Abstract
Rhoptry associated protein 1 (RAP1) and 2 (RAP2), together with a poorly described third protein RAP3, form the low molecular weight complex within the rhoptries of Plasmodium falciparum. These proteins are thought to play a role in erythrocyte invasion by the extracellular merozoite and are important vaccine candidates. We used gene-targeting technology in P.falciparum blood-stage parasites to disrupt the RAP1 gene, producing parasites that express severely truncated forms of RAP1. Immunoprecipitation experiments suggest that truncated RAP1 species did not complex with RAP2 and RAP3. Consistent with this were the distinct subcellular localizations of RAP1 and 2 in disrupted RAP1 parasites, where RAP2 does not traffic to the rhoptries but is instead located in a compartment that appears related to the lumen of the endoplasmic reticulum. These results suggest that RAP1 is required to localize RAP2 to the rhoptries, supporting the hypothesis that rhoptry biogenesis is dependent in part on the secretory pathway in the parasite. The observation that apparently host-protective merozoite antigens are not essential for efficient erythrocyte invasion has important implications for vaccine design.
Keywords: malaria/molecular parasitology/targeted gene disruption/vaccine antigens
Introduction
Plasmodium falciparum causes the most lethal form of malaria in humans and development of a vaccine to this parasite is a priority. A number of antigens from P.falciparum have been tested as vaccines in animal models, including rhoptry associated proteins 1 (RAP1) and RAP2 (Perrin et al., 1985; Ridley et al., 1990a). Both molecules are located in the rhoptry organelles of the invasive merozoite form of P.falciparum (Howard et al., 1984; Clark et al., 1987; Bushell et al., 1988; Howard and Reese, 1990; Jaikaria et al., 1993). Ultrastructural and biochemical studies have suggested that rhoptries play an essential role in the invasion process. The electron-dense rhoptries are connected to the surface of the apical end of the merozoite by a duct-like structure and their contents are expelled during erythrocyte invasion (Aikawa et al., 1978). Both RAP1 and 2 are important vaccine candidates because it has been shown that antibodies to RAP1 are able to block merozoite invasion in vitro (Schofield et al., 1986; Harnyuttanakorn et al., 1992; Howard et al., 1998a). Additionally, monkeys immunized with RAP1 and 2 are partially protected against parasite challenge (Perrin et al., 1985; Ridley et al., 1990a).
The RAP1 protein has an apparent mol. wt of 82 kDa (Ridley et al., 1990b; Howard and Schmidt, 1995), and forms a complex with the 42 kDa protein RAP2 (Howard and Reese, 1990). Antibodies to RAP1 immunoprecipitate both RAP1 and RAP2 and an as yet uncharacterized third protein of 37 kDa that has been termed RAP3 (Howard and Reese, 1990). The precise function of the RAP complex, also known as the low molecular weight rhoptry complex, is unknown, but it is thought to play an important role in invasion and hence in the maintenance of the blood-stage cycle.
Subcellular organelle development can be controlled by two pathways that target proteins and membranes to the developing structure. First, the proteins can be imported post-translationally into preformed organelles where membrane development is a separate event. This process occurs for mitochondria and peroxisomes, and new organelles are generated by membrane fission (Fujiki et al., 1984; Hartl and Neupert, 1990). Secondly, proteins can be co-translationally translocated into the endoplasmic reticulum (ER) and transported via membrane vesicles as either soluble or membrane-bound proteins (Walter and Blobel, 1981). These proteins move through other subcellular compartments such as the Golgi where protein maturation and sorting can occur. Finally, vesicles fuse to form or enlarge the organelle, and examples of this mode of organellar biogenesis include lysosomes and secretory granules (Farquhar, 1985). There is evidence to suggest that development of rhoptries occurs via this second mechanism using the secretory pathway (Howard and Schmidt, 1995). The rhoptry proteins that have been identified have structural characteristics of proteins transported within the secretory pathway, including short hydrophobic N-terminal sequences that resemble membrane translocation signal sequences (Lustigman et al., 1988; Peterson et al., 1989; Howard and Reese, 1990; Saul et al., 1992).
In this report we describe targeted disruption (Crabb et al., 1997a; Lobo et al., 1999) of the RAP1 gene in P.falciparum. This event led to the expression of severely truncated forms of RAP1, and the loss of its ability to interact with RAP2 and RAP3. Also, RAP2 was found to be localized to a compartment resembling the lumen of the ER in these mutant parasites, showing that interaction with RAP1 is required for rhoptry localization of this protein. These results have important implications for protein trafficking and morphogenesis of organelles such as the rhoptries in the merozoite of this parasite. They also demonstrate that the low molecular weight rhoptry complex is not necessary for erythrocyte invasion by merozoites.
Results
Targeted disruption of the RAP1 gene
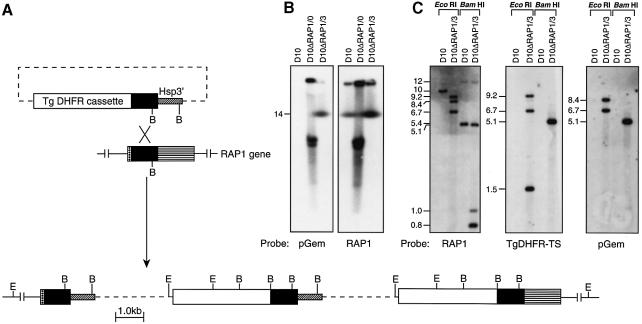
We have studied the function of the RAP1–RAP2 complex (Howard et al., 1984; Ridley et al., 1990b) by constructing a P.falciparum line in which the single wild-type copy of the RAP1 gene has been disrupted. The transfection vector pHC1ΔRAP1 (see Materials and methods; Figure 1A) was used to introduce a mutant RAP1 gene into the cloned parasite line D10. Transfected parasites were selected in pyrimethamine, followed by several cycles of cultivation without drug (to permit loss of episomal plasmid), and with drug to select parasites in which the plasmid had integrated into the genome (Crabb and Cowman, 1996; Wu et al., 1996; Crabb et al., 1997a). Chromosomes were analysed from the D10 parent and transfected populations D10ΔRAP1/0 and D10ΔRAP1/3, corresponding to parasites isolated shortly after transfection or after three rounds of cycling, respectively (Figure 1). As shown in Figure 1B, the pGEM plasmid probe did not recognize the parental D10 line, but hybridized with several bands in D10ΔRAP1/0, corresponding to episomal plasmids that typically migrate near chromosomes 6–8 under these electrophoresis conditions (Cowman et al., 1994). In contrast, D10ΔRAP1/3 DNA showed a single hybridizing band migrating with chromosome 14, suggesting that the plasmid had integrated into the RAP1 gene. The other hybridizing band belongs to DNA remaining in the agarose block.
Fig. 1. Disruption of the RAP1 gene. (A) Integration of plasmid pHC1ΔRAP1 by single-site homologous recombination produces a ‘pseudodiploid’, in which the upstream copy of RAP1 is truncated, while the downstream copy lacks a promoter element (Crabb and Cowman, 1996; Wu et al., 1996; Crabb et al., 1997a,b). Two copies of the plasmid have been integrated as shown. E, EcoRI; B, BamHI. (B) Hybridization of a pulsed-field gel electrophoresis blot, containing separated P.falciparum chromosomes (Corcoran et al., 1986), with pGEM plasmid sequences alone (left) reveals multiple transgenic sequences in D10ΔRAP1/0, corresponding to episomal DNA (Crabb and Cowman, 1996; Crabb et al., 1997a). After cycling cultures with/without pyrimethamine, stable parasite line D10ΔRAP1/3 exhibits a single band that co-migrates with chromosome 14. The upper band on both blots corresponds to DNA remaining in the wells. Hybridization of an identical blot with labelled RAP1 (right) confirms that the pGEM sequences are present on the same chromosome as RAP1. (C) Digestion of genomic DNA with EcoRI or BamHI confirms that the RAP1 gene has been disrupted in D10ΔRAP1/3, yielding the expected restriction pattern.
Hybridization of the RAP1-containing probe to restriction-digested genomic DNA from each line showed that the RAP1 gene was indeed disrupted (Figure 1C). The RAP1 probe in D10 detected a single 10 kb EcoRI band, whereas D10ΔRAP1/3 showed a 9.2 kb band and two smaller hybridizing bands. Similarly, the RAP1 probe detected a 5.4 kb BamHI fragment in D10, whereas in D10ΔRAP1/3 fragments of 5.1, 1.0 and 0.8 kb were obtained. Hybridization of the TgDHFR-TS and pGem probes to the same restriction digests (Figure 1C) enabled mapping of the region surrounding the integration event. These results are consistent with a single-site homologous recombination between the transfection plasmid and the endogenous locus (Figure 1A). An identical restriction pattern was observed in two independent clonal isolates derived from D10ΔRAP1/3 (designated D10ΔRAP1c1 and D10ΔRAP1c2), and these lines were used for all further experiments. The resulting ‘pseudodiploid’ (Crabb and Cowman, 1996; Wu et al., 1996) produces a truncated RAP1 gene predicted to encode only 344 amino acids of the 782 amino acid wild-type RAP1 protein.
Disruption of RAP1 leads to the expression of severely truncated forms of the RAP1 protein
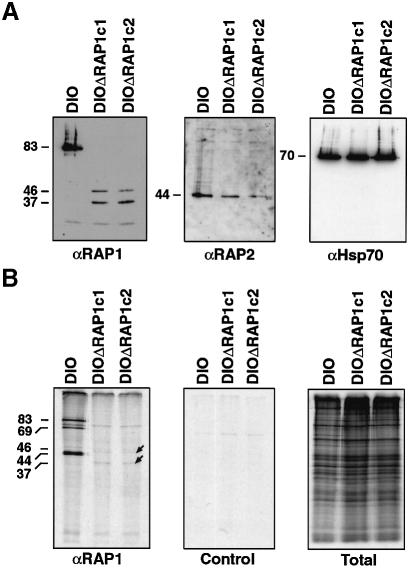
In order to determine whether D10ΔRAP1c1 and D10ΔRAP1c2 transfectants produce mutant RAP1 protein, schizont stages were analysed by SDS–PAGE and western analysis using anti-RAP1 antibodies (Figure 2A). The expected 83 kDa RAP1 protein was readily detected in D10 (Clark et al., 1987; Bushell et al., 1988; Ridley et al., 1991; Howard et al., 1998b), but the D10ΔRAP1c1 and D10ΔRAP1c2 transgenic lines expressed anti-RAP1-reactive proteins of ∼46 and 37 kDa, and no wild-type protein. The 37 kDa band corresponds to the size expected for the 344 amino acid truncated protein. The 46 kDa protein probably results from inefficient translation termination in the Hsp86 3′ sequence (see Figure 1A), as described previously (Triglia et al., 1998). This protein cannot be derived from the 3′ end of the RAP1 gene (downstream-most RAP1 fragment in Figure 1A), because this gene fragment lacks a promoter and an in-frame initiation codon and open reading frame large enough to encode a 46 kDa protein. Additionally, it has a number of ATG initiation codons that could only result in out of frame expression of small polypeptides. However, in order to confirm that it was not possible to express any RAP1 peptides from this promoter and partial 3′ RAP1 fragment, we performed western blots on the initial uncloned transfectant line that contains the plasmid as an episome. No peptides were detected that would indicate expression from the transfected RAP1 (data not shown).
Fig. 2. RAP1 and RAP2 expression and association in wild type and ΔRAP1 mutants. (A) Purified schizonts of the D10 parental parasites and two clonal mutants (ΔRAP1c1 and ΔRAP1c2) were separated by SDS–PAGE, immunoblotted and probed with antibodies specific for RAP1 (monoclonal 7H8/50) (Schofield et al., 1986; Howard et al., 1998b), RAP2 (monoclonal IC3/94) or Hsp70 (polyclonal antiserum). The D10ΔRAP1 mutants produced no wild-type RAP1 protein (left) and only low levels of two truncated RAP1 proteins (∼46 and 37 kDa). The mutants produced reduced levels of wild-type RAP2 (centre), but normal levels of Hsp70 (right). (B) Purified trophozoites were metabolically labelled for 6 h with [35S]methionine and immunoprecipitated with monoclonal antibody 7H8/50 (left panel); immunoprecipitation with a negative control monoclonal antibody is shown (centre panel), total labelled protein is also shown (right panel). RAP2 and RAP3 proteins co-immunoprecipitated with RAP1 in wild-type D10 parasites, but not in the ΔRAP1 mutants.
Truncation of RAP1 disrupts the interaction with RAP2 and RAP3
The probing of identical immunoblots with antibodies against RAP2 showed the expected 44 kDa RAP2 protein (Ridley et al., 1991; Saul et al., 1992). Interestingly, the RAP2 signal in both mutants was consistently lower than in the D10 controls, suggesting that translation or, more likely, stability of this protein had been affected (Figure 2A, centre panel). The size and steady-state levels of Hsp70 expression were identical in all three lines (Figure 2A, right).
In order to compare the association of RAP1 with RAP2 and RAP3 in wild-type D10 parasites with the truncated protein(s) observed in D10ΔRAP1c1 and D10ΔRAP1c2 (ΔRAP1 mutants parasites), [35S]methionine-labelledparasite extracts were immunoprecipitated with anti-RAP1 antibodies (Figure 2B). In D10 parasites, both RAP2 and RAP3 (doublet at ∼44 kDa) co-precipitated with RAP1 (83 kDa) (Schofield et al., 1986; Howard et al., 1998b). The band at 69 kDa corresponds to a processed form of RAP1 (Ridley et al., 1990b, 1991; Harnyuttanakorn et al., 1992). In contrast, only low levels of the truncated RAP1 peptides were detected (consistent with lower expression levels and decreased methionine content), and neither RAP2 nor RAP3 was co-immunoprecipitated in the ΔRAP1 mutants. It thus appears that truncation of the RAP1 protein in the ΔRAP1 mutants retains the epitope for the RAP1 monoclonal antibody, but disrupts the interaction between RAP1 and the RAP2–RAP3 molecules. It appears that these intermolecular interactions are not required for normal parasite replication in vitro.
Subcellular localization of truncated RAP1 and RAP2 in ΔRAP1 parasites
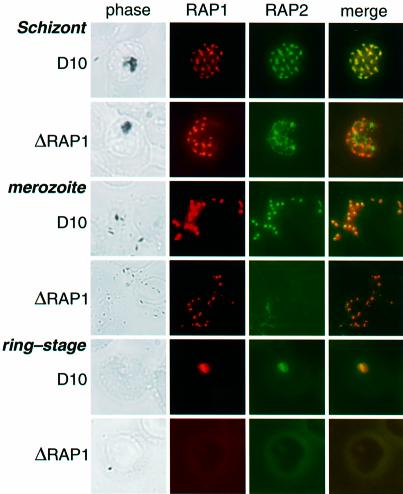
Immunofluorescence microscopy of D10 schizonts using either anti-RAP1 or anti-RAP2 antibodies reveals a punctate fluorescence typical of rhoptry staining (Figure 3, top panel) (Howard et al., 1984, 1998b; Schofield et al., 1986; Clark et al., 1987; Ridley et al., 1990b). ΔRAP1 mutant parasites showed a similar fluorescence pattern for the truncated RAP1 in mature schizonts (Figure 3, second panel), suggesting that the N-terminal 344 amino acids of RAP1 are sufficient for trafficking to the rhoptries. RAP2 failed to localize to the rhoptries, however, exhibiting a diffuse fluorescence that did not co-localize with RAP1 (Figure 3, second panel). We conclude that the correct targeting of RAP2 is dependent on its association with RAP1.
Fig. 3. RAP1 specifies rhoptry localization of RAP2. Individual rows show D10 and D10ΔRAP1c1 schizonts, merozoites and ring-stage parasites, as labelled. Each row shows phase-contrast images, staining with polyclonal rabbit anti-RAP1 (red), staining with monoclonal anti-RAP2 (green), and merged RAP1 + RAP2 staining (yellow indicates co-localization) for the same parasite. Top panel: D10 schizont showing punctate rhoptry localization of both RAP1 and RAP2. Second panel: the truncated RAP1 protein in D10ΔRAP1c1 schizonts (see Figure 2) still localizes to the rhoptries, but RAP2 exhibits diffuse non-rhoptry fluorescence. Third panel: RAP1 and RAP2 co-localize in D10 merozoites. Fourth panel: RAP1 exhibits similar (presumably rhoptry) focal staining in D10ΔRAP1c1 parasites, but RAP2 is not detectable. Fifth panel: in wild-type parasites, both RAP1 and RAP2 proteins are carried into ring-stage parasites after infection. Sixth panel: neither RAP1 nor RAP2 are carried into the erythrocyte during merozoite infection. Ring-stage parasites were identified by staining with DAPI (not shown).
RAP2 in D10ΔRAP1c1 and D10ΔRAP1c2 could not be detected in extracellular merozoites of mutant parasites (Figure 3, fourth panel). In contrast, both RAP1 and RAP2 in wild-type merozoites (Figure 3, third panel) and RAP1 in the merozoites of ΔRAP1 mutants (Figure 3, fourth panel) exhibit the distinctive paired dots characteristic of rhoptry staining (Howard et al., 1984, 1998b; Schofield et al., 1986; Clark et al., 1987; Ridley et al., 1990b). RAP1 has previously been shown to be carried into ring-stage parasites during erythrocyte invasion (Howard et al., 1984, 1998b), as shown in Figure 3 (fifth panel). RAP2 is also carried into the erythrocyte, where it continues to co-localize with RAP1 (Figure 3, fifth panel). Interestingly, neither RAP1 nor RAP2 was detected in the ring stages of ΔRAP1 parasites (Figure 3, sixth panel). The absence of RAP2 is not surprising, as this antigen was absent from the merozoite (see above). However, despite its apparently correct localization within merozoites (Figure 3, fourth panel), the truncated RAP1 protein appears to be lost during the invasion process.
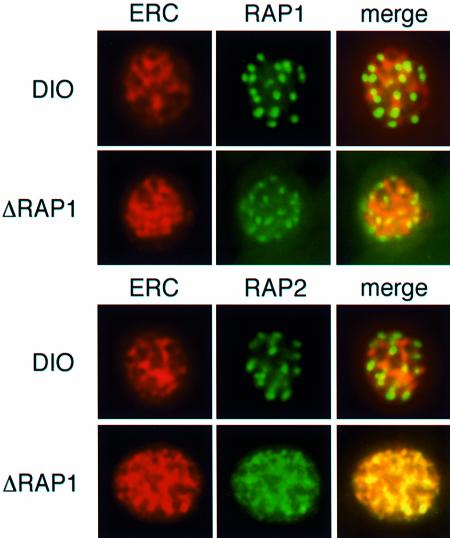
In order to determine the subcellular localization of RAP2 in the ΔRAP1 mutants we performed co-localization studies using antibodies to P.falciparum proteins that have previously been localized to specific compartments of the parasitized red cell. Localization of RAP2 in D10ΔRAP1c1 and D10ΔRAP1c2 transfected parasites was similar but not identical to the parasitophorous vacuole protein S-antigen (Cowman et al., 1985) and MSP3 (McColl and Anders, 1997) (data not shown). The P.falciparum ERC protein (PfERC) binds calcium and has previously been shown to be localized in the lumen of the ER (La Greca et al., 1997). This protein is most likely a functional homologue of reticulocalbin (Ozawa and Muramatsu, 1997). PfERC has a C-terminal IDEL amino acid motif, consistent with its residency and retention within the ER lumen. Antibodies to PfERC yield a typical ER pattern very different to the punctate rhoptry pattern seen for RAP1 and RAP2 in D10 parasites (Figure 4). Localization of PfERC in the ΔRAP1 parasites (D10ΔRAP1c1 and D10ΔRAP1c2) shows the same ER localization as D10. The truncated RAP1 peptides in ΔRAP1 parasites do not co-localize with PfERC as they are trafficked to the rhoptries; however, RAP2 co-localizes with PfERC. These results strongly suggest that RAP2, in the ΔRAP1 parasites, traffics to the lumen of the ER and cannot move to the rhoptries in the absence of functional RAP1. Also, it is interesting that most of the ER appears to be lost at schizont rupture and release of the merozoites; however, there is a small amount of labelling of merozoites with the anti-PfERC antibodies (data not shown).
Fig. 4. RAP2 is located in the ER lumen of ΔRAP1 parasites. Individual rows show mature schizonts of D10 and ΔRAP1 parasites (D10ΔRAP1c1) as labelled. Each row shows staining with polyclonal rabbit anti-PfERC (red) and anti-RAP1 (green) or staining with monoclonal anti-RAP2 (green). Merged images are PfERC + RAP1 or RAP2 (yellow indicates co-localization) for the same parasite. Top panel: PfERC and RAP1 do not co-localize in D10 schizonts. Second panel: PfERC and RAP1 do not co-localize in ΔRAP1 schizonts. Third panel: PfERC and RAP2 do not co-localize in D10 schizonts. Fourth panel: PfERC and RAP2 co-localize in ΔRAP1 schizonts.
Genetic ablation of the major Toxoplasma gondii rhoptry antigen ROP1, which shows no sequence similarity to P.falciparum RAP1 or RAP2, has been shown to alter rhoptry morphology in this parasite (Soldati et al., 1995). D10 and D10ΔRAP1 merozoites were analysed by electron microscopy in order to determine whether the truncation of RAP1 and the absence of RAP2 from the rhoptries had any effect on rhoptry structure. This analysis did not reveal any differences in the electron density or gross ultrastructure of the rhoptries, implying that the low molecular weight complex is not essential for defining these features (data not shown).
ΔRAP1 parasites show no in vitro growth defect
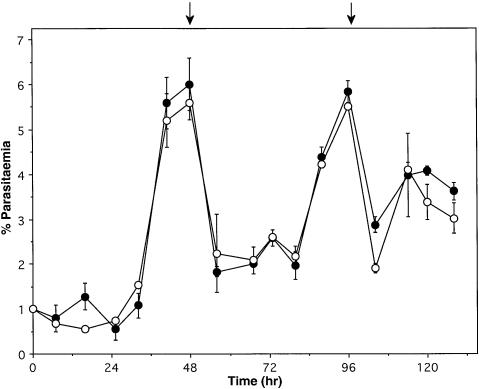
The parental D10 line was compared with D10ΔRAP1c1 and D10ΔRAP1c2 mutants to determine whether truncation of RAP1 and incorrect localization of RAP2 had any deleterious effect on the ability of these parasites to invade and propagate. Cultures were synchronized as ring-stage parasites and the growth and stage distribution were followed over a 132 h period. No detectable difference was observed between wild-type and mutant parasites in either growth rates (Figure 5) or rings/trophozoites/schizont stage distribution (not shown). Therefore, truncation of RAP1 and incorrect localization of RAP2 in the ΔRAP1 mutant parasites have not affected the efficiency of in vitro blood-stage growth.
Fig. 5. Comparison of the growth rates of the parental D10 line and the RAP1 mutants. Synchronized parasites of D10 and D10ΔRAP1c1 were plated at 1% parasitaemia. Smears were taken every 8 h and parasitaemia counted for 132 h. Closed circles correspond to D10 and open circles to D10ΔRAP1c1. The arrows indicate when the parasites were subcultured to 2%.
The invasion of erythrocytes by P.falciparum merozoites is a complex process believed to incorporate redundancies (Miller et al., 1977; Hadley et al., 1986; Mitchell et al., 1986). Three independent pathways of invasion have been described to date (Camus and Hadley, 1985; Hadley et al., 1987; Dolan et al., 1994), defined by entry via the erythrocyte surface proteins glycophorin A, glycophorin B and an unknown ‘receptor X’. To determine whether invasion via any of these pathways is affected by disruption of RAP1, we examined the ability of ΔRAP1 mutants to invade human erythrocytes that had been pre-treated with neuraminidase and/or trypsin. Neuraminidase removes sialic acid and blocks invasion via both glycophorin A and B, whereas trypsin removes both the glycophorin A and the X receptor pathways (Dolan et al., 1994). As shown in Table I, no statistically significant difference from D10 controls was observed in the ability of either the D10ΔRAP1c1 or D10ΔRAP1c2 clones to invade untreated versus neuraminidase- and/or trypsin-treated erythrocytes. It is interesting, however, to note that the mutants consistently invaded both neuraminidase- and trypsin-treated erythrocytes better than the D10 parental line.
Table I. The effect of enzyme treatmenta on invasionb of D10 and ΔRAP1 mutant P.falciparum into human red blood cells.
| Neuraminidase |
Trypsin |
Neuraminidase + trypsinc |
Trypsin + neuraminidasec |
|||||
|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | % | n | |
| D10 | 45 ± 11 | 8 | 9 ± 8 | 8 | 2 ± 2 | 4 | 1 ± 1 | 4 |
| D10ΔRAP1c1 | 62 ± 7 | 8 | 20 ± 7 | 8 | 3 ± 3 | 4 | 3 ± 3 | 4 |
| D10ΔRAP1c2 | 58 ± 7 | 8 | 18 ± 6 | 8 | 2 ± 2 | 4 | 5 ± 5 | 4 |
aSamples of erythrocytes treated with neuraminidase were tested for lack of agglutination with influenza virus to ensure that the sialic acid had been digested by the enzyme treatment. Enzyme concentations used were: trypsin, 1 mg/ml; soybean trypsin inhibitor, 0.5 mg/ml; neuraminidase, 0.25 U/108 erythrocytes.
bThe invasion data are presented as a percentage of control invasion into untreated, normal erythrocytes and the number (n) of independent experiments with the SEM. Experiments were performed as described previously (Dolan, 1994).
cThe enzymes are listed in the order that they were applied to the erythrocytes.
Discussion
The rhoptry organelles in the invasive merozoite form of Plasmodium species have been strongly implicated in a central role in erythrocyte invasion (Aikawa et al., 1978). Consequently, the role of rhoptry-localized proteins in the function of this organelle and as mediators of host protective immune responses has been of major interest. RAP1 and RAP2 are present as a complex in the rhoptries of the merozoite, but despite their importance as vaccine candidates, no function has been assigned to these proteins (Howard et al., 1984; Howard and Reese, 1990; Jaikaria et al., 1993). We have used the ability to modify P.falciparum genetically by transfection (Crabb and Cowman, 1996; Wu et al., 1996; Crabb et al., 1997a) to disrupt the RAP1 gene. Our results show that RAP1 controls the trafficking of RAP2 to the rhoptries, and the results are consistent with the idea that the secretory pathway regulates transport of these proteins and, in part, controls rhoptry development.
Two possible pathways can control the targeting of proteins to the developing rhoptries and previous analysis of RAP1 transport has supported the hypothesis that rhoptry targeting is directed via the secretory pathway (Howard and Schmidt, 1995). These results predicted a model for protein trafficking and rhoptry biogenesis where RAP1 and other rhoptry proteins are co-translationally translocated into the lumen of the ER, correctly folded, and transported by membrane vesicles from the ER to the Golgi. At one or more points between the ER and Golgi, the rhoptry proteins including RAP1 are directed to the forming rhoptries by specific targeting signals on the proteins. RAP1 would associate with RAP2 and RAP3 somewhere within this pathway. Finally, the vesicles would fuse into rhoptries, releasing their contents into the lumen of the developing organelle. This secretory pathway-dependent mode of rhoptry formation is consistent with retention of RAP2 in the ER of ΔRAP1 parasites, as observed in the present study.
The localization of RAP2 in the ER of ΔRAP1 parasites suggests that interaction of RAP1 with RAP2 occurs in the ER followed by trafficking of the protein complex to the rhoptries. The retention of RAP2 in the ER of ΔRAP1 parasites must be due to an inability to be directed to rhoptries rather than a specific ER retention signal such as the amino acid motif KDEL (Andres et al., 1991), which is not present in RAP2. There are two possible reasons why RAP2 cannot be trafficked to the rhoptries in the mutant parasites. First, it is possible that the RAP2 protein, accumulating in the ER of ΔRAP1 parasites, is not folded correctly in the absence of interacting RAP1. The misfolded RAP2 would not be trafficked to the rhoptries and would accumulate in the ER, as has been described previously for a number of other proteins in mammalian cells (Gething and Sambrook, 1992). Consistent with this was the lower amount of RAP2 protein detected in ΔRAP1 mutant parasites, suggesting active degradation. It is unlikely that the disruption of the RAP1 gene has affected the level of expression of the RAP2 gene as they are on different chromosomes. Thus, accumulation of incorrectly folded RAP2, in the absence of RAP1, would result in the degradation of this protein in the ER. Secondly, it is possible that localization to the rhoptries is dependent on timing of expression and no localization signals are required. In this hypothesis, retention of RAP2 in the ER of ΔRAP1 parasites is due to incorrect folding of RAP2 and non-association with full-length RAP1.
The remaining N-terminal 344 amino acids of RAP1 are sufficient to specify rhoptry targeting, but insufficient for association with RAP2 or entry into the erythrocyte with the invading merozoite. The inability of anti-RAP1 antibodies to co-precipitate RAP2 and the different subcellular localization of these proteins indicate that the RAP1 truncated peptides do not interact with RAP2 in ΔRAP1 parasites. Additionally, the truncated RAP1 sequence is sufficient for RAP1 localization into rhoptries of the paired organelles in each merozoite. These results demonstrate that RAP1 is required for RAP2 targeting to the rhoptries.
It is interesting that the vast majority of the PfERC protein in the ER lumen was lost during schizont rupture. There was a small punctate dot of anti-PfERC reactivity in free merozoites (data not shown), suggesting that during cell division only a small proportion of the total ER is retained in each daughter cell, from which the complete ER can develop in more mature stages of the next parasite cycle. The rest of the ER is discarded into the medium on schizont rupture and merozoite release.
The loss of RAP1 from ΔRAP1 parasites during invasion suggests that the RAP1–RAP2 complex discharges from the rhoptries during invasion. In wild-type parasites, this complex is present in young ring-stage parasites. This may result from binding to erythrocyte or parasite components, which are then carried inside the red blood cell (RBC) in association with the invading merozoite. In the ΔRAP1 mutants, we assume (but have not demonstrated) that the truncated RAP1 is unable to bind to the parasite or erythrocyte, and diffuses into the medium. Disappearance of the truncated RAP1 proteins during the invasion process also strongly suggests that these peptides play no part in this process and that RAP1 is not required for normal merozoite invasion. However, we cannot rule out the possibility that a small portion of the total RAP1 content of the parasite is all that is required for binding the RBC and performing subsequent steps in invasion.
Disruption of the RAP1 protein, and as a consequence accumulation of RAP2 in the ER, have shown that neither rhoptry-localized RAP2 nor full-length RAP1 is required for normal parasite growth and invasion in vitro. This is a surprising result as previous investigators have shown that RAP1 and RAP2 can protect Saimiri monkeys from challenge with P.falciparum (Perrin et al., 1985; Ridley et al., 1990a) and that monoclonal antibodies specific for RAP1 can inhibit invasion into erythrocytes (Schofield et al., 1986; Harnyuttanakorn et al., 1992; Howard et al., 1998a). These data suggested that members of the low molecular weight complex might be essential for parasite survival and, by their conserved nature, be useful as vaccine antigens (Howard, 1992; Howard and Peterson, 1996). This work demonstrates that intact RAP1–RAP2–RAP3 complexes are not essential for invasion and growth in human erythrocytes by P.falciparum in vitro, and while in vivo studies remain to be carried out, this evidence must temper enthusiasm for RAP antigens as vaccine candidates.
It remains a possibility that RAP1 and RAP2 are involved in alternative invasion pathways yet to be defined (Dolan et al., 1994), or in vivo parasite growth in the human host, but we have not been able to observe any alteration of parasite viability or replication rates in vitro. Analysis of the known invasion pathways using enzyme-treated RBCs has shown no statistically significant difference, although it is interesting that the ability of the ΔRAP1 mutants to invade trypsin- and neuraminidase-treated RBCs was consistently higher than that of the parental parasites. It is possible that inhibition of merozoite invasion by RAP1 antibodies occurs via steric hindrance of the process rather than specific inhibition of an essential function. This would explain the ability of RAP1 and RAP2 to protect monkeys against P.falciparum challenge (Perrin et al., 1985; Ridley et al., 1990a), but raises the possibility of obtaining breakthrough parasites that have deleted the expression of RAP1.
The rhoptry organelle is an electron-dense structure that is visible in electron micrographs at the apical end of the merozoite (Aikawa et al., 1978). The electron density of the rhoptries was identical for both parental and ΔRAP1 mutant parasites, showing that full-length RAP1 and RAP2 play no role in this physical property. Also, the rhoptries were not detectably different in structure in wild-type D10 and the ΔRAP1 parasites, again suggesting that neither rhoptry protein plays a dominant role in the organelle’s structure. In contrast, when expression of ROP1 in T.gondii was disrupted, an alteration in structure was observed, but no other phenotypic changes were detected (Soldati et al., 1995).
In summary, we have used transfection in P.falciparum to show that RAP1 is required for targeting of RAP2 to the rhoptries, results consistent with the hypothesis that rhoptry biogenesis is controlled in part by the secretory pathway. The truncated RAP1 contains sufficient sequence to direct itself to the rhoptries and bind to the RAP1 monoclonal antibody, but fails to interact with full-length RAP2. It is clear that P.falciparum parasites containing truncated RAP1 molecules and intact RAP2 molecules remaining predominantly in the ER are still capable of normal in vitro growth and invasion. These findings raise important questions about the sequence of events during RBC invasion and, as a result, have implications for both of these proteins as blood-stage vaccine candidates against P.falciparum infection. This paper, which describes the first functional disruption of a merozoite antigen, argues for a better understanding of antigen function before major decisions relating to the components of a malaria vaccine are made.
Materials and methods
Parasites and transfection
Plasmodium falciparum was cultivated (Trager and Jensen, 1978) and synchronized by standard procedures (Lambros and Vanderberg, 1979). Parasites were transfected as described previously (Crabb and Cowman, 1996; Wu et al., 1996; Crabb et al., 1997a, b) with 150 µg of plasmid that had been purified using CsCl density gradients (Sambrook et al., 1989). Transfected parasites containing integrated forms of the plasmid were selected by successive cycles of growth on increasing amounts of pyrimethamine (0.1, 1 and 2 µM) followed by single-cell cloning.
The transfection plasmid pHC1ΔRAP1 was constructed by cloning a 908 bp RAP1 insert into the pHC1 transfection vector (Crabb et al., 1997b). This fragment was amplified using D10 genomic DNA as a template and the following oligonucleotides: forward primer, CTCGAGATGATTACAATTATTGGACACC; reverse primer, CTCGAGATATTCTTGATAGGTTGCACCTAC (nucleotides 128–149 and 1012–1035 of the coding region). A XhoI restriction enzyme site (shown in bold) was introduced for cloning at both ends of the fragment. A schematic representation of the construct is shown in Figure 1A; the solid block represents the amplified, truncated RAP1 domain, which includes the repeat region (Howard, 1992), but none of the Cys residues.
Nucleic acids and pulsed-field gel electrophoresis
Genomic DNA was extracted from trophozoites as described previously (Corcoran and Kemp, 1986). The analysis of nucleic acids by Southern blot hybridization and recombinant DNA techniques was carried out using standard procedures. Chromosomes were separated by pulsed-field gel electrophoresis (Chu et al., 1986) as has been described previously (Corcoran et al., 1986).
Antisera and immunoblot assays
Proteins from synchronized Percoll-purified parasites were separated on SDS–10% PAGE and transferred to nitrocellulose membrane, according to standard procedures (Cowman et al., 1991). RAP1 (7H8/50) (Schofield et al., 1986) and RAP2 (IC3/94) monoclonal antibodies were diluted 1:500 and 1:200, respectively, and polyclonal rabbit anti-Hsp70 diluted 1:5000, in phosphate-buffered saline (PBS) with 5% w/v skim milk powder and used as primary antibodies. Sheep anti-mouse and sheep anti-rabbit Ig horseradish peroxidase (HRP)-conjugated antibodies (Silenus, Melbourne, Australia) were used as secondary antibodies. Antigen–antibody complexes were detected by ECL (Amersham, Buckinghamshire, UK) and autoradiography.
Biosynthetic radiolabelling and immunoprecipitation
Trophozoites from synchronized parasite lines were incubated with 300 µCi/ml [35S]methionine (NEN, Boston, MA) for 6 h and proteins extracted as described previously (Howard et al., 1998b). Immunoprecipitations were performed using the monoclonal antibodies 7H8/50 or IC3/94 (Schofield et al., 1986) and protein G–Sepharose (Pharmacia Biotech, Uppsala, Sweden). Proteins were separated by SDS–PAGE and visualized by enhancement with sodium salicylate (Ajax Chemicals, NSW, Australia) and autoradiography.
Immunofluorescence
Immunofluorescence of synchronized parasitized erythrocytes was performed on air-dried thin blood smears after fixation at –20°C in acetone/methanol (9:1) for 5 min. RAP1 was detected using rabbit antibodies raised to a glutathione S-transferase–RAP1 fusion protein and rhodamine-labelled goat anti-rabbit antibodies (Chemicon, Temecula, CA). RAP2 was detected using mouse monoclonal 7B2/1H1 and fluorescein isothiocyanate-labelled sheep anti-mouse antibodies (Silenus, Melbourne, Australia). Rabbit anti-PfERC antibodies were a gift from Dr Leanne Tilley and have been described previously (La Greca et al., 1997). All parasites were also labelled with the 4′,6-diamidino-2-phenylindole (DAPI) DNA stain (Molecular Probes Inc., Eugene, OR).
Enzymatic treatment of erythrocytes and invasion assays
Erythrocytes were treated as reported previously (Dolan et al., 1994) with 1 mg/ml TPCK-treated trypsin (Sigma, St Louis, MO), 0.5 mg/ml soybean trypsin inhibitor (Sigma, St Louis, MO) and 0.25 U Vibrio cholerae neuraminidase (Calbiochem, La Jolla, CA). The efficacy of neuraminidase treatment was assessed by the inability of a highly haemagglutinating influenza A virus [Mem71H-BelN(R); kindly supplied by M.Anders (Anders et al., 1990)] to agglutinate erythrocytes. Invasion assays were conducted essentially as described (Dolan et al., 1994). Briefly, 2 × 106 synchronized, Percoll-purified parasites were mixed with 2 × 107 erythrocytes to make a final volume of 150 µl with 50/50 culture medium in a 96 well U-bottomed microtitre plate. Assays were performed in duplicate and chicken erythrocytes, refractory to invasion by P.falciparum, were included as a control. After 24–28 h incubation at 37°C, a thin smear was made and stained with Giemsa.
Growth assays
Parasites were sorbitol synchronized twice, at 4 h intervals, and then plated at 1% parasitaemia in 4% haematocrit, in duplicate. Thin blood smears were made every 8 h. Fresh medium was added every 24 h, and every 48 h the cultures were subbed 1:5 with fresh 4% haematocrit.
Acknowledgments
Acknowledgements
We thank Sonia Caruana for expert technical assistance, Allan Saul for advice throughout the course of this study, and Leanne Tilley for advice and provision of the PfERC antibody. Research was supported by the National Health and Medical Research Council of Australia and the National Institutes of Health USA (1 RO1 AI44008). We would like to thank the Red Cross Blood Service (Melbourne, Australia) for supply of human RBCs and serum. D.S.R. is a Burroughs Wellcome Scholar in Molecular Parasitology.
References
- Aikawa M., Miller,L.H., Johnson,J. and Rabbege,J. (1978) Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J. Cell Biol., 77, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders E.M., Hartley,C.A. and Jackson,D.C. (1990) Bovine and mouse serum B inhibitors of influenza A viruses are mannose-binding lectins. Proc. Natl Acad. Sci. USA, 87, 4485–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres D.A., Rhodes,J.D., Meisel,R.L. and Dixon,J.E. (1991) Characterisation of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J. Biol. Chem., 266, 14277–14288. [PubMed] [Google Scholar]
- Bushell G.R., Ingram,L.T., Fardoulys,C.A. and Cooper,J.A. (1988) An antigenic complex in the rhoptries of Plasmodium falciparum. Mol. Biochem. Parasitol., 28, 105–112. [DOI] [PubMed] [Google Scholar]
- Camus D. and Hadley,T.J. (1985) A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science, 230, 553–556. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath,D. and Davis,R. (1986) Separation of large DNA molecules by contour clamped homogeneous electric fields. Science, 234, 1582–1585. [DOI] [PubMed] [Google Scholar]
- Clark J.T., Anand,R., Akoglu,T. and McBride,J.S. (1987) Identification and characterisation of proteins associated with the rhoptry organelles of Plasmodium falciparum merozoites. Parasitol. Res., 73, 425–434. [DOI] [PubMed] [Google Scholar]
- Corcoran L.M. and Kemp,D.J. (1986) Chromosomes of Plasmodium falciparum. PNG Med. J., 29, 95–101. [PubMed] [Google Scholar]
- Corcoran L.M., Forsyth,K.P., Bianco,A.E., Brown,G.V. and Kemp,D.J. (1986) Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell, 44, 87–95. [DOI] [PubMed] [Google Scholar]
- Cowman A.F., Saint,R.B., Coppel,R.L., Brown,G.V., Anders,R.F. and Kemp,D.J. (1985) Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum.Cell, 40, 775–783. [DOI] [PubMed] [Google Scholar]
- Cowman A.F., Karcz,S., Galatis,D. and Culvenor,J.G. (1991) A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J. Cell Biol., 113, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A.F., Galatis,D. and Thompson,J.K. (1994) Selection for mefloquine resistance in Plasmodium falciparum is linked to amplification of the pfmdr1 gene and cross-resistance to halofantrine and quinine. Proc. Natl Acad. Sci. USA, 91, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B.S. and Cowman,A.F. (1996) Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc. Natl Acad. Sci. USA, 93, 7289–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb B.S. et al. (1997a) Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell, 89, 287–296. [DOI] [PubMed] [Google Scholar]
- Crabb B.S., Triglia,T., Waterkeyn,J.G. and Cowman,A.F. (1997b) Stable transgene expression in Plasmodium falciparum. Mol. Biochem. Parasitol., 90, 131–144. [DOI] [PubMed] [Google Scholar]
- Dolan S.A., Proctor,J.L., Alling,D.W., Okubo,Y., Wellems,T.E. and Miller,L.H. (1994) Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol., 64, 55–63. [DOI] [PubMed] [Google Scholar]
- Farquhar M.G. (1985) Progress in unravelling pathways of Golgi traffic. Annu. Rev. Cell Biol., 1, 489–530. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski,R.A. and Lazarow,P.B. (1984) Synthesis of a major integral membrane protein polypeptide of rat liver peroxisomes on free polysomes. Proc. Natl Acad. Sci. USA, 81, 7127–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M.-J. and Sambrook,J. (1992) Protein folding in the cell. Nature, 355, 33–44. [DOI] [PubMed] [Google Scholar]
- Hadley T.J., Erkmen,Z., Kaufman,B.M., Futrovsky,S., McGuinnis,M.H., Graves,P., Sadoff,J.C. and Miller,L.H. (1986) Factors influencing invasion of erythrocytes by Plasmodium falciparum parasites: the effects of an N-acetyl glucosamine neoglycoprotein and an anti-glycophorin A antibody. Am. J. Trop. Med. Hyg., 35, 898–905. [DOI] [PubMed] [Google Scholar]
- Hadley T.J., Klotz,F.W., Pasvol,G., Haynes,J.D. and McGinniss,M.H. (1987) Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J. Clin. Invest., 80, 1190–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnyuttanakorn P., McBride,J.S., Donachie,S., Heidrich,H.-G. and Ridley,R.G. (1992) Inhibitory monoclonal antibodies recognise epitopes adjacent to a proteolytic cleavage site on the RAP-1 protein of Plasmodium falciparum. Mol. Biochem. Parasitol., 55, 177–186. [DOI] [PubMed] [Google Scholar]
- Hartl F.-U. and Neupert,W. (1990) Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science, 247, 930–938. [DOI] [PubMed] [Google Scholar]
- Howard R.F. (1992) The sequence of the p82 rhoptry protein is highly conserved between two Plasmodium falciparum isolates. Mol. Biochem. Parasitol., 51, 327–330. [DOI] [PubMed] [Google Scholar]
- Howard R.F. and Peterson,C. (1996) Limited RAP-1 sequence diversity in field isolates of Plasmodium falciparum. Mol. Biochem. Parasitol., 77, 95–98. [DOI] [PubMed] [Google Scholar]
- Howard R.F. and Reese,R.T. (1990) Plasmodium falciparum: hetero-oligomeric complexes of rhoptry polypeptides. Exp. Parasitol., 71, 330–342. [DOI] [PubMed] [Google Scholar]
- Howard R.F. and Schmidt,C.M. (1995) The secretory pathway of Plasmodium falciparum regulates transport of p82/RAP-1 to the rhoptries. Mol. Biochem. Parasitol., 74, 43–54. [DOI] [PubMed] [Google Scholar]
- Howard R.F., Stanley,H.A., Campbell,G.H. and Reese,R.T. (1984) Proteins responsible for a punctate fluorescence pattern in Plasmodium falciparum merozoites. Am. J. Trop. Med. Hyg., 33, 1055–1059. [DOI] [PubMed] [Google Scholar]
- Howard R.F., Jacobson,K.C., Rickel,E. and Thurman,J. (1998a) Analysis of inhibitory epitopes in the Plasmodium falciparum rhoptry protein RAP-1 including identification of a second inhibitory epitope. Infect. Immun., 66, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R.F., Narum,D.L., Blackman,M. and Thurman,J. (1998b) Analysis of the processing of Plasmodium falciparum rhoptry-associated protein 1 and localization of Pr86 to schizont rhoptries and p67 to free merozoites. Mol. Biochem. Parasitol., 92, 111–122. [DOI] [PubMed] [Google Scholar]
- Jaikaria N.S., Rozario,C., Ridley,R.G. and Perkins,M.E. (1993) Biogenesis of rhoptry organelles in Plasmodium falciparum. Mol. Biochem. Parasitol., 57, 269–280. [DOI] [PubMed] [Google Scholar]
- La Greca N., Hibbs,A.R., Riffkin,C., Foley,M. and Tilley,L. (1997) Identification of an endoplasmic reticulum-resident calcium-binding protein with multiple EF-hand motifs in asexual stages of Plasmodium falciparum. Mol. Biochem. Parasitol., 89, 283–293. [DOI] [PubMed] [Google Scholar]
- Lambros C. and Vanderberg,J.P. (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol., 65, 418–420. [PubMed] [Google Scholar]
- Lobo C.-A., Fujioka,H., Aikawa,M. and Kumar,N. (1999) Disruption of the Pfg27 locus by homologous recombination leads to loss of the sexual phenotype in P.falciparum. Mol. Cell, 3, 795–798. [DOI] [PubMed] [Google Scholar]
- Lustigman S., Anders,R.F., Brown,G.V. and Coppel,R.L. (1988) A component of an antigenic rhoptry complex of Plasmodium falciparum is modified after merozoite invasion. Mol. Biochem. Parasitol., 30, 217–224. [DOI] [PubMed] [Google Scholar]
- McColl D.J. and Anders,R.F. (1997) Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol. Biochem. Parasitol., 90, 21–31. [DOI] [PubMed] [Google Scholar]
- Miller L.H., Haynes,J.D., McAuliffe,F.M., Shiroishi,T. and Durocher,J.R. (1977) Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J. Exp. Med., 146, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G.H., Hadley,T.J., McGinniss,M.H., Klotz,F.W. and Miller,L.H. (1986) Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood, 67, 1519–1521. [PubMed] [Google Scholar]
- Ozawa M. and Muramatsu,T. (1997) Reticulocalbin, a novel endoplasmic reticulum resident Ca2+-binding protein in the sarcoplasmic/reticulum of muscle and non-muscle cells. J. Biol. Chem., 268, 699–705. [PubMed] [Google Scholar]
- Perrin L.H., Merkli,B., Gabra,M.S., Stocker,J.W., Chizzolini,C. and Richle,R. (1985) Immunization with a Plasmodium falciparum merozoite surface antigen induces a partial immunity in monkeys. J. Clin. Invest., 75, 1718–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M.G., Marshall,V.M., Smythe,J.A., Crewther,P.E., Lew,A., Silva,A., Anders,R.F. and Kemp,D.J. (1989) Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol. Cell. Biol., 9, 3151–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley R.G., Takacs,B., Etlinger,H. and Scaife,J.G. (1990a) A rhoptry antigen of Plasmodium falciparum is protective in Saimiri monkeys. Parasitology, 101, 187–192. [DOI] [PubMed] [Google Scholar]
- Ridley R.G., Takacs,B., Lahm,H.W., Delves,C.J., Goman,M., Certa,U., Matile,H., Woollett,G.R. and Scaife,J.G. (1990b) Characterisation and sequence of a protective rhoptry antigen from Plasmodium falciparum. Mol. Biochem. Parasitol., 41, 125–134. [DOI] [PubMed] [Google Scholar]
- Ridley R.G., Lahm,H.W., Takács,B. and Scaife,J.G. (1991) Genetic and structural relationships between components of a protective rhoptry antigen complex from Plasmodium falciparum. Mol. Biochem. Parasitol., 47, 245–246. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 12.4–12.29. [Google Scholar]
- Saul A., Cooper,J., Hauquitz,D., Irving,D., Cheng,Q., Stowers,A. and Limpaiboon,T. (1992) The 42-kilodalton rhoptry-associated protein of Plasmodium falciparum. Mol. Biochem. Parasitol., 50, 139–150. [DOI] [PubMed] [Google Scholar]
- Schofield L., Bushell,G.R., Cooper,J.A., Saul,A.J., Upcroft,J.A. and Kidson,C. (1986) A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol. Biochem. Parasitol., 18, 183–195. [DOI] [PubMed] [Google Scholar]
- Soldati D., Kim,K., Kampmeier,J., Dubremetz,J.F. and Boothroyd,J.C. (1995) Complementation of a Toxoplasma gondii ROP1 knock-out mutant using phleomycin selection. Mol. Biochem. Parasitol., 74, 87–97. [DOI] [PubMed] [Google Scholar]
- Trager W. and Jensen,J.B. (1978) Cultivation of malarial parasites. Nature, 273, 621–622. [DOI] [PubMed] [Google Scholar]
- Triglia T., Wang,P., Sims,P.F.G., Hyde,J.E. and Cowman,A.F. (1998) Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J., 17, 3807–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P. and Blobel,G. (1981) Translocation of proteins across the endoplasmic reticulum II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in vitro-assembled polysomes synthesising secretory machinery. J. Cell Biol., 91, 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Kirkman,L.A. and Wellems,T.E. (1996) Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl Acad. Sci. USA, 93, 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]