Abstract
Hormones and neurotransmitters mobilize Ca2+ from the endoplasmic reticulum via inositol trisphosphate (IP3) receptors, but how a single target cell encodes different extracellular signals to generate specific cytosolic Ca2+ responses is unknown. In pancreatic acinar cells, acetylcholine evokes local Ca2+ spiking in the apical granular pole, whereas cholecystokinin elicits a mixture of local and global cytosolic Ca2+ signals. We show that IP3, cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) evoke cytosolic Ca2+ spiking by activating common oscillator units composed of IP3 and ryanodine receptors. Acetylcholine activation of these common oscillator units is triggered via IP3 receptors, whereas cholecystokinin responses are triggered via a different but converging pathway with NAADP and cyclic ADP-ribose receptors. Cholecystokinin potentiates the response to acetylcholine, making it global rather than local, an effect mediated specifically by cyclic ADP-ribose receptors. In the apical pole there is a common early activation site for Ca2+ release, indicating that the three types of Ca2+ release channels are clustered together and that the appropriate receptors are selected at the earliest step of signal generation.
Keywords: cyclic ADP-ribose/inositol trisphosphate/NAADP/oscillator units/pancreatic acinar cells
Introduction
Crabtree (1999), reviewing generic Ca2+ signals and specific outcomes, has pointed out that, ‘A surprising and yet vexing outcome of the rapid progress made in understanding signal transduction is the observation that while activation of specific receptors leads to highly specific biologic responses, these receptors seem to use ubiquitous signalling intermediates’. One of the ubiquitous signalling intermediates is Ca2+ (Pozzan et al., 1994; Berridge, 1997; Parekh and Penner, 1997). It is generally accepted that hormone- or neurotransmitter-elicited intracellular Ca2+ release is mediated via inositol 1,4,5-trisphosphate (IP3) generation and activation of IP3 receptors in the endoplasmic reticulum (ER) (Berridge, 1997). However, in pancreatic acinar cells, acetylcholine (ACh) and cholecystokinin (CCK) can induce specific cytosolic Ca2+ signatures. ACh elicits repetitive short-lasting cytosolic Ca2+ spikes in the apical granular pole. CCK evokes the same type of repetitive short-lasting local Ca2+ spikes, but at certain intervals a short spike triggers a much longer lasting global Ca2+ transient. The frequency of such global transients increases with increasing CCK concentrations, within the physiological range (1–10 pM) (Osipchuk et al., 1990; Petersen,C. et al., 1991; Thorn et al., 1993; Petersen,O. et al., 1994). These agonist-selective Ca2+ signatures cannot be explained simply by the IP3 pathway. Different patterns of IP3 generation (Hirose et al., 1999) or phosphorylation of the IP3 receptors (LeBeau et al., 1999), or different couplings of Ca2+ release to Ca2+ entry (Parekh and Penner, 1997) could play a role, but it would also seem reasonable to consider the possibility that other Ca2+-releasing messengers may be of functional importance.
Both cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) release Ca2+ from sea-urchin egg microsomes (Galione, 1993; Genazzani and Galione, 1997; Lee, 1997), and in pancreatic acinar cells it has been shown that both cADPR (Thorn et al., 1994) and NAADP (Cancela et al., 1999) can elicit cytosolic Ca2+ spiking. Furthermore, both functional cADPR and NAADP receptors are essential for the cytosolic Ca2+ spiking responses evoked by CCK (Cancela et al., 1998, 1999). Nevertheless, it would appear that the action of CCK is also dependent on functional IP3 receptors, since the responses are inhibited by blocking these receptors with heparin (Thorn and Petersen, 1993).
In general, the actions of most hormones and neurotransmitters, which release Ca2+ from internal stores, are blocked by heparin used as an IP3 receptor antagonist, but the possible involvement of cADPR and NAADP receptors has not been tested (Petersen and Cancela, 1999). It is therefore not known whether the more complex mechanism of action that has emerged recently for the action of CCK on pancreatic acinar cells (Cancela et al., 1998, 1999) is generally valid or whether different neurotransmitters and hormones could have different ways of mobilizing Ca2+ from internal stores. The pancreatic acinar cell represents an excellent model system to investigate this problem, as two different extracellular agonists, the hormone CCK and the neurotransmitter ACh, both act to release Ca2+ from intracellular stores (Petersen et al., 1994).
We show here that whereas the CCK-induced Ca2+ signalling response is completely dependent on NAADP, cADPR and IP3 receptors, the ACh-elicited Ca2+ spiking cannot be inhibited by blocking NAADP and cADPR receptors, but does depend on functional IP3 receptors. We also show that both ACh- and CCK-elicited Ca2+ signalling are completely dependent on both IP3 and ryanodine receptors, since all responses can be blocked both by ryanodine and caffeine. We demonstrate that CCK markedly potentiates ACh-elicited responses, by changing the repetitive local Ca2+ spikes to much longer lasting global transients, and show that this novel phenomenon is specifically dependent on functional cADPR receptors. Finally, using a fast confocal acquisition protocol, we have identified a common early activation site for ACh- and CCK-induced Ca2+ release in the apical pole. We conclude that in a single target cell, separate neurotransmitter/hormone-evoked cytosolic Ca2+ signals are encoded by different combinations of intracellular Ca2+ release channels, which serve as separate routes to stimulate common Ca2+ oscillator units made up of IP3 and ryanodine receptors.
Results
The importance of cADPR receptors
The pyridine nucleotide metabolite cADPR releases Ca2+ from sea-urchin egg microsomes (Lee, 1997) by acting on ryanodine receptors to stimulate Ca2+-induced Ca2+ release (Galione et al., 1991; Meszaros et al., 1993). cADPR can elicit cytosolic Ca2+ spiking in pancreatic acinar cells (Thorn et al., 1994) and cADPR receptors are involved in the response to a physiological CCK stimulus, since the cADPR antagonist 8-NH2-cADPR (Walseth and Lee, 1993; Cancela and Petersen, 1998) blocks Ca2+ spiking induced by this hormone (Cancela et al., 1998). It is not known whether cADPR receptors are generally involved in agonist-elicited Ca2+ signalling or only play a role in certain pathways. We have therefore compared the effect of intracellular 8-NH2-cADPR on the responses elicited by ACh and CCK, using the patch–clamp whole-cell recording configuration (Hamill et al., 1981). With this technique, putative Ca2+-releasing messengers and antagonists placed in the pipette solution have access to the cytosol, and the Ca2+-dependent current, which has previously been shown to be a sensitive measure of local cytosolic Ca2+ concentration changes in the apical pole of pancreatic acinar cells, can be monitored (Petersen, 1992; Thorn et al., 1993, 1994, 1996; Petersen et al., 1994; Tinel et al., 1999).
In all experiments, we used low levels of CCK and ACh, just above threshold concentrations. The CCK concentrations used (2 and 5 pM) are physiological (Walsh, 1994). There are two types of CCK receptor: A and B. The type A receptor mediates the Ca2+ signal, which activates the enzyme secretion, and has a much higher affinity for CCK than gastrin. The type B receptor is also referred to as the gastrin receptor, as it has equal affinities for CCK and gastrin (Jensen, 1994). The cytosolic Ca2+ signals elicited by CCK in pancreatic acinar cells are clearly due to activation of type A receptors, since: (i) the gastrin concentrations required to elicit Ca2+ signals (and the resulting membrane potential and conductance changes) are several orders of magnitude higher than the CCK concentrations needed to induce such effects (Petersen, 1980; Jensen, 1994); and (ii) only the CCK type A, and not the type B receptor is expressed in normal mouse pancreatic acinar cells (Saillan-Barreau et al., 1998).
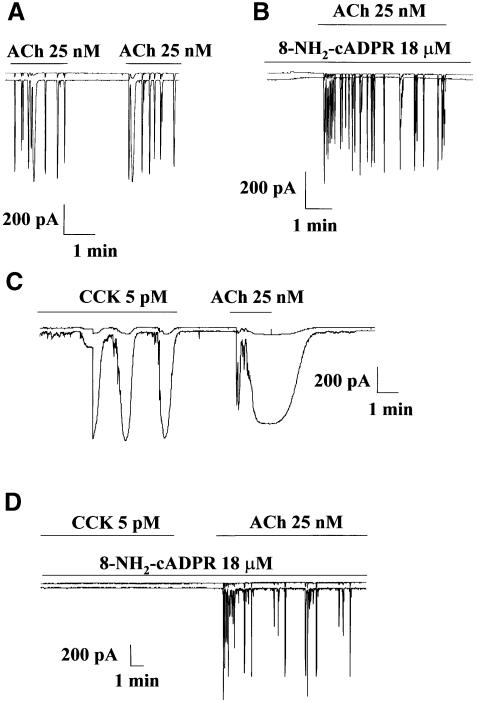
Figure 1A shows the repetitive spikes of Ca2+-sensitive current evoked by a very low (just above threshold) concentration of ACh in freshly isolated mouse pancreatic acinar cells (Petersen,C. et al., 1991; Petersen,O. et al., 1994). When the cADPR antagonist 8-NH2-cADPR was included in the intracellular pipette solution, the ACh-evoked response was not blocked (Figure 1B). In the control cells, ACh (25 nM) evoked repetitive spiking responses in all nine cells tested. When 8-NH2-cADPR was present in the intracellular pipette solution at a concentration of 18 µM [a concentration previously shown to block Ca2+ spiking evoked by 5 pM CCK (Cancela et al., 1998) and 10 µM cADPR, but not 10 µM IP3 (Cancela and Petersen, 1998)], all 10 cells tested also responded to ACh by firing repetitive spikes of Ca2+-dependent current.
Fig. 1. Blockade of cADPR receptors by 8-NH2-cADPR abolishes Ca2+ spiking elicited by CCK, but not by ACh. Repetitive spikes of Ca2+-sensitive current were evoked by a low, just above threshold, concentration of ACh (A). When the cADPR antagonist 8-NH2-cADPR was included in the intracellular pipette solution, the ACh-evoked response was not blocked (B). In a series of experiments in which the effects of ACh and CCK were tested in the same cells, CCK was initially applied and a response was observed. Shortly thereafter, ACh was applied and a much larger response than normal was obtained (C). In the presence of 8-NH2-cADPR in the intracellular pipette solution, CCK failed to evoke any response, but the subsequent ACh application evoked a normal, non-potentiated, response (D).
We also tested the effects of ACh and CCK in the same cells. In the control experiments, CCK in a physiological concentration (5 pM) evoked a clear response (Figure 1C). When ACh (25 nM) was applied shortly after a period of CCK stimulation, the response obtained was much larger than normal (Figure 1C). The ACh-elicited short-lasting Ca2+ spikes (Figure 1A and B) had been converted into a prolonged continuous Ca2+ increase (Figure 1C). This CCK-evoked potentiation of the ACh response was observed in five of the seven cells tested using this protocol. In the presence of 18 µM 8-NH2-cADPR in the intracellular pipette solution, 5 pM CCK failed, as expected (Cancela and Petersen, 1998; Cancela et al., 1998), to evoke any response in the five cells tested, but subsequent ACh application evoked a normal, non-potentiated, response in all of the same five cells (Figure 1D). The CCK-evoked potentiation of the ACh response therefore depends on cADPR receptor activation. In a previous study (Petersen et al., 1991), in which the actions of CCK and ACh were studied in the same cells, CCK potentiation of a subsequent ACh response was not observed. Interestingly, in that study (Petersen et al., 1991), the intracellular pipette solution always contained 10 mM glucose, which is now known to abolish cADPR-elicited, but not IP3-induced, Ca2+ spiking (Cancela et al., 1998). Although potentiation of ACh responses involves cADPR receptors, it is clear that the basic action of ACh, in contrast to the case for CCK, does not depend on functional intracellular cADPR receptors (Figure 1).
The importance of intracellular NAADP receptors
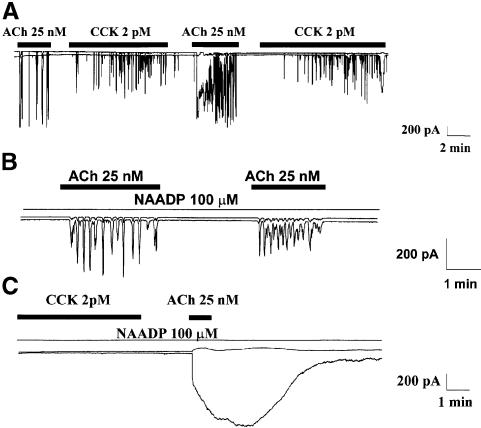
The next step was to investigate the role of NAADP, a novel Ca2+-releasing agent in sea urchin eggs (Chini et al., 1995; Lee and Aarhus, 1995; Genazzani and Galione, 1997; Lee, 1997). In sea urchin eggs, it would appear that the NAADP-sensitive stores are physically separated from those sensitive to IP3 and cADPR (Genazzani and Galione, 1997; Lee, 1997). In pancreatic acinar cells, NAADP has recently been shown to be involved in CCK-evoked Ca2+ signalling (Cancela et al., 1999), but it is not known whether NAADP receptors are generally involved in agonist-evoked Ca2+ release, like IP3 and ryanodine receptors, or whether they are only used in certain specific pathways. NAADP-sensitive signalling exhibits a remarkable self-desensitization mechanism (Aarhus et al., 1996; Genazzani et al., 1996; Cancela et al., 1999), which provides a good tool. When a high desensitizing concentration (100 µM) of NAADP was included in the pipette solution, the response to CCK (2 and 5 pM) was markedly inhibited or abolished (Cancela et al., 1999). However, as seen in Figure 2, the response to ACh was not inhibited by the high intracellular NAADP concentration. In the control series (without NAADP), 11 of the 16 cells tested responded to ACh (25 nM). In the test series of 16 cells, with 100 µM NAADP in the pipette, ACh evoked the usual responses in 11 cells.
Fig. 2. Blockade of NAADP receptors by a high desensitizing concentration of NAADP abolishes Ca2+ spiking in response to CCK, but not to ACh stimulation. A low, physiological, CCK concentration (2 pM) evoked a potentiation of the ACh response (A). The response to ACh was not inhibited by 100 µM NAADP in the intracellular pipette solution (B). The CCK mediated potentiation was not blocked by NAADP, even though NAADP had abolished the Ca2+ signal evoked by CCK (C).
We also tested the effects of both CCK and ACh in the same cells with or without NAADP in the pipette solution. As seen in Figure 2A, CCK (2 pM) potentiated the ACh response, while ACh did not potentiate the CCK response (n = 4). The CCK-mediated potentiation was not blocked by 100 µM NAADP in the cell interior, even though NAADP had abolished the Ca2+ signal normally evoked by this CCK concentration (Figure 2C). CCK (2 pM) evoked responses in four of the five control cells tested, but no responses were recorded in the three test cells (with NAADP) although they all subsequently responded vigorously to ACh (potentiated response). These results indicate that ACh-evoked Ca2+ signal generation, in contrast to CCK-induced Ca2+ spiking, is independent of NAADP receptors. It is also clear that NAADP receptors, in contrast to cADPR receptors, do not play any role in CCK-evoked potentiation of the ACh response. In pancreatic acinar cells, the NAADP receptors are therefore specifically of importance for CCK-elicited Ca2+ signal generation.
The importance of ryanodine receptors
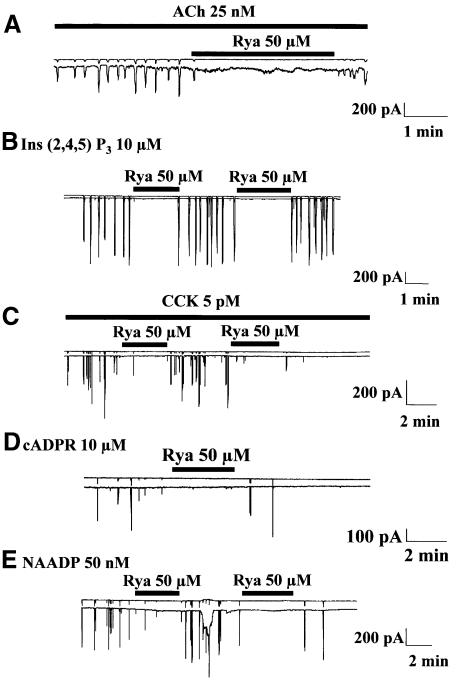
There is evidence for the existence of intimate functional relationships between ryanodine and IP3 receptors (Boittin et al., 1998). In pancreatic acinar cells, ryanodine has been shown to inhibit responses elicited by ACh, CCK or cADPR, but in a previous study, ryanodine (10 µM) failed to inhibit IP3-induced Ca2+ spiking, although ruthenium red (50 µM) markedly reduced the spiking frequency (Thorn et al., 1994). We have tested the effects of ryanodine on the Ca2+ spiking responses elicited by NAADP and reinvestigated the actions of ryanodine on the Ca2+ oscillations elicited by ACh, IP3, CCK and cADPR. In general, ryanodine has complex effects on the ryanodine receptors, since low concentrations tend to open the channels, whereas higher concentrations are more likely to evoke closure. Ryanodine also binds more easily to open channels than to closed ones; this causes the use-dependence phenomenon (Sutko et al., 1997). Initially using ryanodine concentrations of 10–50 µM, we observed enhanced spiking in the presence of 10 µM ryanodine, whereas at 50 µM, ryanodine evoked inhibition of Ca2+ spiking; we therefore focused our attention on this concentration in later studies [25 nM ACh, n = 7; 10 µM IP3 (the non-metabolizable analogue inositol 2,4,5-trisphosphate was used), n = 3; 5 pM CCK, n = 6; 10 µM cADPR, n = 5; 50 nM NAADP, n = 3]. Figure 3 illustrates the strongly inhibitory or blocking effects of ryanodine on Ca2+ spiking. It would appear that ryanodine receptor involvement is required for all Ca2+ spiking responses in pancreatic acinar cells.
Fig. 3. Blockade of ryanodine receptors by ryanodine inhibits all Ca2+ spiking responses. Effects of ryanodine (Rya) (applied externally) on Ca2+ spiking in response to ACh (A), inositol 2,4,5-trisphosphate [Ins(2,4,5)P3] (B), CCK (C), cADPR (D) and NAADP (E).
The importance of IP3 receptors
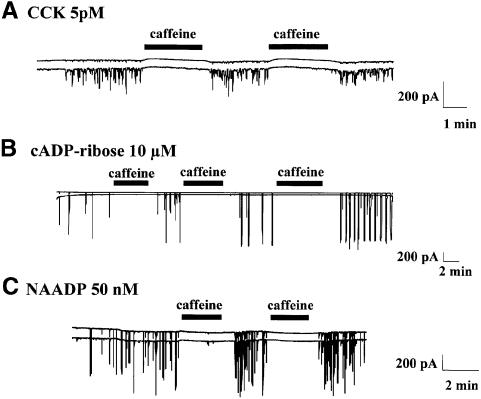
Recent investigations have indicated that not only CCK, but also cADPR and NAADP, could recruit IP3 receptors through a Ca2+-induced Ca2+ release process (Cancela et al., 1999; Petersen and Cancela, 1999). However, the conclusion that the IP3 receptor is involved was based on the inhibitory effect of heparin. Unfortunately, heparin is not only an IP3 antagonist, but also has other effects. For example, it can interact with GTP-binding proteins and therefore uncouple the plasma membrane receptors (Petersen and Cancela, 1999). Caffeine, although best known as an activator of ryanodine receptors, also inhibits the opening of IP3 receptors (Wakui et al., 1990; Parker and Ivorra, 1991; Brown et al., 1992; Ehrlich et al., 1994; Petersen and Cancela, 1999). This effect is clearly not mediated by an increase in the intracellular cyclic AMP concentration (Wakui et al., 1990; Brown et al., 1992), but is likely to be a direct effect on the IP3 receptor or a closely associated protein, since it has been observed in single-channel current studies of isolated IP3 receptors from cerebellum (Ehrlich et al., 1994). Furthermore, caffeine has the advantage of being extremely membrane permeant. It can therefore be applied externally and its effects are rapidly reversible (Wakui et al., 1990; Petersen and Cancela, 1999). It has previously been shown that high caffeine concentrations (10–20 mM) inhibit Ca2+ spiking evoked by ACh, IP3 and various CCK analogues, as well as the responses induced by low caffeine concentrations, which are most likely initiated by activation of ryanodine receptors (Wakui et al., 1990; Thorn et al., 1994; Petersen and Cancela, 1999). This inhibitory effect of a high caffeine concentration is not due to store depletion as the effect is rapidly reversible even in the complete absence of external Ca2+ (Wakui et al., 1990). We have now tested the effects of caffeine on the actions of normal CCK, cADPR and NAADP (Figure 4).
Fig. 4. Blockade of IP3 receptors by caffeine inhibits Ca2+ spiking responses. Effect of caffeine on Ca2+-dependent current spikes evoked by CCK, cADPR and NAADP. Caffeine (20 mM) was applied externally. CCK (5 pM)-evoked Ca2+ spikes were reversibly inhibited by caffeine (A). cADPR and NAADP were present in the internal patch pipette solution at 10 µM or 50 nM, respectively, and application of caffeine reversibly blocked the Ca2+ spikes evoked by both cADPR (B) and NAADP (C).
When caffeine was applied externally to the bath solution, during continuous CCK application, the Ca2+-dependent current spiking was abolished; this inhibitory effect was fully reversible (n = 4). Caffeine reversibly inhibited the cADPR-evoked Ca2+ spikes (n = 7) and reversibly inhibited the Ca2+ spikes evoked by 50 nM NAADP (n = 12). Caffeine and heparin are not very selective drugs, but to our knowledge the only action shared by these two very different compounds is the antagonism of IP3 receptors. Our new caffeine data, taken in conjunction with the previously published heparin data (Wakui et al., 1990; Cancela et al., 1999; Petersen and Cancela, 1999), therefore provide fresh evidence for the involvement of IP3 receptors in the CCK, cADPR and NAADP responses.
Localization of early activation sites
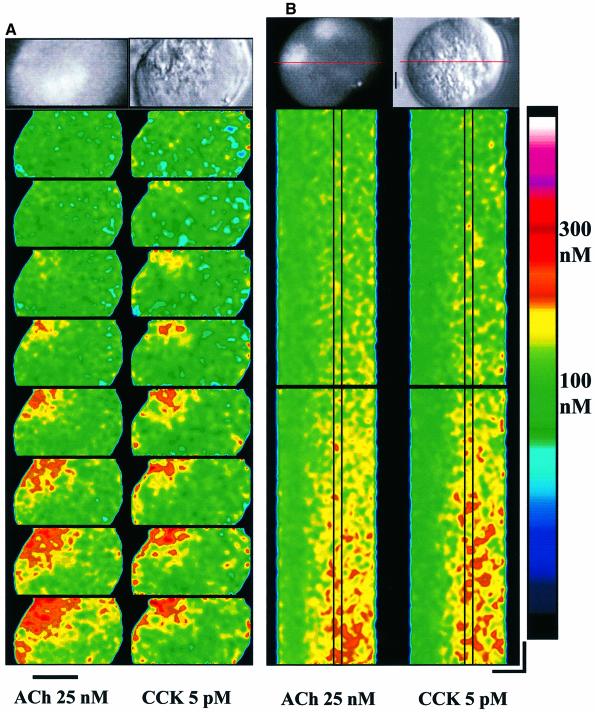
We investigated whether the Ca2+ signalling responses to ACh and CCK share the same Ca2+ release sites. High (supramaximal) concentrations of ACh or CCK elicit global cytosolic Ca2+ increases and these always start in the apical area and then spread as waves towards the basal part of the cells (Kasai and Augustine, 1990; Mogami et al., 1997; Ito et al., 1999). We have now looked at the initiation of the Ca2+ signals at the low concentrations of ACh and CCK (just above threshold) employed in our electropharmacological experiments (Figures 1–4). Figure 5A shows a band-scan experiment, where the effects of ACh and CCK have been compared in the same cell. In both cases the signal is initiated in a part of the secretory pole (∼4 × 4 µm) close to the apical luminal membrane and spreads out in the secretory region in much the same way (n = 4). The band-scan experiments do not allow us to follow the evolution of the Ca2+ signals continuously. We therefore also carried out line-scan experiments. We applied ACh or CCK twice in the same cell and observed that repeated application of the same agonist led to similar initial signal evolutions (ACh, n = 5; CCK, n = 5). We then compared the effects of ACh and CCK in the same cells. Figure 5B shows the result of such an experiment where the scan line goes through one of the nuclei and the apical region. An area with a width of 2 µm, in which early activation sites for Ca2+ release can be seen, has been highlighted. As for other cells (Parker and Yao, 1991; Yao et al., 1995; Parker et al., 1996; Berridge, 1997; Marchant et al., 1999), the evolution of a Ca2+ signal starts with a low frequency of small and short-lasting discrete events in one (or a few) area(s). The earliest events in the pancreatic acinar cells last for ∼20–50 ms, have amplitudes of ∼100–150 nM and spread out less than ∼2 µm. The frequency and amplitude of these events then gradually increase and events begin to occur also in other neighbouring areas, so that there is Ca2+ release in virtually all parts of the granular region (Figure 5B), in general agreement with descriptions in other systems (Parker et al., 1996; Berridge, 1997). As seen in Figure 5, the initial parts of the ACh- and CCK-elicited Ca2+ signal generation were very similar (n = 16), suggesting the existence of converging early release sites stimulated by different routes.
Fig. 5. Confocal fluorescence microscopy reveals similar positions of early activation sites for Ca2+ release by ACh and CCK. Using band scan (A, 8 bands/s; bar corresponds to 10 µm) or line scan (B, horizontal 10 µm, vertical 200 ms), ACh and CCK released Ca2+ from similar early activation sites in the same cells. At the top of each panel are shown, on the right, a transmitted light picture of the cell investigated (the bar corresponds to 2 µm) and, on the left, the fluorescence image showing the position of the nucleus (nuclei). Calibration of the colour coding of the cytosolic Ca2+ concentration for both panels is shown on the right.
Discussion
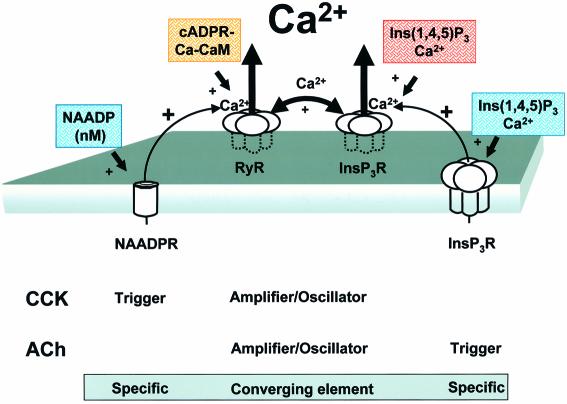
Our experiments demonstrate both the unity and diversity in the agonist-induced cytosolic Ca2+ signalling systems operating in a classic cell biological model, namely the normal pancreatic acinar cell (Figure 6). The common feature of the ACh and the CCK response is the short-lasting Ca2+ spike in the apical pole (Petersen et al., 1991; Thorn et al., 1993). Our new results show that these local Ca2+ spikes evoked by ACh or CCK stimulation always depend on concerted activity of IP3 and ryanodine receptors (Figures 3 and 4). Although IP3, cADPR and NAADP are each capable of eliciting cytosolic Ca2+ spikes (Petersen and Cancela, 1999), our data show that the Ca2+-releasing actions of directly injected IP3, cADPR or NAADP all depend on concerted activation of IP3 and ryanodine receptors. Closely clustered IP3 and ryanodine receptors therefore function as common oscillator units (Figure 6).
Fig. 6. Schematic model showing that CCK and ACh initiate Ca2+ spiking from units of ryanodine and IP3 receptors (InsP3R) via separate intracellular pathways. The peptide hormone CCK initiates Ca2+ release through a specific combination of intracellular Ca2+ release channels with separate functions, where the NAADP receptor (NAADPR) is the trigger, the cADPR receptor via the ryanodine receptor (RyR) is an amplifier and the combination of IP3 receptors and RyRs is the oscillator. The neurotransmitter ACh also acts through a combination of intracellular Ca2+ channels, where the InsP3R is the trigger and the RyR–IP3 complex is the amplifier/oscillator. The RyR is probably recruited by the action of Ca2+ released from the InsP3R, but another unknown messenger cannot be excluded. CaM, calmodulin.
Our data demonstrate that the route to the common oscillator units is quite different for ACh and CCK stimulation. Although functional cADPR and NAADP receptors clearly are not required in order for ACh to evoke repetitive cytosolic Ca2+ spikes, these receptors are obligatory for the Ca2+ spiking responses elicited by CCK (Figures 1 and 2). In sea urchin eggs, NAADP-induced Ca2+ release does not behave as a Ca2+-induced Ca2+ release system, suggesting that the only mechanism for activation of these channels would be by NAADP itself (Chini and Dousa, 1996). Furthermore, in pancreatic acinar cells, NAADP-induced Ca2+ spiking is blocked by a cADPR antagonist, whereas the cADPR-evoked response is not blocked by a high desensitizing concentration of NAADP (Cancela et al., 1999). For the responses initiated by CCK, the NAADP receptors are therefore probably the triggers and recruit cADPR/ryanodine and finally IP3 receptors (Figure 6). Since it is clear that neither cADPR nor NAADP receptors are involved in the pathway initiated by ACh stimulation, it would be simplest to propose that IP3 receptors are activated initially and therefore are the triggers in this pathway (Figure 6).
In pancreatic acinar cells, the stimulant-evoked, local short-lasting Ca2+ spikes in the apical pole can continue for a long time in the complete absence of external Ca2+ (Wakui et al., 1989, 1990). This can most easily be explained by effective Ca2+ tunnelling through a highly interconnected ER store, providing a large mobilizable intracellular Ca2+ reservoir (Villa et al., 1991; Terasaki et al., 1994; Mogami et al., 1997). However, there are systems in which Ca2+ stores may not be so well coupled (Gennazani and Galione, 1997; Golovina and Blaustein, 1997; Lee, 1997) and such differences may account for observations showing that Ca2+ spiking from ryanodine receptors can be independent of IP3 (Malgaroli et al., 1990) or, as for pancreatic acinar cells, depend on concerted action of the IP3 and ryanodine receptors (Figure 6). Separate dynamic Ca2+ stores may also exist (Villa et al., 1991; Gennazani and Galione, 1997; Lee, 1997). We cannot, therefore, exclude the possibility that the high NAADP concentration, which blocks the CCK, but not the ACh effect in our experiments (Figure 2), empties a small Ca2+ pool specifically linked to the NAADP receptor. This particular interpretation of our result would not, however, change the conclusion that the CCK response, in contrast to the ACh response, depends specifically on intracellular functional NAADP receptors (Figure 6).
In pancreatic acinar cells, ACh and CCK elicit specific Ca2+ signals (Osipchuk et al., 1990; Petersen,C. et al., 1991; Petersen,O. et al., 1994). CCK not only stimulates secretion of digestive enzymes, but also promotes pancreatic growth, whereas ACh mainly stimulates secretion (Petersen et al., 1994). It is possible that the growth-promoting action of CCK is due to global Ca2+ transients, although there is no specific evidence for this. It is well established that global increases in cytosolic Ca2+, which can display complex patterns, such as waves and oscillations, result from the co-ordinated activity of elementary Ca2+ release units (Lechleiter et al., 1991; Parker et al., 1996; Thomas et al., 1996; Berridge, 1997). Elementary Ca2+ release units are of two types: (i) ‘Ca2+ sparks’, involving only ryanodine receptors in cardiac muscle cells (Cheng et al., 1993; Cannell and Soeller, 1999); and (ii) ‘Ca2+ puffs’ in, for example, Xenopus oocytes and PC12 cells (Yao et al., 1995; Parker et al., 1996; Reber and Schindelholz, 1996), involving only IP3 receptors. In portal vein smooth muscle cells, it has been shown that elementary Ca2+ release sites can also be composed of a mixture of IP3 and ryanodine receptors (Boittin et al., 1998). Our imaging data from pancreatic acinar cells (Figure 5) indicate that the Ca2+ spikes originate from the same part of the apical region and are basically similar, irrespective of agonist type. The earliest Ca2+ release events last for 20–50 ms and have an amplitude of 100–150 nM with a spread of 2 µm. Like all channel events, the small Ca2+ releases occur stochastically, but the probability of observing such events increases throughout the period following the start of agonist application (Parker et al., 1996).
It would appear that early Ca2+ release sites contain IP3, ryanodine, cADPR and NAADP receptors, and represent the basic units of Ca2+ signal generation in pancreatic acinar cells. We have, therefore, in one unique normal cell, two different intracellular Ca2+ mobilization pathways, which converge on the same oscillator units consisting of IP3 and ryanodine receptors. The agonist can therefore specifically pick up the appropriate receptors giving rise to a specific Ca2+ signal. In view of the limited range of Ca2+ diffusion (Baker, 1978; Allbritton et al., 1992; Kasai and Petersen, 1994), an interesting consequence of this particular organization of the intracellular receptors is the possibility of cross-talk between agonists, as observed in our study. In this particular case, CCK markedly potentiates the ACh response and this potentiation phenomenon requires cADPR receptors (Figure 1). Previous studies of pancreatic acinar cells show that whereas short-lasting (a few seconds duration) Ca2+ spikes are local, longer lasting transients inevitably spread throughout the whole cytosol (Osipchuk et al., 1990; Thorn et al., 1993; Tinel et al., 1999). The cADPR-mediated potentiation of the ACh response, elicited by CCK (Figure 1), must therefore involve a major spatial extension of the cytosolic Ca2+ increase from the apical pole to the whole of the cell.
On the basis of our data, a general model for Ca2+ spike generation in the apical pole can be produced. To generate a Ca2+ spike by ACh or CCK, concerted activity of IP3 and ryanodine receptors is needed. ACh stimulates the production of IP3, which in turn releases Ca2+ through IP3 receptors and thereby also recruits ryanodine receptors, via Ca2+-induced Ca2+ release, although the involvement of another unknown messenger cannot be excluded. Physiological concentrations of CCK (5–10 pM) may not generate IP3 (Matozaki et al., 1990), but may instead activate NAADP and cADPR receptors by messenger formation or by sensitization of these Ca2+ release channels via other unknown mechanisms. The enzyme ADPR cyclase, which is responsible for NAADP (and cADPR) formation, has been found in pancreatic acinar cells (Cancela et al., 1999). In these cells, it is not known whether CCK stimulation results in an increase in intracellular NAADP or cADPR concentration, but in longitudinal intestinal smooth muscle cells, it has been shown that the CCK receptor is coupled to ADPR cyclase (Kuemmerle and Makhlouf, 1995). Ca2+ release from channels regulated by NAADP and cADPR (ryanodine receptors) in the vicinity of the IP3 receptors could sensitize the latter and lead to the major Ca2+ release generating the spikes.
One characteristic of CCK signals is that the Ca2+ release becomes global at some point (Thorn et al., 1993). IP3 receptors of all three subtypes are localized in the apical pole (Nathanson et al., 1994; Lee et al., 1997), in agreement with the finding that the local ACh-evoked response is blocked by caffeine and heparin (Wakui et al., 1990). However, the existence of a low density of IP3 receptors in the basolateral part of the cell cannot be excluded and is indeed likely (Kasai et al., 1993; Thorn et al., 1993). There are at present no data about NAADP receptors or their localization in pancreatic acinar cells or in any other cell type. cADPR activates ryanodine receptors of subtype 2 (Meszaros et al., 1993), which are known to be present in pancreatic acinar cells (Leite et al., 1999). In contrast to what has been shown for IP3 receptors, ryanodine receptors seem to be widely distributed in acinar cells (Leite et al., 1999). It would appear, therefore, that there are different receptor ratios in different parts of the cell. The apical pole seems to be richer in IP3 than in ryanodine receptors, whereas the basolateral part of the cell is richer in ryanodine receptors. This could explain the global response to CCK stimulation, which is normally dependent on cADPR activation of ryanodine receptors.
Active mitochondria play a crucial role in confining IP3-elicited Ca2+ spikes generated in the apical granular pole to this part of the cell (Tinel et al., 1999). In general, the ability of mitochondria to take up Ca2+ released from internal stores would depend on close proximity between the part of the ER store providing the released Ca2+ and the mitochondria (Pozzan et al., 1994; Rizzuto et al., 1998; Csordas et al., 1999). Because of the different receptor pathways used by ACh and CCK stimulation, it is possible that the ability of CCK to produce global responses, which becomes more pronounced at higher agonist concentrations (Petersen et al., 1994), could be due to part of the intracellular Ca2+ release channel population being relatively far away from the mitochondrial ring surrounding the granular pole (Tinel et al., 1999).
Finally, the ability of CCK to produce global responses may also rely on the particular distribution of NAADP receptors. Being a trigger, the localization of NAADP receptors may be of crucial importance in order to initiate the Ca2+ response at the appropriate time and place. In more general terms, the specific location of the trigger, whether it is IP3 or NAADP, may be crucial in shaping the Ca2+ signals generated by the agonists.
Materials and methods
Isolation of pancreatic acinar cells
Isolated single and double mouse pancreatic acinar cells were prepared and loaded with Fura red AM as described previously (Gerasimenko et al., 1996).
Patch–clamp recordings
Cells were investigated using the whole-cell patch–clamp configuration. From a holding potential of –30 mV, steps were made to 0 mV, the reversal potential of the two Ca2+-dependent currents through Cl– and non-selective cation channels (Thorn and Petersen, 1992). Using our solutions, the reversal potential of both the Cl– and non-selective cation currents was at 0 mV (for use as a potential control) (Petersen et al., 1991). Small deviations in ECl and Ecation and in the holding potential sometimes produce small inward or outward currents at 0 mV. At –30 mV we obtained a measure of both the Ca2+-dependent currents, which are an index of the cytosolic Ca2+ changes (Thorn et al., 1993; Tinel et al., 1999). The extracellular Na+-rich solution contained (in mM): 140 NaCl, 4.7 KCl, 1.13 MgCl2, 10 glucose, 1 CaCl2 and 10 HEPES–NaOH pH 7.2. CCK octapeptide or ACh was added to the external solution as indicated. The internal solution contained (in mM): 140 KCl, 1.13 MgCl2, 0.05 EGTA, 2 ATP and 10 HEPES–KOH pH 7.2. Extracellular application of CCK, ACh, ryanodine and caffeine was performed by means of a gravity perfusion system.
Confocal imaging
Fluorescence measurements and calcium concentration calibration on Fura red loaded cells (Gerasimenko et al., 1996) were performed using a Noran Odyssey confocal microscope. The KD for Fura red–Ca2+ at room temperature was assumed to be 140 nM (Molecular Probes). An objective (60×) with n.a. 1.4 and slit 25 µm was used in all experiments. For fast scanning experiments, 8 or 16 frames/s final scanning speed was used together with quarter of screen mode and online averaging 4 in slow mode (400–800 ns). For line scanning experiments, the slowest speed, 4 ms/line in slow mode, was used. Image processing was performed using TwoD Analysis (Noran Inc.). Images were shade corrected (divided by first image) and inverted (subtracted from saturated image) (Gerasimenko et al., 1996). A linear colour scale was used in all cases.
Chemicals
NAADP, cADPR, CCK, ACh and caffeine were purchased from Sigma. Ryanodine mixture was obtained from Calbiochem. Fura red and 8-NH2-cADPR were from Molecular Probes.
Acknowledgments
Acknowledgements
We thank N.Burdakova for technical support. This work was supported by an MRC Programme Grant. O.H.P. is an MRC Research Professor.
References
- Aarhus R., Dickey,D.M., Graeff,R.M., Gee,K.R., Walseth,T.F. and Lee,H.C. (1996) Activation and inactivation of Ca2+ release by NAADP. J. Biol. Chem., 271, 8513–8516. [DOI] [PubMed] [Google Scholar]
- Allbritton N.L., Meyer,T. and Stryer,L. (1992) Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science, 258, 1812–1815. [DOI] [PubMed] [Google Scholar]
- Baker P.F. (1978) The regulation of intracellular calcium in giant axons of Loligo and Myxicola. Ann. NY Acad. Sci., 307, 250–268. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. (1997) Elementary and global aspects of calcium signalling. J. Physiol., 499, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin F.X., Coussin,F., Macrez,N., Mironneau,C. and Mironneau,J. (1998) Inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channel-dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium, 23, 303–311. [DOI] [PubMed] [Google Scholar]
- Brown G.R., Sayers,L.G., Kirk,C.J., Michell,R.H. and Michelangeli,F. (1992) The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem. J., 282, 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela J.M. and Petersen,O.H. (1998) The cyclic ADP-ribose antagonist 8-NH2-cADP-ribose blocks cholecystokinin-evoked cytosolic Ca2+ spiking in pancreatic acinar cells. Pflüger’s Arch., 435, 746–748. [DOI] [PubMed] [Google Scholar]
- Cancela J.M., Mogami,H., Tepikin,A.V. and Petersen,O.H. (1998) Intracellular glucose switches between cyclic ADP-ribose and inositol trisphosphate triggering of cytosolic Ca2+ spiking. Curr. Biol., 8, 865–868. [DOI] [PubMed] [Google Scholar]
- Cancela J.M., Churchill,G.C. and Galione,A. (1999) Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature, 398, 74–76. [DOI] [PubMed] [Google Scholar]
- Cannell M.B. and Soeller,C. (1999) Mechanisms underlying calcium sparks in cardiac muscle. J. Gen. Physiol., 113, 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer,W.J. and Cannell,M.B. (1993) Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science, 262, 740–744. [DOI] [PubMed] [Google Scholar]
- Chini E.N. and Dousa,T.P. (1996) Nicotinate-adenine dinucleotide phosphate-induced Ca2+ release does not behave as a Ca2+-induced Ca2+ release system. Biochem. J., 316, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini E.N., Beers,K.W. and Dousa,T.P. (1995) Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J. Biol. Chem., 270, 3216–3223. [DOI] [PubMed] [Google Scholar]
- Crabtree G.R. (1999) Generic signals and specific outcomes: signaling through Ca2+, calcineurin and NF-AT. Cell, 96, 611–614. [DOI] [PubMed] [Google Scholar]
- Csordas G., Thomas,A.P. and Hajnoczky,G. (1999) Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J., 18, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B.E., Kaftan,E., Bezprozvannaya,S. and Bezprozvanny,I. (1994) The pharmacology of intracellular Ca2+ release channels. Trends Pharmacol. Sci., 15, 145–148. [DOI] [PubMed] [Google Scholar]
- Galione A. (1993) Cyclic ADP-ribose: a new way to control calcium. Science, 259, 325–326. [DOI] [PubMed] [Google Scholar]
- Galione A., Lee,H.C. and Busa,W.B. (1991) Ca2+-induced Ca2+ release in sea urchin egg homogenates and its modulation by cyclic ADP-ribose. Science, 253, 1143–1146. [DOI] [PubMed] [Google Scholar]
- Genazzani A.A. and Galione,A. (1997) A Ca2+ release mechanism gated by the novel pyridine nucleotide, NAADP. Trends Pharmacol. Sci., 18, 108–110. [DOI] [PubMed] [Google Scholar]
- Genazzani A.A., Empson,R.M. and Galione,A. (1996) Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem., 271, 11599–11602. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O.V., Gerasimenko,J.V., Petersen,O.H. and Tepikin,A.V. (1996) Short pulses of acetylcholine stimulation induce cytosolic Ca2+ signals that are excluded from the nuclear region in pancreatic acinar cells. Pflüger’s Arch., 432, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Golovina V.A. and Blaustein,M.P. (1997) Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science, 275, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Hamill O.P., Marty,A., Neher,E., Sakman,B. and Sigworth,F.J. (1981) Improved patch–clamp techniques for high-resolution current recording from cells and cell-free membranes patches. Pflüger’s Arch., 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Hirose K., Kadowaki,S., Tanabe,M., Takeshima,H. and Iino,M. (1999) Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science, 284, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Ito K., Miyashita,Y. and Kasai,H. (1999) Kinetic control of multiple forms of Ca2+ spikes by inositol trisphosphate in pancreatic acinar cells. J. Cell Biol., 146, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.T. (1994) Receptors on pancreatic acinar cells. In Johnson,L.R. (ed.), Physiology of the Gastrointestinal Tract, 3rd edn. Raven Press, New York, NY, pp. 1377–1446. [Google Scholar]
- Kasai H. and Augustine,G.J. (1990) Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature, 348, 735–738. [DOI] [PubMed] [Google Scholar]
- Kasai H. and Petersen,O.H. (1994) Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers. Trends Neurosci., 17, 95–101. [DOI] [PubMed] [Google Scholar]
- Kasai H., Li,Y.X. and Miyashita,Y. (1993) Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell, 74, 669–677. [DOI] [PubMed] [Google Scholar]
- Kuemmerle J.F. and Makhlouf,G.M. (1995) Agonist-stimulated cyclic ADP ribose. Endogenous modulator of Ca2+-induced Ca2+ release in intestinal longitudinal muscle. J. Biol. Chem., 270, 25488–25494. [DOI] [PubMed] [Google Scholar]
- LeBeau A.P., Yule,D.I., Groblewski,G.E. and Sneyd,J. (1999) Agonist-dependent phosphorylation of the inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol., 113, 851–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard,S., Clapham,D. and Peralta,E. (1991) Subcellular patterns of calcium release determined by G protein-specific residues of muscarinic receptors. Nature, 350, 505–508. [DOI] [PubMed] [Google Scholar]
- Lee H.C. (1997) Mechanisms of calcium signalling by cyclic ADP-ribose and NAADP. Physiol. Rev., 77, 1133–1164. [DOI] [PubMed] [Google Scholar]
- Lee H.C. and Aarhus,R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphophate and cyclic ADP-ribose. J. Biol. Chem., 270, 2152–2157. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Xu,X., Zeng,W., Diaz,J., Wojcikiewicz,J.H., Kuo,T.H., Wuytack,F., Racymaekers,L. and Muallem,S. (1997) Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. J. Biol. Chem., 272, 15765–15770. [DOI] [PubMed] [Google Scholar]
- Leite M.F., Dranoff,J.A., Gao,L. and Nathanson,M.H. (1999) Expression and subcellular localization of the ryanodine receptor in rat pancreatic acinar cells. Biochem. J., 337, 305–309. [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Fesce,R. and Meldolesi,J. (1990) Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J. Biol. Chem., 265, 3005–3008. [PubMed] [Google Scholar]
- Marchant J., Callamaras,N. and Parker,I. (1999) Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J., 18, 5285–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T., Goke,B., Tsunoda,Y., Rodriguez,M., Martinez,J. and Williams,J.A. (1990) Two functionally distinct cholecystokinin receptors show different modes of action on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. Studies using a new cholecystokinin analog, JMV-180. J. Biol. Chem., 265, 6247–6254. [PubMed] [Google Scholar]
- Meszaros L.G., Bak,J. and Chu,A. (1993) Cyclic ADP-ribose as an endogeneous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature, 364, 76–79. [DOI] [PubMed] [Google Scholar]
- Mogami H., Nakano,K., Tepikin,A.V. and Petersen,O.H. (1997) Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell, 88, 49–55. [DOI] [PubMed] [Google Scholar]
- Nathanson M.H., Fallon,M.B., Padfield,P.J. and Maranto,A.R. (1994) Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J. Biol. Chem., 269, 4693–4696. [PubMed] [Google Scholar]
- Osipchuk Y.V., Wakui,M., Yule,D.I., Gallacher,D.V. and Petersen,O.H. (1990) Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+-dependent Cl– current recording in single pancreatic acinar cells. EMBO J., 9, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Parker I. and Ivorra,I. (1991) Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J. Physiol., 433, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I. and Yao,Y. (1991) Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc. R. Soc. London Ser. B, 246, 269–274. [DOI] [PubMed] [Google Scholar]
- Parker I., Choi,J. and Yao,Y. (1996) Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium, 20, 105–121. [DOI] [PubMed] [Google Scholar]
- Petersen C.C.H., Toescu,E.C. and Petersen,O.H. (1991) Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cells: dependence on receptor type, agonist concentration and intracellular Ca2+ buffering. EMBO J., 10, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.H. (1980) The Electrophysiology of Gland Cells. Academic Press, London, UK. [Google Scholar]
- Petersen O.H. (1992) Stimulus–secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J. Physiol., 448, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.H. and Cancela,J.M. (1999) New Ca2+-releasing messengers: are they important in the nervous system? Trends Neurosci., 22, 488–494. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Petersen,C.C.H. and Kasai,H. (1994) Calcium and hormone action. Annu. Rev. Physiol., 56, 297–319. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular Ca2+ stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Reber B.F.X. and Schindelholz,A. (1996) Detection of a trigger zone of bradykinin-induced fast calcium waves in PC12 neurites. Pflüger’s Arch., 432, 893–903. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton,P., Carrington,W., Fay,F.S., Fogarty,K.E., Lifshitz,L.M., Tuft,R.A. and Pozzan,T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial responses. Science, 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Saillan-Barreau C., Clerc,P., Adato,M., Escrieut,C., Vaysse,N., Fourmy,D. and Dufresne,M. (1998) Transgenic CCK-B/gastrin receptor mediates murine exocrine pancreatic secretion. Gastroenterology, 115, 988–996. [DOI] [PubMed] [Google Scholar]
- Sutko J.L., Airey,J.A., Welch,W. and Ruest,L. (1997) The pharmacology of ryanodine and related compounds. Pharmacol. Rev., 49, 53–98. [PubMed] [Google Scholar]
- Terasaki M., Slater,N.T., Fein,A., Schmidek,A. and Reese,T.S. (1994) Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA, 91, 7510–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.P., Bird,G.S.J., Hajnoczky,G., Robb-Gaspers,L.D. and Putney,J.W. (1996) Spatial and temporal aspects of cellular calcium signalling. FASEB J., 10, 1505–1517. [PubMed] [Google Scholar]
- Thorn P. and Petersen,O.H. (1992) Activation of nonselective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J. Gen. Physiol., 100, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P and Petersen,O.H. (1993) Calcium oscillations in pancreatic acinar cells, evoked by the cholecystokinin analogue JMV-180, depend on functional inositol 1,4,5-trisphosphate receptors. J. Biol. Chem., 268, 23219–23221. [PubMed] [Google Scholar]
- Thorn P., Lawrie,A.M., Smith,P.M., Gallacher,D.V. and Petersen,O.H. (1993) Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell, 74, 661–668. [DOI] [PubMed] [Google Scholar]
- Thorn P., Gerasimenko,O. and Petersen,O.H. (1994) Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. EMBO J., 13, 2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Moreton,R. and Berridge,M.J. (1996) Multiple, co-ordinated Ca2+ release events underlie the inositol trisphosphate-induced local Ca2+ spikes in mouse pancreatic acinar cells. EMBO J., 15, 999–1003. [PMC free article] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Gerasimenko, O.V., Tepikin,A.V. and Petersen,O.H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini,P., Clegg,D.O., Pozzan,T. and Meldolesi,J. (1991) Intracellular Ca2+ stores in chicken Purkinje neurons—differential distribution of the low affinity–high capacity Ca2+ binding protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, BIP. J. Cell Biol., 113, 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Potter,B.V.L. and Petersen,O.H. (1989) Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature, 339, 317–320. [DOI] [PubMed] [Google Scholar]
- Wakui M., Osipchuk,Y.V. and Petersen,O.H. (1990) Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2+-induced Ca2+ release. Cell, 63, 1025–1032. [DOI] [PubMed] [Google Scholar]
- Walseth T.F. and Lee,H.C. (1993) Synthesis and characterization of antagonists of cyclic ADP-ribose. Biochim. Biophys. Acta, 1178, 235–242. [DOI] [PubMed] [Google Scholar]
- Walsh J.H. (1994) Gastrointestinal hormones. In Johnson,L.R. (ed.), Physiology of the Gastrointestinal Tract, 3rd edn. Raven Press, New York, NY, pp. 1–128. [Google Scholar]
- Yao Y., Choi,J. and Parker,I. (1995) Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol., 482, 533–553. [DOI] [PMC free article] [PubMed] [Google Scholar]