) (Figure 1). One of the major caveats in the above studies is that the activation of specific Rho-GTPases is inferred from the appearance of specific cytoskeletal changes, rather than by direct biochemical observations. This problem has been circumvented recently by measuring directly the activation of various Rho-GTPases by quantitative protein–protein interaction assays based on the ability of specific protein domains to bind the GTP-bound forms of the GTPases preferentially (Manser et al., 1998). By using these assays, Sander et al. (1999) showed that activation of Rac leads to the inhibition of Rho (Figure 1).
A logical consequence of the above findings is that activation of Rho must proceed, at least in some cases, through a Rac-independent mechanism. In addition, some levels of co-regulation of Rac and Rho activation must exist, as suggested by studies in neuronal cells (Jalink et al., 1994; Kozma et al., 1997; Leeuwen et al., 1997). A potential candidate for such a role is Trio, a multifunctional protein originally isolated as an interactor of the tyrosine phosphatase LAR (Debant et al., 1996). It contains three catalytic domains: a serine/threonine kinase, and two GEF domains, displaying Rac and Rho specificity, respectively (Bellanger et al., 1998). Thus, Trio represents a unique member of the Rho-GEF family that can regulate Rac and Rho signaling pathways coordinately in a way that is not strictly epistatic.
Similar mechanisms of signal integration might also be at work in the transduction of signals from Cdc42 to Rac. The best known candidate for such a function is PIX (Pak-interacting exchange factor) (Bagrodia et al., 1998; Manser et al., 1998; Obermeier et al., 1998). PIX is part of a macromolecular complex involving RTKs, PI3-K and adaptor proteins, and its GEF activity is modulated by RTKs, most likely via PI3-K (Yoshii et al., 1999). Thus, the function of PIX might not be restricted to the Cdc42→Rac pathway and might extend to the regulation of other aspects of the control of the Rho-GTPase network.
In summary, we seem to be faced with a situation in which in some cell types a linear hierarchical arrangement of Rho-GTPases is responsible for propagation of signals, while in other types a more complex integrated circuitry is at work. It is possible that the simple epistatic arrangement, first detected in Swiss 3T3 cells, constitutes the basic framework on which additional levels of complexity are built. Under this scenario, the exact regulator/effector molecular machinery, which can be cell type specific, and the nature of the activating stimulus (growth factor versus a GEF versus an active mutant, for instance) might determine the precise biochemical outcome.
Conclusions
As our knowledge of signaling pathways advances, it becomes increasingly clear that linear cascades of events cannot account for the complexity of functions regulated through signal transduction. Instead, a network of pathways with multiple overlaps, feedback loops, and uni- and bidirectional signals provides a picture that is closer to reality. However, this higher level of complexity might not fully represent the cellular machinery, since signaling networks are organized efficiently in multimolecular complexes. In this framework, small GTPases function as critical relays through which branches of the various pathways are switched on or off. The definition of the molecular mechanisms of cross-talk among GTPases will provide us with the opportunity to interfere with specific branches of the transduction machinery in those diseases, most notably cancer, in which subversion of signaling pathways has a prevalent role.
Acknowledgments
Acknowledgements
We thank Pier Giuseppe Pelicci and Pascale Romano for critically reviewing the manuscript.
References
- Bagrodia S., Taylor,S.J., Jordon,K.A., Van Aelst,L. and Cerione,R.A. (1998) A novel regulator of p21-activated kinases. J. Biol. Chem., 273, 23633–23636. [DOI] [PubMed] [Google Scholar]
- Barbacid M. (1990) ras oncogenes: their role in neoplasia. Eur. J. Clin. Invest., 20, 225–235. [DOI] [PubMed] [Google Scholar]
- Bellanger J.M., Lazaro,J.B., Diriong,S., Fernandez,A., Lamb,N. and Debant,A. (1998) The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo.Oncogene, 16, 147–152. [DOI] [PubMed] [Google Scholar]
- Boriack-Sjodin P.A., Margarit,S.M., Bar-Sagi,D. and Kuriyan,J. (1998) The structural basis of the activation of Ras by Sos. Nature, 394, 337–343. [DOI] [PubMed] [Google Scholar]
- Carpenter C.L. and Cantley,L.C. (1996) Phosphoinositide kinases. Curr. Opin. Cell Biol., 8, 153–158. [DOI] [PubMed] [Google Scholar]
- Cerione R.A. and Zheng,Y. (1996) The Dbl family of oncogenes. Curr. Opin. Cell Biol., 8, 216–222. [DOI] [PubMed] [Google Scholar]
- Chant J. and Stowers,L. (1995) GTPase cascades choreographing cellular behavior: movement, morphogenesis and more. Cell, 81, 1–4. [DOI] [PubMed] [Google Scholar]
- Chuang T.H., Xu,X., Knaus,U.G., Hart,M.J. and Bokoch,G.M. (1993) GDP dissociation inhibitor prevents intrinsic and GTPase activating protein-stimulated GTP hydrolysis by the Rac GTP-binding protein. J. Biol. Chem., 268, 775–778. [PubMed] [Google Scholar]
- Debant A., Serra-Pages,C., Seipel,K., O’Brien,S., Tang,M., Park,S.H. and Streuli,M. (1996) The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc. Natl Acad. Sci. USA, 93, 5466–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J. (1994) The GRB2/Sem-5 adaptor protein. FEBS Lett., 338, 113–117. [DOI] [PubMed] [Google Scholar]
- Fan W.T., Koch,C.A., de Hoog,C.L., Fam,N.P. and Moran,M.F. (1998) The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr. Biol., 8, 935–938. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi,K., Hori,Y., Fujioka,H., Araki,S., Ueda,T., Kikuchi,A. and Takai,Y. (1990) Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene, 5, 1321–1328. [PubMed] [Google Scholar]
- Habets G.G., Scholtes,E.H., Zuydgeest,D., van der Kammen,R.A., Stam,J.C., Berns,A. and Collard,J.G. (1994) Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP–GTP exchangers for Rho-like proteins. Cell, 77, 537–549. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Han J. et al. (1998) Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science, 279, 558–560. [DOI] [PubMed] [Google Scholar]
- Hart M.J., Maru,Y., Leonard,D., Witte,O.N., Evans,T. and Cerione,R.A. (1992) A GDP dissociation inhibitor that serves as a GTPase inhibitor for the Ras-like protein CDC42Hs. Science, 258, 812–815. [DOI] [PubMed] [Google Scholar]
- Hawkins P.T. et al. (1995) PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr. Biol., 5, 393–403. [DOI] [PubMed] [Google Scholar]
- Hirao M., Sato,N., Kondo,T., Yonemura,S., Monden,M., Sasaki,T., Takai,Y. and Tsukita,S. (1996) Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J. Cell Biol., 135, 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti M., Zippel,R., Brambilla,R. and Sturani,E. (1999) CDC25 (Mm)/Ras-GRF1 regulates both Ras and Rac signaling pathways. FEBS Lett., 460, 357–362. [DOI] [PubMed] [Google Scholar]
- Jalink K., van Corven,E.J., Hengeveld,T., Morii,N., Narumiya,S. and Moolenaar,W.H. (1994) Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol., 126, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono M., Satoh,T. and Kaziro,Y. (1999) G protein β γ subunit-dependent Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25 (Mm). Proc. Natl Acad. Sci. USA, 96, 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K. et al. (1994) Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J., 13, 2313–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R., Ahmed,S., Best,A. and Lim,L. (1995) The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol., 15, 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R., Sarner,S., Ahmed,S. and Lim,L. (1997) Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1 and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol., 17, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Thomas,D.Y. and Whiteway,M. (1997) Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev., 7, 59–66. [DOI] [PubMed] [Google Scholar]
- Leeuw T., Fourest-Lieuvin,A., Wu,C., Chenevert,J., Clark,K., Whiteway,M., Thomas,D.Y. and Leberer,E. (1995) Pheromone response in yeast: association of Bem1p with proteins of the MAP kinase cascade and actin. Science, 270, 1210–1213. [DOI] [PubMed] [Google Scholar]
- Leeuwen F.N., Kain,H.E., Kammen,R.A., Michiels,F., Kranenburg,O.W. and Collard,J.G. (1997) The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol., 139, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E., Loo,T.H., Koh,C.G., Zhao,Z.S., Chen,X.Q., Tan,L., Tan,I., Leung,T. and Lim,L. (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell, 1, 183–192. [DOI] [PubMed] [Google Scholar]
- Margolis B. and Skolnik,E.Y. (1994) Activation of Ras by receptor tyrosine kinases. J. Am. Soc. Nephrol., 5, 1288–1299. [DOI] [PubMed] [Google Scholar]
- Marshall M. (1995) Interactions between Ras and Raf: key regulatory proteins in cellular transformation. Mol. Reprod. Dev., 42, 493–499. [DOI] [PubMed] [Google Scholar]
- McCormick F. (1994) Activators and effectors of ras p21 proteins. Curr. Opin. Genet. Dev., 4, 71–76. [DOI] [PubMed] [Google Scholar]
- McGlade J., Brunkhorst,B., Anderson,D., Mbamalu,G., Settleman,J., Dedhar,S., Rozakis-Adcock,M., Chen,L.B. and Pawson,T. (1993) The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J., 12, 3073–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema R.H., de Laat,W.L., Martin,G.A., McCormick,F. and Bos,J.L. (1992) GTPase-activating protein SH2–SH3 domains induce gene expression in a Ras-dependent fashion. Mol. Cell. Biol., 12, 3425–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnual A.S., Yatsula,B.A. and Bar-Sagi,D. (1998) Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science, 279, 560–563. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hawkins,P., Stephens,L. and Hall,A. (1995) Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J. Cell Sci., 108, 225–233. [DOI] [PubMed] [Google Scholar]
- Obermeier A., Ahmed,S., Manser,E., Yen,S.C., Hall,C. and Lim,L. (1998) PAK promotes morphological changes by acting upstream of Rac. EMBO J., 17, 4328–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano C., Gallo,R., Carbone,R., Di Fiore,P.P., Falcone,G., Castellani,L. and Alema,S. (1998) Eps8, a tyrosine kinase substrate, is recruited to the cell cortex and dynamic F-actin upon cytoskeleton remodeling. Exp. Cell Res., 242, 186–200. [DOI] [PubMed] [Google Scholar]
- Qiu R.G., Abo,A., McCormick,F. and Symons,M. (1997) Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol. Cell. Biol., 17, 3449–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J., Self,A.J., Kasmi,F., Paterson,H.F., Hall,A., Marshall,C.J. and Ellis,C. (1993) rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo.EMBO J., 12, 5151–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Marte,B.M., Warne,P.H. and Downward,J. (1996) Phosphatidylinositol 3′ kinase: one of the effectors of Ras. Philos. Trans. R. Soc. Lond. B Biol. Sci., 351, 225–231. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Warne,P.H., Khwaja,A., Marte,B.M., Pappin,D., Das,P., Waterfield,M.D., Ridley,A. and Downward,J. (1997) Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell, 89, 457–467. [DOI] [PubMed] [Google Scholar]
- Sander E.E., ten Klooster,J.P., van Delft,S., van der Kammen,R.A. and Collard,J.G. (1999) Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol., 147, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Kunz,J. and Hall,M.N. (1996) TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl Acad. Sci. USA, 93, 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G., Nordstrom,J., Carbone,R., Tenca,P., Giardina,G., Gutkind,S., Bjarnegard,M., Betsholtz,C. and Di Fiore,P.P. (1999) EPS8 and E3B1 transduce signals from Ras to Rac. Nature, 401, 290–293. [DOI] [PubMed] [Google Scholar]
- Settleman J., Narasimhan,V., Foster,L.C. and Weinberg,R.A. (1992) Molecular cloning of cDNAs encoding the GAP-associated protein p190: implications for a signaling pathway from ras to the nucleus. Cell, 69, 539–549. [DOI] [PubMed] [Google Scholar]
- Soisson S.M., Nimnual,A.S., Uy,M., Bar-Sagi,D. and Kuriyan,J. (1998) Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell, 95, 259–268. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sasaki,T., Mammoto,A., Takaishi,K., Kameyama,T., Tsukita,S. and Takai,Y. (1997) Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem., 272, 23371–23375. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sasaki,T., Mammoto,A., Hotta,I., Takaishi,K., Imamura,H., Nakano,K., Kodama,A. and Takai,Y. (1998) Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl. Oncogene, 16, 3279–3284. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sasaki,T., Tanaka,K. and Nakanishi,H. (1995) Rho as a regulator of the cytoskeleton. Trends Biochem. Sci., 20, 227–231. [DOI] [PubMed] [Google Scholar]
- Tolias K.F. and Cantley,L.C. (1999) Pathways for phosphoinositide synthesis. Chem. Phys. Lipids, 98, 69–77. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Oishi,K., Sato,N., Sagara,J. and Kawai,A. (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J. Cell Biol., 126, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Kikuchi,A., Ohga,N., Yamamoto,J. and Takai,Y. (1990) Purification and characterization from bovine brain cytosol of a novel regulatory protein inhibiting the dissociation of GDP from and the subsequent binding of GTP to rhoB p20, a ras p21-like GTP-binding protein. J. Biol. Chem., 265, 9373–9380. [PubMed] [Google Scholar]
- Van Aelst L. and D’Souza-Schorey,C. (1997) Rho GTPases and signaling networks. Genes Dev., 11, 2295–2322. [DOI] [PubMed] [Google Scholar]
- Wennstrom S., Hawkins,P., Cooke,F., Hara,K., Yonezawa,K., Kasuga,M., Jackson,T., Claesson-Welsh,L. and Stephens,L. (1994) Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr. Biol., 4, 385–393. [DOI] [PubMed] [Google Scholar]
- Whitehead I.P., Campbell,S., Rossman,K.L. and Der,C.J. (1997) Dbl family proteins. Biochim. Biophys. Acta, 1332, F1–F23. [DOI] [PubMed] [Google Scholar]
- Xu N., McCormick,F. and Gutkind,J.S. (1994) The non-catalytic domain of ras-GAP inhibits transformation induced by G protein coupled receptors. Oncogene, 9, 597–601. [PubMed] [Google Scholar]
- Yoshii S. et al. (1999) αPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene, 18, 5680–5690. [DOI] [PubMed] [Google Scholar]
- Yuspa S.H., Dlugosz,A.A., Cheng,C.K., Denning,M.F., Tennenbaum,T., Glick,A.B. and Weinberg,W.C. (1994) Role of oncogenes and tumor suppressor genes in multistage carcinogenesis. J. Invest. Dermatol., 103, 90S–95S. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Bender,A. and Cerione,R.A. (1995a) Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae.J. Biol. Chem., 270, 626–630. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Hart,M.J. and Cerione,R.A. (1995b) Guanine nucleotide exchange catalyzed by dbl oncogene product. Methods Enzymol., 256, 77–84. [DOI] [PubMed] [Google Scholar]
- Zhou K., Wang,Y., Gorski,J.L., Nomura,N., Collard,J. and Bokoch,G.M. (1998) Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J. Biol. Chem., 273, 16782–16786. [DOI] [PubMed] [Google Scholar]
- Zohn I.M., Campbell,S.L., Khosravi-Far,R., Rossman,K.L. and Der,C.J. (1998) Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene, 17, 1415–1438. [DOI] [PubMed] [Google Scholar]