Abstract
The aorta–gonad–mesonephros (AGM) region is a potent hematopoietic site within the mammalian embryo body, and the first place from which hematopoietic stem cells (HSCs) emerge. Within the complex embryonic vascular, excretory and reproductive tissues of the AGM region, the precise location of HSC development is unknown. To determine where HSCs develop, we subdissected the AGM into aorta and urogenital ridge segments and transplanted the cells into irradiated adult recipients. We demonstrate that HSCs first appear in the dorsal aorta area. Furthermore, we show that vitelline and umbilical arteries contain high frequencies of HSCs coincident with HSC appearance in the AGM. While later in development and after organ explant culture we find HSCs in the urogenital ridges, our results strongly suggest that the major arteries of the embryo are the most important sites from which definitive HSCs first emerge.
Keywords: AGM/AML1/embryo/Runx1/vitelline
Introduction
Definitive hematopoietic stem cells (HSCs) are the cells responsible for the continuous production of all mature blood cells during the entire adult life span of an individual (Lemischka, 1991; Spangrude et al., 1991). They are clinically important cells in transplantation protocols used in therapies for blood-related diseases. Experimentally, HSCs are defined as cells that can confer long-term reconstitution of the entire hematopoietic system of an irradiated adult recipient. To understand the biology of HSCs better, particularly the factors leading to their induction, expansion and maintenance, much research has focused on their developmental origins (Dzierzak et al., 1998). During mouse ontogeny, the first HSCs appear in the intra-embryonic aorta–gonad–mesonephros (AGM) region at day 10 of gestation (E10) (Muller et al., 1994; Medvinsky and Dzierzak, 1996). From E11 onwards, HSCs are also found in the yolk sac and fetal liver (Moore and Metcalf, 1970; Huang and Auerbach, 1993; Muller et al., 1994; Medvinsky and Dzierzak, 1996). However, the AGM region is the most potent source of adult repopulating HSCs (Medvinsky and Dzierzak, 1996), which are thought to colonize the fetal liver and subsequently the bone marrow, the main site of hematopoiesis in the adult.
The AGM region is an anatomically complex tissue forming several organ systems in addition to hematopoietic cells. The AGM is derived from the mesodermal germ layer and generally extends from the forelimbs to the hindlimbs of the E9.5–E12.5 mouse embryo. The first discernible tissue formed in the corresponding region of the E8 mouse embryo is the vasculature of the paired dorsal aortas (Rugh, 1990; Kaufman, 1992). At E8.5, the aortas are linked to the yolk sac vasculature through the vitelline artery, and at E9 the paired aortas fuse to form a single midline dorsal aorta (Garcia-Porrero et al., 1995). The umbilical artery forms the connection between the dorsal aorta and the placenta. The urogenital system begins to form at E9 (Rugh, 1990; Kaufman, 1992). Differentiation of the embryonic excretory system begins with the pronephric ducts at E9 and the mesonephric ducts at E10. By late E11, the pro/mesonephric ducts are regressing and by E12 the metanephros (definitive kidney) has become prominent in the caudal aspect of the embryo. The gonads begin to be discernible at E10 (Rugh, 1990; Kaufman, 1992) and primordial germ cells (PGCs) migrate into them. By E12 the gonads have enlarged significantly and are prominent ventral to the mesonephroi. As hematopoietic cells have been detected in association with each of the three developing organ systems (see below), it is of great interest to determine where within the actively differentiating AGM region the first HSCs develop. Insight into the spatial and temporal occurrence of this process is a prerequisite for further studies into the cellular and molecular interactions involved in the generation of definitive HSCs.
Descriptive studies performed initially in the chick (Dieterlen-Lievre and Martin, 1981) and subsequently in mammalian embryos (Garcia-Porrero et al., 1995; Tavian et al., 1996) suggest that the aorta may be the site where definitive hematopoietic cells emerge. These investigations demonstrated that discrete clusters of hematopoietic cells are closely associated with the ventral wall of the dorsal aorta. In the mouse and the human these clusters are found from E9.5 to E11.5 (Garcia-Porrero et al., 1995) and from E27 to E40 (Tavian et al., 1999), respectively, and cells within the clusters express hematopoietic markers (Tavian et al., 1996; Wood et al., 1997; Garcia-Porrero et al., 1998; Marshall et al., 1999; North et al., 1999). Genetic analyses support the hypothesis that the intra-aortic clusters represent definitive hematopoietic cells. For example, mutation of the Tie-2 (Takakura et al., 1998) and Cbfa2 (known also as AML1 and Runx1) (North et al., 1999) genes disrupts both definitive hematopoiesis and the formation of intra-aortic clusters. Finally, in vivo labeling experiments in the chick suggest a precursor–progeny relationship between aortic endothelial cells and intra-aortic clusters (Jaffredo et al., 1998).
Several descriptive and functional studies indicate that the urogenital ridges (UGRs) may also serve as hematopoietic tissues. In non-mammalian vertebrates the presumptive mesonephric anlagen generate hematopoietic precursors and the pro/mesonephros serves as a hematopoietic site (Turpen et al., 1981). The abundant presence of hematopoietic cells together with the expression of hematopoietic related genes such as SCL, GATA-2, Flk-1 and Fli-1 in the amphibian (Walmsley et al., 1994; Bertwistle et al., 1996) and fish (Gering et al., 1998; Brown et al., 2000) pronephros suggests that this site is important in the formation of the definitive adult hematopoietic system. In the mouse, CFU-S8 (colony-forming unit-spleen day 8) (Medvinsky et al., 1996) and CFU-S11 (M.de Bruijn, M.Peeters, T.Luteijn, P.Visser, N.Speck and E.Dzierzak, submitted) are found in the UGRs, as well as in the aorta, starting at E10 of development. It is unclear whether CFU-S are seeded into or are generated in situ in the UGRs since the circulation is contiguous and blood cells actively move throughout the embryo beginning at E8.5 (Cumano et al., 1996). Several hematopoietic markers are expressed in the mouse UGRs at the time of AGM HSC generation, including SCL (Sanchez et al., 1999) and GATA-3 (Lakshmanan et al., 1999), which are expressed in all mesonephric tubules, Ly-6E (Sca-1) in the anterior mesonephric tubules (Miles et al., 1997), and CD34 throughout the mesonephros (Wood et al., 1997). In addition, GATA-2 appears to play a critical role in urogenital development (Zhou et al., 1998). However, the functional relevance of these genes in the development of HSCs within the UGRs of mammals remains to be established.
It has also been suggested that PGCs in the developing gonads may contribute to the hematopoietic lineage (Rich, 1995). Mouse PGCs not only express the c-kit receptor tyrosine kinase (Geissler et al., 1988), a common hematopoietic marker expressed on HSCs (Sanchez et al., 1996), but can also, under certain culture conditions, yield embryonic stem (ES)-like cells that can be differentiated in vitro into hematopoietic cells (Rich, 1995). In summary, the available data suggest that the aorta, mesonephros and gonads are all possible sites for the development of hematopoietic cells. However, the precise site from which definitive HSCs first emerge is unknown.
Here we present the results of experiments in which we determine the localization of HSCs within the mouse AGM region. We subdissected AGM regions from genetically marked E11–E12 embryos into parts containing either the aorta or the UGRs, and tested cell suspensions prepared from these tissues for functional HSCs by in vivo transplantion into irradiated adult mice. We find that at E11 HSCs reside only in the aorta region of the subdissected AGM. Results of transplantation experiments with other major embryonic vessels, the vitelline and umbilical arteries, indicate that these tissues also contain HSCs. Thus, the major arterial regions of the mouse embryo are the first and most potent tissues involved in the development of HSCs. At E12 the UGRs contain HSCs as well. Data obtained from the transplantation of cultures of E11 UGR explants suggest that the HSCs increase autonomously in the UGRs. The studies presented here are the first to demonstrate functionally that definitive HSCs develop from the major arterial regions (dorsal aorta, vitelline and umbilical vessels) of the vertebrate embryo and strongly support a close relationship between the developing hematopoietic and vascular systems.
Results
HSCs develop within the dorsal aorta and surrounding mesenchyme of the subdissected E11 AGM
To locate HSCs within the AGM region we subdissected it longitudinally, separating the dorsal aorta with its surrounding mesenchyme from the UGRs (Figure 1). E11 was chosen as the earliest time for analysis since the developing tissues are well demarcated, allowing precise subdissections. Also, at E11 HSCs are present in sufficiently high numbers to be frequently detected in freshly isolated tissues (Muller et al., 1994). To compare the frequency of HSCs in the aorta with that in the UGRs, cell suspensions were made from the subdissected tissues of genetically marked embryos and injected intravenously at limiting dilutions into irradiated adult recipient mice. Mice were analyzed for donor-type hematopoietic reconstitution at 4–9 months post-transplantation by a donor-specific semi-quantitative PCR assay on DNA isolated from peripheral blood.
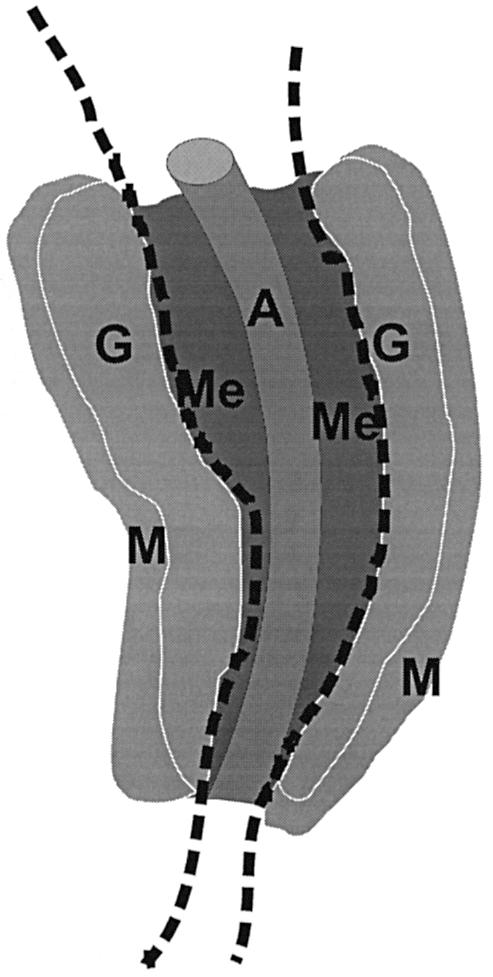
Fig. 1. Schematic representation of subdissected regions of the AGM. E11 and E12 AGM tissue is dissected along the dotted lines to separate the dorsal aorta and surrounding mesenchyme from the urogenital ridges, the latter of which contain the developing gonads and pro/mesonephroi. A, dorsal aorta; Me, mesenchyme; G, gonad; M, mesonephros.
As shown in Table I, direct transplantation of 1 or 0.3 embryo equivalents (e.e.) of E11 aorta and mesenchyme yielded high level donor-type repopulation. Successfully transplanted recipients of either 1 or 0.3 e.e. of dorsal aorta (7/17 and 4/15, respectively) showed high level engraftment (average 90%) in the peripheral blood (Figure 2A). In contrast, at the same embryonic donor tissue equivalents, HSCs could not be detected, not even at low levels (<10%) of engraftment in recipients transplanted with UGR cells (Table I). To determine whether a small number of HSCs are present in the UGRs, high numbers of UGR tissue equivalents were also transplanted. No donor cell derived engraftment was found when up to 5 e.e. of UGR cells were transplanted, suggesting that at E11 the UGR contains very little or no HSC activity.
Table I. HSC activity in directly transplanted and cultured E11 and E12 aorta and UGR.
| No. of mice repopulated/total transplanteda |
|||||
|---|---|---|---|---|---|
| Tissue | Embryo equivalent | E11 | E12 | E11 culturedb | E12 culturedb |
| Aortac | 1 | 7/17 | 17/29 | 17/28 | 6/6 |
| 0.3 | 4/15 | 1/6 | 8/20 | 0/6 | |
| UGR | 4.3d | 0/3 | |||
| 1 | 0/10 | 5/26 | 18/26 | 2/6 | |
| 0.3 | 0/9 | 0/4 | 8/19 | 2/5 | |
| AGM | 1 | 7/8 | 7/9 | 9/11 | 4/5 |
| 0.3 | 1/6 | 1/2 | 5/11 | 4/5 | |
aOnly mice with ≥10% donor repopulation in the peripheral blood at 4–9 months after transfer were included as positive. Data are from a total of 21 independent experiments.
bOrgan explants were cultured for 3 days prior to transplantation.
cAorta region: includes surrounding mesenchyme.
dAn average of 4.3 e.e. were transplanted in two experiments (range 4–5).
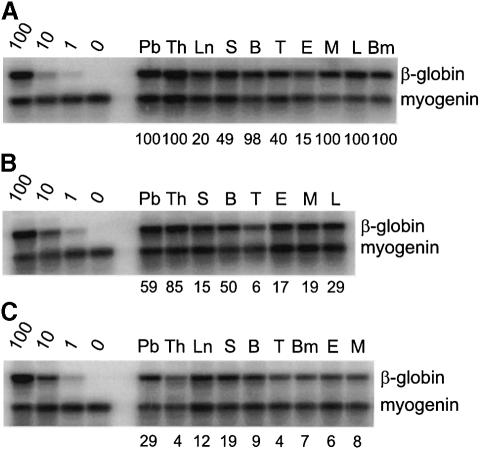
Fig. 2. Multilineage repopulation analysis of transplant recipients engrafted with aorta- or UGR-derived HSCs. Southern blot analysis was performed on products obtained from donor-marker specific PCRs. PCR reactions were performed on DNA isolated from hematopoietic tissues and sorted hematopoietic cells from representative transplant recipients receiving cells from (A) E11 aorta, (B) E12 aorta and (C) E12 UGR. At >4 months post-transplantation, transplant recipients were killed and hematopoietic tissues isolated. Lymphoid, myeloid and erythroid lineage cells were sorted and DNAs examined for the presence of the donor cell genetic marker (human β-globin). Percentage contribution was controlled by mixes of the donor-transgene marker with unmarked DNA (100, 10, 1 and 0%). Donor-marker contribution was determined by PhosphorImager quantitation of specific 32P hybridizing probe. The donor signal was normalized to the hybridization signal for the myogenin PCR product. Percentage donor marker contributions are indicated below each lane. Pb, peripheral blood; Th, thymus; Ln, lymph nodes; S, spleen; B, splenic B lymphocytes; T, splenic T lymphocytes; E, bone marrow erythroid precursors; M, bone marrow myeloid precursors; L, bone marrow lymphoid precursors; Bm, bone marrow.
To test whether the transplantation of E11 aorta region cells results in complete, multilineage hematopoietic repopulation, recipient mice were killed at >4 months post-transplantation and several hematopoietic tissues and cell lineages were tested for the presence of the donor genetic marker. As shown in Figure 2A, PCR analysis of DNAs from all samples shows high level (up to 100%) contribution by donor-marked cells. Furthermore, the bone marrow of such primary recipient mice yields high level hematopoietic engraftment when transplanted into secondary recipients (data not shown), fulfilling the stringent criteria of definitive HSCs. Thus, adult-type HSCs first develop within the dorsal aorta area of the AGM region.
HSCs are also present in the UGRs at E12
We previously found a change in the anterior–posterior distribution of HSCs from E10 to E11 in the mouse AGM region (Medvinsky and Dzierzak, 1996; A.Medvinsky, personal communication). At E10 HSCs were found only in the anterior half of the AGM region, whereas at E11 they were spread throughout the AGM region. Here we examined whether HSC activity expands in a lateral direction during development. We dissected E12 AGMs into aorta and UGRs, and transplanted the cells as described above. In contrast to the E11 UGR, transplantation of 1 e.e. of E12 UGR cells did result in high level (40–100%) donor-type HSC repopulation in five out of 26 mice (Table I). Multilineage hematopoietic repopulation of all hematopoietic tissues was found (Figure 2C), and secondary transplantion of bone marrow from the primary UGR transplanted mice yielded high level peripheral blood contribution (40% engraftment). Thus, the UGR harbors and/or generates true HSCs at E12. However, the highest number of HSCs is still found in the aorta region (17 out of 29 mice were high level engrafted with 1 e.e. of E12 aorta; Table I). Repopulation by aorta region-derived HSCs is multilineage (Figure 2B) and the HSCs can be serially transplanted into secondary recipients yielding up to 80% peripheral blood contribution (not shown). Hence, the high frequency of HSCs in the E12 aorta region suggests that it remains the major site of HSC development.
The complex differentiation of the excretory and reproductive systems within the UGR begins around E10/11. At E12 it is possible to dissect the gonads cleanly from the pro/mesonephros. In addition, the metanephroi (definitive kidneys) are now easily identified and isolated. To determine in which part of the UGR HSCs are localized, we isolated gonads, mesonephroi and metanephroi from E12 genetically marked embryos and transplanted cell suspensions of these tissues into irradiated adult recipients. At >4 months post-transplantation, hematopoietic engraftment was found mainly in mice transplanted with 1–2 e.e. of mesonephroi (four out of 11 recipients) (Table II). Only one out of 10 recipients transplanted with 1–2 e.e. of gonads was positive. Interestingly, one out of two recipient mice transplanted with a high dose (5 e.e.) of metanephric cells was positive. These data demonstrate that the majority of HSCs in the E12 UGR are harbored in the mesonephroi.
Table II. Spatial distribution of HSCs within the E12 UGR.
| Tissue | No. of mice repopulated/total transplanteda |
|---|---|
| Gonads | 1/10 |
| Mesonephroi | 4/11 |
| Metanephroi | 1/2b |
| UGRc | 3/7 |
| Aortad | 9/11 |
| AGMc | 5/6 |
aTransplanted mice received 1–2 e.e. of subdissected tissue. Only mice with ≥10% donor repopulation in the peripheral blood at 4–5 months after transfer were included as positive. Data are from a total of two independent experiments.
bFive embryo equivalents transplanted per mouse.
cNeither UGR nor AGM include metanephroi.
dAorta region: includes surrounding mesenchyme.
HSC numbers increase autonomously in cultured E11 UGRs
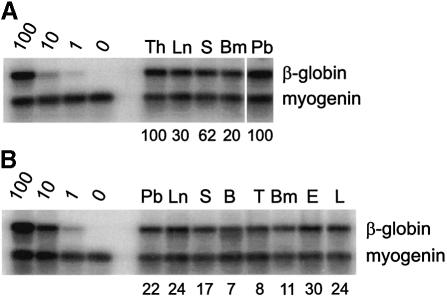
There are several possible explanations for the 1 day delay in the appearance of HSCs in the UGR. (i) The UGR is seeded by definitive HSCs between E11 and E12. (ii) The UGR is seeded by very low numbers of HSCs that migrated there before E11. These HSCs then expand in the UGR, reaching detectable levels by E12. (iii) HSCs develop autonomously in the UGR. (iv) The UGR is seeded by pre-definitive HSCs by E11 (Medvinsky and Dzierzak, 1999). To begin to discriminate between these possibilities we isolated E11 UGRs and performed an organ explant culture before transplantation. We previously showed that the organ explant culture allows for the expansion of detectable HSC activity (Medvinsky and Dzierzak, 1996). Separation of the E11 UGR from the remaining embryonic tissues, followed by explant culture, will prevent migration of HSCs into the UGR between E11 and E12. As shown in Table I, a remarkable increase in HSC activity was found in the cultured E11 UGR compared with the uncultured E11 UGR (18/26 versus 0/10; 1 e.e./mouse). Cultures of E11 aorta region, performed for comparison, also showed an increase in HSC activity, though less dramatic than the increase in the UGR cultures. The engraftment by cultured E11 UGR and aorta is due to definitive HSCs, since they confer multilineage hematopoietic repopulation (see Figure 3). HSC activity is maintained at the same levels in E12 cultured UGR compared with uncultured E12 UGR (2/6 versus 5/26). These explant culture data indicate that HSC numbers can be dramatically increased in E11 UGR, independent of HSC influx from the aorta region between E11 and E12. The HSCs in the UGR must therefore have either migrated to the UGR in very limited numbers by E11, or developed autonomously in the UGR during the culture period.
Fig. 3. Multilineage repopulation analysis of transplant recipients engrafted with cultured aorta- or UGR-derived HSCs. DNAs were isolated from hematopoietic tissue and sorted hematopoietic cells from representative transplant recipients receiving cells from (A) E11 cultured aorta and (B) E11 cultured UGRs. At >4 months post-transplantation, transplant recipients were killed and hematopoietic tissues isolated. Lymphoid, myeloid and erythroid lineage cells were sorted and DNAs examined for the presence of the donor cell genetic marker (human β-globin) as described in Figure 2.
To determine whether signaling between the aorta and UGR influences in a positive or negative manner the HSC activity in either tissue at E11, we prepared co-cultures of genetically marked aorta with unmarked UGR explants, and of unmarked aorta with marked UGR explants. One subdissected UGR was placed on each side of the aorta region to restore the normal AGM architecture. Following 3 days of co-culture the cells were tested for HSC activity (Table III). The repopulating activity found in 1 e.e. of UGR cultured alone (8/14 recipients) versus 1 e.e. of UGR cultured with aorta (7/14) was similar. The repopulating activity in the 1 e.e. of aorta cultured alone (7/14) compared with that of 1 e.e. of aorta cultured in the presence of the UGR (5/14) was also similar. Thus, HSC generation in either the aorta or UGR appears to occur at the same efficiency regardless of whether the aorta and UGR are cultured alone or in direct juxtaposition, suggesting that there is neither an activating nor repressing signal delivered by one tissue that impacts on HSC generation in the other.
Table III. HSC activity in co-cultured E11 aorta and UGR.
| No. of mice repopulated/total transplanteda |
|||
|---|---|---|---|
| Marked tissue | Embryo equivalent | Cultured alone | Co-culturedb |
| Aorta | 1 | 7/14 | 5/14 |
| 0.3 | 4/12 | 3/11 | |
| UGR | 1 | 8/14 | 7/14 |
| 0.3 | 5/11 | 4/14 | |
aOnly mice with ≥10% donor repopulation in peripheral blood at 4–6 months after transfer were included as positive. Data are from a total of five independent experiments.
bMarked aorta regions were cultured for 3 days with unmarked UGRs, and marked UGRs with unmarked aortas. Marked and unmarked co-cultured tissues were injected together.
The spatial and temporal expression pattern of Cbfa2 in the AGM coincides with the presence of HSCs
The expression patterns of known markers of definitive HSCs have been studied to identify the source of HSCs in complex tissues. One such recently described marker is CBFα2, a transcription factor important in the development of definitive hematopoietic cells. Homozygous disruption of the Cbfa2 gene leads to a complete deficiency in all definitive hematopoietic lineages in the developing mouse embryo, resulting in fetal liver anemia and lethality at E12 (Okuda et al., 1996; Wang et al., 1996). No HSCs are found in the AGM of Cbfa2-deficient embryos, suggesting that the AGM is the site of the primary defect (Z.L.Cai, M.de Bruijn, X.Ma, B.Dortland, T.Luteijn, J.R.Downing and E.Dzierzak, submitted). Cbfa2 is expressed in the ventral endothelium of the dorsal aorta, the ventral para-aortic mesenchyme and in the clusters of hematopoietic cells associated with the ventral wall of the aorta (North et al., 1999). Furthermore, Cbfa2 is required for the formation of intra-aortic hematopoietic clusters (North et al., 1999). Taken together, these results suggest that Cbfa2 is expressed by the first HSCs as they arise in the mouse embryo. Since we observe HSCs in the UGRs of E11 cultured and E12 uncultured AGM regions, we tested whether Cbfa2 was expressed in the UGRs at these times.
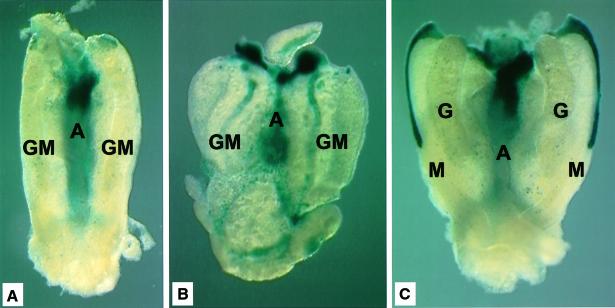
Embryos that express lacZ from the Cbfa2 gene (North et al., 1999) were dissected and stained with the β-galactosidase substrate X-gal. As shown in Figure 4A, a representative E11 AGM region shows a high degree of staining in the aorta and surrounding mesenchyme. However, little staining (at the anterior-most tips of the pronephros) is observed in the UGR, although serial sections reveal the presence of isolated Cbfa2-expressing cells scattered throughout the UGR (not shown). At E11 HSCs are found in the directly transplanted aorta, but not in the UGRs (Table I). In contrast, strong X-gal staining is observed in the UGRs of E11 cultured and E12 uncultured AGMs (Figure 4B and C, respectively), times at which functional HSCs are present there (Table I). In the UGRs of the E12 AGM, Cbfa2 expression is most prominent in the Mullerian ducts in the mesonephroi, again coincident with the presence of HSCs in the mesonephroi (Table II). Thus, Cbfa2 expression coincides with the spatial and temporal presence of HSCs in the aorta and UGRs.
Fig. 4. Cbfa2-LacZ expression in subregions of the AGM. X-gal staining was performed on (A) E11 AGM directly after dissection, (B) E11 AGM after 3 days in organ explant culture and (C) E12 AGM directly after dissection. Abundant blue staining is observed in the area containing the dorsal aorta and mesenchyme in all three tissues. However, the UGRs stained brightly only at E12 or after explant culture of E11 AGMs. The stripe of expression in the anterior mesonephros at E12 is from the Mullerian duct. Whether HSC activity is located in the strongly X-gal-positive Mullerian ducts or elsewhere in the mesonephroi remains to be determined.
HSCs are present in the vitelline and umbilical arteries at high frequency beginning at E10.5
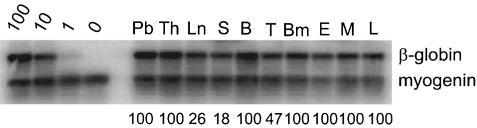
The enrichment of HSCs in the aorta region of the AGM prompted us to extend our analyses to the other major arteries present at this time in the mouse conceptus: the vitelline and umbilical vessels. Histological and functional studies (Garcia-Porrero et al., 1995; Takakura et al., 1998), as well as the Cbfa2 expression pattern (North et al., 1999), suggested the presence of definitive hematopoietic cells in these vessels, but the presence of HSCs had not been assessed. We isolated the vitelline and umbilical vessels from E10 through E12 embryos and assayed them by adoptive transfer for the presence of HSCs. At early E10 (<35 somite pairs) no HSC activity is detectable in the vitelline and umbilical arteries even when large numbers of cells (up to 4.5 e.e.) are transplanted. However, beginning at E10.5, HSCs are found in limiting numbers (Table IV). Direct transplantation of 2.5–5 e.e. of the vitelline and umbilical vessels resulted in four highly engrafted recipients out of 35 transplanted. The frequency of successfully engrafted recipients increases at E11 (three out of eight recipients with 1 e.e.) and remains high at E12 (10 out of 22 with 1 e.e.). Mice engrafted with vitelline and umbilical arteries show long-term multilineage hematopoietic repopulation of all hematopoietic tissues (Figure 5), as well as high level hematopoietic repopulation of secondary recipients with bone marrow from primary engrafted animals (not shown). These results demonstrate that the vitelline and umbilical vessels harbor true HSCs. To determine whether HSCs are generated autonomously in this tissue, explant cultures were attempted, but were not successful. Nevertheless, since HSCs were not found in E10–12 body remnants or in the circulating blood by direct transplantation (our unpublished results; Muller et al., 1994), the data indicate that the vitelline and umbilical vessels are in fact a potent source of definitive HSCs. At all time points the frequencies of HSCs in the vitelline plus umbilical vessels and AGM regions transplanted from the same embryos were very similar. Thus, the vitelline and umbilical arteries, along with the dorsal aorta region of the AGM, are a major source of definitive HSCs.
Table IV. HSC activity in directly transplanted vitelline/umbilical arteries.
| Embryo | No. of mice repopulated/total transplanteda |
|||
|---|---|---|---|---|
| Tissue | equivalent | E10.5 | E11.5 | E12.5 |
| Vitelline/umbilical | 2.5–5 | 4/35b | ||
| 3 | 3/7 | 22/24 | ||
| 1 | 3/8 | 10/22 | ||
| 0.3 | 1/3 | 2/16 | ||
| 0.1 | 2/8 | |||
| AGM | 2.5–3 | 1/12 | 16/16 | |
| 1 | 4/6 | 14/22 | ||
| 0.3 | 3/6 | 6/14 | ||
| 0.1 | 1/3 | 0/10 | ||
| Liverc | 3 | 0/3 | 2/2 | |
| 1 | 2/6 | |||
| 0.3 | 1/2 | |||
aWith one exception (see below), only mice with ≥10% donor repopulation at 4–7 months after transfer were included as positive. Data are from a total of 12 independent experiments.
bOne mouse transplanted with 5 e.e. of umbilical arteries was engrafted at high levels (100%) at 3 months post-transplantation, but died before the >4 month bleed could be performed.
cLiver was used in these experiments as a positive control and thus is not mentioned in the text of the Results.
Fig. 5. Multilineage repopulation analysis of a transplant recipient engrafted with E10 vitelline and umbilical arteries. At >4–7 months post-transplantation, transplant recipients that received vitelline and umbilical arteries were killed and hematopoietic tissues isolated. The results from a representative multilineage analysis are shown. Lymphoid, myeloid and erythroid lineage cells were sorted and DNAs examined for the presence of the donor cell genetic marker (human β-globin) as described in Figure 2.
Discussion
Vascular arterial localization of definitive HSCs
The ontogenic association of hematopoietic cells with the vasculature has long been observed in the blood islands of the mammalian yolk sac (Sabin, 1917) and together with genetic studies has led to the notion that endothelial and hematopoietic cells differentiate from a common progenitor. The targeted disruption of the vascular endothelial growth factor (VEGF) (Carmeliet et al., 1996; Ferrara et al., 1996) and the Flk-1 receptor tyrosine kinase (Shalaby et al., 1995, 1997) genes in mouse embryos results in complete deficiencies in both the vascular and hematopoietic systems, strongly implicating a close developmental relationship between these lineages. However, the acceptance of an intra-embryonic origin for adult mammalian hematopoiesis is only recent (Dzierzak and Medvinsky, 1995). Hence, the origin of adult hematopoietic cells (and in particular definitive HSCs) within the specific vascular regions of the mammalian embryo body is highly speculative. Many descriptive, immuno- and histochemical analyses in mouse and human embryos using markers associated with definitive HSCs and progenitors have demonstrated clusters of hematopoietic cells in close association with the floor of the dorsal aorta (Garcia-Porrero et al., 1995; Tavian et al., 1996). The expression of hematopoietic markers such as CD34, SCL, CD31, AA4.1 and CBFα2 is shared with endothelial cells lining the dorsal aorta (Baldwin et al., 1994; Wood et al., 1997; Marshall et al., 1999; North et al., 1999; Petrenko et al., 1999; Sanchez et al., 1999) and closely parallels results observed in the avian species (Drake et al., 1997; McNagny et al., 1997; Jaffredo et al., 1998). Targeted disruption of Tie-2 selectively disrupts vascular remodeling and definitive hematopoiesis (Sato et al., 1995; Takakura et al., 1998), and disruption of the Cbfa2 gene blocks both definitive hematopoiesis (Okuda et al., 1996; Wang et al., 1996) and the formation of intra-aortic clusters (North et al., 1999). Taken together, these studies strongly suggest that the mammalian dorsal aortic endothelium and/or surrounding mesenchymal cells are the source of definitive hematopoietic cells. However, none of these studies directly investigated whether functional definitive HSCs, the putative source of the adult hematopoietic system, are localized at high frequencies within the mammalian dorsal aorta.
We demonstrate here that the dorsal aorta with its surrounding mesenchyme is indeed a potent site of functional HSC generation. Subdissections of the E11 AGM region show that only the aortic region contains definitive HSCs at this time in development. At a later developmental time, the aortic region remains the most potent source of HSCs within the AGM region. We also show for the first time that functional definitive HSCs are present within the vitelline and umbilical arteries. The presence of HSCs in these vessels at E10.5 coincides with their first appearance in the AGM. Furthermore, our data show that the number of HSCs within the vitelline and umbilical vessels appears to be similar to the number of HSCs in the AGM region. Thus, the first and most potent sites of definitive HSC activity are associated with the major vasculature of the mouse embryo: the dorsal aorta and the vitelline and umbilical arteries.
Remarkably, these are the only vessels in both avian and mouse embryos in which clusters of hematopoietic cells were found to be attached to the lumenal wall (Dieterlen-Lievre and Martin, 1981; Garcia-Porrero et al., 1995). In addition, the CBFα2 transcription factor is expressed in the clusters of hematopoietic cells and in the endothelial cells lining both the ventral wall of the dorsal aorta and the vitelline and umbilical arteries (North et al., 1999). The hematopoietic cells appear to be budding from the aortic endothelium, thus suggesting an endothelial precursor to definitive hematopoietic cells. Direct labeling studies in chick embryos show that cells lining the dorsal aorta, most likely endothelial cells, give rise to intra-aortic hematopoietic clusters (Jaffredo et al., 1998). If hemogenic endothelial cells do in fact exist (Dieterlen-Lievre, 1998; Nishikawa et al., 1998a,b), they may be present only at specific developmental times and anatomical positions. Since the first adult repopulating HSCs appear in the dorsal aorta when it is an intact and functioning vessel, it is likely that the immediate precursor to the definitive HSC is an endothelial cell that maintains plasticity and potential for the hematopoietic lineage. However, at this developmental time the aorta is continuing to increase in length as well as diameter (Kaufman, 1992). Hemangioblasts may still be present and contribute to this vascular growth as well as give rise to HSCs in the inductive microenvironment of the existing ventral endothelium. Another alternative for the direct precursor to adult repopulating HSCs may be a type of multipotential hematopoietic cell that acquires characteristics needed for adult hematopoietic repopulation. Putative precursors may include the neonatal repopulating cells found in the E9 AGM and yolk sac (YS) (Yoder et al., 1997), or the in vitro clonable multipotential progenitors found in E7.5 paraaortic splanchnopleura and E8.5 YS (Cumano et al., 1996).
Sublocalization of other hematopoietic progenitors within the AGM
Recently, several other hematopoietic progenitors have been sublocalized within the mouse AGM region. In general, these immature and more mature hematopoietic progenitors are localized within the aortic region. The spatial and temporal mapping of CFU-S8 show that these progenitors are present in both the aorta and the UGRs at E10 (Medvinsky et al., 1996). Although the frequency of CFU-S8 is always highest in aortic tissue, by late E10 the absolute number of CFU-S8 in the UGRs is higher than in the aorta (2- to 4-fold), suggesting that these progenitors may be made in the aortic area but preferentially colonize and/or expand in the UGR. The distribution of the more immature CFU-S11 progenitors over the E10 and E11 AGM has been found to be slightly higher in absolute numbers and in frequency in the aorta region. Later, at E12 in development, equal frequencies are found in the aorta and UGRs. Thus, CFU-S predominate early in the aorta region, with activity spreading to the UGRs.
B-cell precursors also predominate (in absolute number and frequency) in the aorta region, but are present as well in the mesonephros, gonad and mesentery of E10 embryos (Godin et al., 1999). Additionally, in vitro clonable multilineage precursors are found in the aorta region (Godin et al., 1999), but no data are available from the other subdissected regions. Thus, the aorta region contains the greatest numbers of HSCs as well as immature and more mature progenitors, supporting the notion that this vascular region (together with the vitelline and umbilical arteries) is the most potent generator of the definitive adult hematopoietic system. It is interesting to speculate that definitive HSCs, along with the variety of other functional hematopoietic progenitors detected in the aorta region, may reside together in the hematopoietic clusters associated with the arterial walls, as suggested by differential expression of glycoprotein IIb–IIIa on the cells within the aortic clusters (Ody et al., 1999).
The mesonephros as a site of hematopoietic cell growth
The presence of functional definitive HSCs in the mesonephros of the mouse embryo is a curious but not unexpected finding. The pronephros in amphibians and fish is a well-described site of hematopoiesis (Turpen et al., 1981; Walmsley et al., 1994; Bertwistle et al., 1996; Gering et al., 1998). IgM-positive B cells are found in the mesonephros of the chick (Zettergren and Cutlan, 1992) and also in Rana pronephros and mesonephros (Zettergren, 1982). Macrophages are detected in UGRs of E11.5 mouse embryos (De Felici et al., 1986), and as described above, hematopoietic progenitors are also found in the UGRs of E10 and E11 mouse embryos. Since the mesonephros is formed from intermediate mesoderm (Larsen, 1997), the presence of HSCs in the mesonephros suggests that plasticity for the hematopoietic lineage may be maintained in the mesoderm. Indeed, many hematopoietic-associated genes (Cbfa2, GATA-2, GATA-3, SCL, Sca-1, CD34, Flk-1) (Miles et al., 1997; Wood et al., 1997; Robert et al., 1998; Zhou et al., 1998; Lakshmanan et al., 1999; North et al., 1999; Sanchez et al., 1999) are expressed in the developing excretory system (mesonephros, metanephros) and particularly in the Wolffian and Mullerian ducts. Since vasculogenesis rather than angiogenesis is thought to occur in the UGRs (Robert et al., 1998), it is interesting to speculate that these vascular precursors may still possess hematopoietic potential and form some HSCs in situ.
Alternatively, localized migration of hematopoietic cells (or cells with hematopoietic potential) into the E11 mesonephros may occur, since it has been reported that cells in the mouse embryo readily migrate from the mesonephros to the gonads at E10/E11 (Martineau et al., 1997; Perez-Aparicio et al., 1998). If the source of definitive HSCs in the mouse is the aorta, the presence of these cells in the mesonephros (and at low frequencies in the gonads and metanephros) would support a lateral pattern of interstitial migration. Regardless of the mechanism by which HSCs appear in the UGR, the UGR is clearly a potent site for HSC activity, as is shown by the dramatic increase in HSC activity in E11 UGR explant cultures. The nature of this increase, be it expansion of limited numbers of HSCs already present or de novo generation of HSCs in isolated UGR, remains to be established.
While our studies do not directly address the question of PGCs as the precursors to HSCs, the frequency of PGCs in the gonads at E12 suggests that they are not a potent source of HSCs. Studies examining the migration and expansion of PGCs in mid-gestation mouse embryos estimate the number of PGCs in the gonads at E12 to be between 2000 and 2500 (Chiquoine, 1954). This number of PGCs, if they were to possess HSC potential, would be readily detected in the gonads by our adoptive transfer method. Our studies show very little hematopoietic repopulation with transplanted gonadal cells. Although we transplanted low embryo equivalents of subdissected gonads and mesonephroi, the number of HSCs found in the subdissected mesonephroi was always similar to the number of HSCs in the whole UGR. Thus, the low frequency of HSCs in the gonads renders it unlikely that the definitive adult hematopoietic system takes its origins from PGCs in this tissue at E12. However, fate mapping and the testing of PGCs in the microenvironment of the aorta, mesenchyme or mesonephros will have to be carried out to address this issue definitively.
In conclusion, we have demonstrated that the vascular regions, the dorsal aorta and the vitelline and umbilical arteries of the mid-gestation mouse embryo are the first and most potent generators of definitive HSCs. The anatomical link between functional HSCs and endothelial and mesenchymal cells in these arterial regions strongly supports the notion that a common progenitor for these lineages exists at this time in development. Our novel functional findings provide important cellular starting points for examining the molecular signals necessary for the generation of the first definitive HSCs.
Materials and methods
Embryo generation
To generate embryos, timed matings were set up between genetically marked male mice and wild-type (C57BL/10 × CBA)F1 or C57BL/10 females. The day of vaginal plugging was counted as day 0. The genetically marked mouse lines used in this study were Ln72 [human β-globin transgenic (Strouboulis et al., 1992)], BL1b [Ly-6E (Sca-1)/LacZ transgenic (Miles et al., 1997)] and Cbfa2lz/+ [Cbfa2-lacZ knock in (North et al., 1999)]. For embryo generation, the transgene was always inherited through the male parent so as to avoid contribution by genetically marked maternal cells in transplantation assays. Animals were housed according to institutional guidelines, with free access to food and water. Animal procedures were carried out in compliance with the Standards for Humane Care and Use of Laboratory Animals.
Cell preparations
Pregnant mice were killed by cervical dislocation. Procedures for embryo and AGM dissection were essentially as described before (Muller et al., 1994) and are given in detail by Dzierzak and de Bruijn (2000). Subdissections were performed using 27 G needles (Becton-Dickinson). Umbilical arteries and veins were severed with scissors at the points of attachment to the placenta and at the embryo proper. Vitelline vessels were also cut with scissors, along with a small external loop of gut. Circulating blood was washed from the vessels. Tissues were either incubated directly with collagenase (type I, Sigma; final concentration 0.12% v/v) for 1 h at 37°C in phosphate-buffered saline (PBS) supplemented with 10% fetal calf serum and penicillin/streptomycin, or cultured in explant organ culture for 3 days (Medvinsky and Dzierzak, 1996) prior to collagenase digestion. Cells were washed, resuspended in PBS, and transplanted intravenously into irradiated mice. To control for the contribution of circulating hematopoietic cells, body remnants or circulating embryonic blood were transplanted at the same cell doses as the test cell populations examined in these studies. No donor hematopoietic contribution could be detected in these transplanted control cell populations (our unpublished results). Cells were counted in a Bürker hemocytometer using Trypan blue to exclude dead cells. Cells were kept on ice at all times subsequent to collagenase digestion until the time of injection.
Analysis of long-term multilineage repopulating activity
Embryonic cell suspensions were assayed for the presence of definitive HSCs by intravenous transfer into irradiated adult recipients as described (Muller et al., 1994; Dzierzak and de Bruijn, 2000). Briefly, (C57BL/10 × CBA)F1 recipients (male or female) were exposed to a split dose of 900 rad of γ-irradiation from a 137Cs source. Adult (C57BL/10 × CBA)F1 spleen cells (2 × 105) were co-injected with the embryonic cells to promote short-term survival. Recipient mice were bled at 1 and >4 months after transfer and analyzed for percentage donor contribution by donor marker-specific PCR on DNA isolated from peripheral blood (Muller et al., 1994; Miles et al., 1997; Dzierzak and de Bruijn, 2000). Reconstitution was evaluated by ethidium bromide staining of agarose gels and in some cases by Southern blot hybridization as described previously (Muller et al., 1994; Medvinsky and Dzierzak, 1996; Dzierzak and de Bruijn, 2000). To test for long-term multilineage hematopoietic repopulation, genomic DNA was isolated from peripheral blood, thymus, lymph node, splenic B and T cells, and bone marrow myeloid, lymphoid and erythroid cells. Percentage donor-cell contribution was analyzed by PCR followed by Southern blotting. Antibodies used for sorting the different cell lineages from spleen and bone marrow were RA3-6B2 (anti-B220 for B cells), a combination of 53-6.7 and H129.19 (anti-CD8a and anti-CD4, respectively, for T cells) and a combination of MEC13.3 and ER-MP20 (anti-CD31 and anti-Ly-6C, respectively, for bone marrow myeloid, lymphoid and erythroid cells; de Bruijn et al., 1998). All antibodies were directly conjugated (PharMingen; ER-MP20–fluorescein isothiocyanate was a kind gift of Dr P.J.M.Leenen, Rotterdam, The Netherlands). Cells were sorted using a FACS Vantage SE (Becton-Dickinson). The purity of the sorted cells exceeded 95%.
Bone marrow cells of primary long-term (>4 months) reconstituted mice were transplanted into 900-rad-irradiated adult (C57BL/10 × CBA)F1 recipients (3 × 106 nucleated cells per mouse) to test for the ability of the donor HSCs to repopulate secondary recipients. Secondary recipients were analyzed at >1 month post-transplantation.
Analysis of Cbfa2 expression
Cbfa2lz/+ mice were described previously (North et al., 1999). β-galactosidase activity in freshly dissected or cultured AGM regions was monitored after staining with the X-gal substrate as described previously (North et al., 1999).
Acknowledgments
Acknowledgements
The authors sincerely thank all the members of the laboratory for help in various aspects of this work, particularly in the lively and challenging discussions. We also thank Dr A.Medvinsky, Dr I.Lemischka, Professor F.Grosveld and Dr D.Meijer for useful suggestions and critical comments on this manuscript. We thank the EDC for care and maintenance of our transplantation mice. This work is supported by the Netherlands Research Council 901-08-090 (M.F.T.R.d.B., E.D.), Nederlandse Kankerbestrijding EUR 99-1965 (M.C.E.P., E.D.), Leukemia Society of America Scholar Award 103-94 (E.D.), National Institutes of Health ROCA58343 and Fogarty Award FO6TW03300 (N.A.S.) and the National Institutes of Health R01 DK51077-01 (E.D.).
References
- Baldwin H.S. et al. (1994) Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development, 120, 2539–2553. [DOI] [PubMed] [Google Scholar]
- Bertwistle D., Walmsley,M.E., Read,E.M., Pizzey,J.A. and Patient,R.K. (1996) GATA factors and the origins of adult and embryonic blood in Xenopus: responses to retinoic acid. Mech. Dev., 57, 199–214. [DOI] [PubMed] [Google Scholar]
- Brown L., Rodaway,A., Schilling,T., Jowett,T., Ingham,P., Patient,R. and Sharrocks,A. (2000) Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor FLi-1 in wild-type and mutant zebrafish embryos. Mech. Dev., 90, 237–252. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature, 380, 435–439. [DOI] [PubMed] [Google Scholar]
- Chiquoine A. (1954) The identification, origin and migration of the primordial germ cells in the mouse embryo. Anat. Rec., 118, 135–146. [DOI] [PubMed] [Google Scholar]
- Cumano A., Dieterlen-Lievre,F. and Godin,I. (1996) Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell, 86, 907–916. [DOI] [PubMed] [Google Scholar]
- de Bruijn M.F.T.R., van Vianen,W., Ploemacher,R.E., Bakker-Woudenberg,I.A., Campbell,P.A., van Ewijk,W. and Leenen,P.J.M. (1998) Bone marrow cellular composition in Listeria monocytogenes infected mice detected using ER-MP12 and ER-MP20 antibodies: a flow cytometric alternative to differential counting. J. Immunol. Methods, 217, 27–39. [DOI] [PubMed] [Google Scholar]
- De Felici M., Heasman,J., Wylie,C.C. and McLaren,A. (1986) Macrophages in the urogenital ridge of the mid-gestation mouse fetus. Cell. Differ., 18, 119–129. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F. (1998) Hematopoiesis: Progenitors and their genetic program. Curr. Biol., 8, R727–R730. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F. and Martin,C. (1981) Diffuse intraembryonic hemopoiesis in normal and chimeric avian development. Dev. Biol., 88, 180–191. [DOI] [PubMed] [Google Scholar]
- Drake C.J., Brandt,S.J., Trusk,T.C. and Little,C.D. (1997) TAL1/SCL is expressed in endothelial progenitor cells/angioblasts and defines a dorsal-to-ventral gradient of vasculogenesis. Dev. Biol., 192, 17–30. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. and de Bruijn,M. (2000) Isolation and analysis of hematopoietic stem cells from mouse embryos. In Klug,C. and Jordan,C. (eds), Methods in Molecular Medicine: Hematopoietic Stem Cell Protocols. Humana Press, Totowa, NJ, in press. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. and Medvinsky,A. (1995) Mouse embryonic hematopoiesis. Trends Genet., 11, 359–366. [DOI] [PubMed] [Google Scholar]
- Dzierzak E., Medvinsky,A. and de Bruijn,M. (1998) Qualitative and quantitative aspects of haemopoietic cell development in the mammalian embryo. Immunol. Today, 19, 228–236. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore,K., Chen,H., Dowd,M., Lu,L., O’Shea,K.S., Powell-Braxton,L., Hillan,K.J. and Moore,M.W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature, 380, 439–442. [DOI] [PubMed] [Google Scholar]
- Garcia-Porrero J.A., Godin,I.E. and Dieterlen-Lievre,F. (1995) Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. (Berl.), 192, 425–435. [DOI] [PubMed] [Google Scholar]
- Garcia-Porrero J.A., Manaia,A., Jimeno,J., Lasky,L.L., Dieterlen-Lievre,F. and Godin,I.E. (1998) Antigenic profiles of endothelial and hemopoietic lineages in murine intraembryonic hemogenic sites. Dev. Comp. Immunol., 22, 303–319. [DOI] [PubMed] [Google Scholar]
- Geissler E.N., Ryan,M.A. and Housman,D.E. (1988) The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell, 55, 185–192. [DOI] [PubMed] [Google Scholar]
- Gering M., Rodaway,A.R., Gottgens,B., Patient,R.K. and Green,A.R. (1998) The SCL gene specifies haemangioblast development from early mesoderm. EMBO J., 17, 4029–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin I., Garcia-Porrero,J.A., Dieterlen-Lievre,F. and Cumano,A. (1999) Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J. Exp. Med., 190, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. and Auerbach,R. (1993) Identification and characterization of hematopoietic stem cells from the yolk sac of the early mouse embryo. Proc. Natl Acad. Sci. USA, 90, 10110–10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T., Gautier,R., Eichmann,A. and Dieterlen-Lievre,F. (1998) Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development, 125, 4575–4583. [DOI] [PubMed] [Google Scholar]
- Kaufman M. (1992) The Atlas of Mouse Development. Academic Press, London, UK. [Google Scholar]
- Lakshmanan G., Lieuw,K.H., Lim,K.C., Gu,Y., Grosveld,F., Engel,J.D. and Karis,A. (1999) Localization of distant urogenital system-, central nervous system- and endocardium-specific transcriptional regulatory elements in the GATA-3 locus. Mol. Cell. Biol., 19, 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen W. (1997) Human Embryology. Churchill Livingstone, New York, NY. [Google Scholar]
- Lemischka I.R. (1991) Clonal, in vivo behavior of the totipotent hematopoietic stem cell. Semin. Immunol., 3, 349–355. [PubMed] [Google Scholar]
- Marshall C.J., Moore,R.L., Thorogood,P., Brickell,P.M., Kinnon,C. and Thrasher,A.J. (1999) Detailed characterization of the human aorta–gonad–mesonephros region reveals morphological polarity resembling a hematopoietic stromal layer. Dev. Dyn., 215, 139–147. [DOI] [PubMed] [Google Scholar]
- Martineau J., Nordqvist,K., Tilmann,C., Lovell-Badge,R. and Capel,B. (1997) Male-specific cell migration into the developing gonad. Curr. Biol., 7, 958–968. [DOI] [PubMed] [Google Scholar]
- McNagny K.M., Pettersson,I., Rossi,F., Flamme,I., Shevchenko,A., Mann,M. and Graf,T. (1997) Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J. Cell Biol., 138, 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A. and Dzierzak,E. (1996) Definitive hematopoiesis is autonomously initiated by the AGM region. Cell, 86, 897–906. [DOI] [PubMed] [Google Scholar]
- Medvinsky A. and Dzierzak,E. (1999) Development of the hematopoietic stem cell: can we describe it? Blood, 94, 3613–3614. [PubMed] [Google Scholar]
- Medvinsky A.L., Gan,O.I., Semenova,M.L. and Samoylina,N.L. (1996) Development of day-8 colony-forming unit-spleen hematopoietic progenitors during early murine embryogenesis: spatial and temporal mapping. Blood, 87, 557–566. [PubMed] [Google Scholar]
- Miles C., Sanchez,M.-J., Sinclair,A. and Dzierzak,E. (1997) Expression of the Ly-6E.1 (Sca-1) transgene in adult hematopoietic stem cells and the developing mouse embryo. Development, 124, 537–547. [DOI] [PubMed] [Google Scholar]
- Moore M.A. and Metcalf,D. (1970) Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br. J. Haematol., 18, 279–296. [DOI] [PubMed] [Google Scholar]
- Muller A.M., Medvinsky,A., Strouboulis,J., Grosveld,F. and Dzierzak,E. (1994) Development of hematopoietic stem cell activity in the mouse embryo. Immunity, 1, 291–301. [DOI] [PubMed] [Google Scholar]
- Nishikawa S.I., Nishikawa,S., Kawamoto,H., Yoshida,H., Kizumoto,M., Kataoka,H. and Katsura,Y. (1998a) In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity, 8, 761–769. [DOI] [PubMed] [Google Scholar]
- Nishikawa S.-I., Nishikawa,S., Hirashima,M., Matsuyoshi,N. and Kodama,H. (1998b) Progressive lineage analysis by cell sorting and culture identifies FLK+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development, 125, 1747–1757. [DOI] [PubMed] [Google Scholar]
- North T., Gu,T.-L., Stacy,T., Wang,Q., Howard,L., Binder,M., Marin-Padilla,M. and Speck,N. (1999) Cbfa2 is required for the formation of intraaortic hematopoietic clusters. Development, 126, 2563–2575. [DOI] [PubMed] [Google Scholar]
- Ody C., Vaigot,P., Quere,P., Imhof,B.A. and Corbel,C. (1999) Glycoprotein IIb–IIIa is expressed on avian multilineage hematopoietic progenitor cells. Blood, 93, 2898–2906. [PubMed] [Google Scholar]
- Okuda T., van Deursen,J., Hiebert,S.W., Grosveld,G. and Downing,J.R. (1996) AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell, 84, 321–330. [DOI] [PubMed] [Google Scholar]
- Perez-Aparicio F.J., Carretero,A., Navarro,M. and Ruberte,J. (1998) The lack of genital ridge vascularization in the early chick embryo: implications in the migration of the primordial germ cells. Anat. Rec., 251, 398–405. [DOI] [PubMed] [Google Scholar]
- Petrenko O., Beavis,A., Klaine,M., Kittappa,R., Godin,I. and Lemischka,I.R. (1999) The molecular characterization of the fetal stem cell marker AA4. Immunity, 10, 691–700. [DOI] [PubMed] [Google Scholar]
- Rich I.N. (1995) Primordial germ cells are capable of producing cells of the hematopoietic system in vitro. Blood, 86, 463–472. [PubMed] [Google Scholar]
- Robert B., St. John,P.L. and Abrahamson,D.R. (1998) Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am. J. Physiol., 275, F164–172. [DOI] [PubMed] [Google Scholar]
- Rugh R. (1990) The Mouse: Its Reproduction and Development. Oxford University Press, Oxford, UK. [Google Scholar]
- Sabin F.R. (1917) Origin and development of the primitive vessels of the chick and of the pig. Contrib. Embryol. Carnegie Inst., 226, 61–124. [Google Scholar]
- Sanchez M., Gottgens,B., Sinclair,A.M., Stanley,M., Begley,C.G., Hunter,S. and Green,A.R. (1999) An SCL 3′ enhancer targets developing endothelium together with embryonic and adult haematopoietic progenitors. Development, 126, 3891–3904. [DOI] [PubMed] [Google Scholar]
- Sanchez M.J., Holmes,A., Miles,C. and Dzierzak,E. (1996) Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity, 5, 513–525. [DOI] [PubMed] [Google Scholar]
- Sato T.N. et al. (1995) Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature, 376, 70–74. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant,J., Yamaguchi,T.P., Gertsenstein,M., Wu,X.F., Breitman,M.L. and Schuh,A.C. (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376, 62–66. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Ho,J., Stanford,W.L., Fischer,K.D., Schuh,A.C., Schwartz,L., Bernstein,A. and Rossant,J. (1997) A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell, 89, 981–990. [DOI] [PubMed] [Google Scholar]
- Spangrude G.J., Smith,L., Uchida,N., Ikuta,K., Heimfeld,S., Friedman,J. and Weissman,I.L. (1991) Mouse hematopoietic stem cells. Blood, 78, 1395–1402. [PubMed] [Google Scholar]
- Strouboulis J., Dillon,N. and Grosveld,F. (1992) Developmental regulation of a complete 70-kb human β-globin locus in transgenic mice. Genes Dev., 6, 1857–1864. [DOI] [PubMed] [Google Scholar]
- Takakura N., Huang,X.L., Naruse,T., Hamaguchi,I., Dumont,D.J., Yancopoulos,G.D. and Suda,T. (1998) Critical role of the TIE2 endothelial receptor in the development of definitive hematopoiesis. Immunity, 9, 677–689. [DOI] [PubMed] [Google Scholar]
- Tavian M., Coulombel,L., Luton,D., Clemente,H.S., Dieterlen-Lievre,F. and Peault,B. (1996) Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood, 87, 67–72. [PubMed] [Google Scholar]
- Tavian M., Hallais,M.F. and Peault,B. (1999) Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development, 126, 793–803. [DOI] [PubMed] [Google Scholar]
- Turpen J.B., Knudson,C.M. and Hoefen,P.S. (1981) The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev. Biol., 85, 99–112. [DOI] [PubMed] [Google Scholar]
- Walmsley M.E., Guille,M.J., Bertwistle,D., Smith,J.C., Pizzey,J.A. and Patient,R.K. (1994) Negative control of Xenopus GATA-2 by activin and noggin with eventual expression in precursors of the ventral blood islands. Development, 120, 2519–2529. [DOI] [PubMed] [Google Scholar]
- Wang Q., Stacy,T., Binder,M., Marin-Padilla,M., Sharpe,A.H. and Speck,N.A. (1996) Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl Acad. Sci. USA, 93, 3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H.B., May,G., Healy,L., Enver,T. and Morriss-Kay,G.M. (1997) CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood, 90, 2300–2311. [PubMed] [Google Scholar]
- Yoder M.C., Hiatt,K., Dutt,P., Mukherjee P., Bodine,D.M. and Orlic,D. (1997) Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity, 7, 335–344. [DOI] [PubMed] [Google Scholar]
- Zettergren L.D. (1982) Ontogeny and distribution of cells in B lineage in the American leopard frog, Rana pipiens. Dev. Comp. Immunol., 6, 311–320. [DOI] [PubMed] [Google Scholar]
- Zettergren L.D. and Cutlan,R.T. (1992) Immunoglobulin-containing cells in chick embryo urogenital tissues: a new site for early B lineage cells in endothermic vertebrates. J. Exp. Zool., 262, 458–461. [DOI] [PubMed] [Google Scholar]
- Zhou Y. et al. (1998) Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J., 17, 6689–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]