Abstract
Yeast plasma membranes contain a small 55 amino acid hydrophobic polypeptide, Pmp3p, which has high sequence similarity to a novel family of plant polypeptides that are overexpressed under high salt concentration or low temperature treatment. The PMP3 gene is not essential under normal growth conditions. However, its deletion increases the plasma membrane potential and confers sensitivity to cytotoxic cations, such as Na+ and hygromycin B. Interestingly, the disruption of PMP3 exacerbates the NaCl sensitivity phenotype of a mutant strain lacking the Pmr2p/Enap Na+-ATPases and the Nha1p Na+/H+ antiporter, and suppresses the potassium dependency of a strain lacking the K+ transporters, Trk1p and Trk2p. All these phenotypes could be reversed by the addition of high Ca2+ concentration to the medium. These genetic interactions indicate that the major effect of the PMP3 deletion is a hyperpolarization of the plasma membrane potential that probably promotes a non-specific influx of monovalent cations. Expression of plant RCI2A in yeast could substitute for the loss of Pmp3p, indicating a common role for Pmp3p and the plant homologue.
Keywords: membrane potential/Pmp3p/Saccharomyces cerevisiae/toxic cation tolerance
Introduction
Proteolipids are a class of extremely hydrophobic proteins distinguished by their solubility in mixtures of organic solvents such as chloroform–methanol (Folch and Lees, 1951). We have previously purified the proteolipidic fraction extracted from yeast plasma membranes and sequenced two small (38 residues) and highly hydrophobic isoproteins, named Pmp1p and Pmp2p (Navarre et al., 1992, 1994). Deletion of both genes, PMP1 and PMP2, significantly decreases the rate of ATP hydrolysis of Pma1p, the plasma membrane H+-ATPase (Navarre et al., 1994). Recently, mutations in Pma1p were reported to be Na+ resistant partly by reducing Na+ uptake, but also by up-regulating the activity of an intracellular Na+/H+ exchanger (Nass et al., 1997; Withee et al., 1998).
Na+ homeostasis in yeast is achieved by the coordinate activity of plasma membrane efflux and influx systems, and by an efficient Na+ sequestration system (see reviews by Gaber, 1992; Rodriguez-Navarro et al., 1994; Niu et al., 1995; Serrano, 1996; Schachtman and Liu, 1999; Serrano et al., 1999).
Na+ efflux is mediated mainly by several P-type ATPases encoded by the PMR2/ENA locus, containing up to five nearly identical genes (Garciadeblas et al., 1993; Wieland et al., 1995). Disruption of the whole PMR2/ENA locus makes cells hypersensitive to NaCl (Wieland et al., 1995), but does not abolish Na+ efflux completely (Haro et al., 1991), indicating the existence of another plasma membrane Na+ efflux system. The plasma membrane Na+/H+ antiporter, Nha1p, which is highly homologous to the fission yeast Sod2p antiporter, mediates the exchange of cytoplasmic Na+ and K+ with external H+ (Prior et al., 1996; Bañuelos et al., 1998). In addition to the plasma membrane Na+ efflux systems, Nhx1p/Nha2p plays an important role in halotolerance by mediating Na+ sequestration in a pre-vacuolar compartment (Nass et al., 1997; Nass and Rao, 1998; Numata et al., 1998). Finally, YJL094c encodes a putative Na+/H+ permease similar to the Enterococcus hirae Na+/H+ permease, NapA (Miosga et al., 1994), although it functions rather as a K+/H+ exchanger since its deletion results in an increased K+ content (Ramirez et al., 1998).
Moreover, Na+ is believed to be taken up by the K+ transporters Trk1p and Trk2p (Gaber et al., 1988; Ko and Gaber, 1991; Ramos et al., 1994). At high Na+ concentrations, Trk1p increases its selectivity, favouring K+ over Na+ (Haro et al., 1993; Mendoza et al., 1994). This change depends upon the function of the phosphatase calcineurin (Mendoza et al., 1994). A non-specific plasma membrane Na+ and K+ uptake channel, independent of Trk1p and Trk2p, has been shown in whole-cell patch–clamp experiments (Bihler et al., 1998; Roberts et al., 1999). The corresponding monovalent cation current NSC1 is highly sensitive to divalent cations (Roberts et al., 1999).
In this report, a novel polypeptide, named Pmp3p, was purified from Saccharomyces cerevisiae plasma membranes after extraction with organic solvent. Deletion of PMP3 confers NaCl hypersensitivity to a mutant strain lacking the major Na+ efflux systems, Pmr2p/Enap and Nha1p. It also restores growth of a strain deficient in K+ uptake, particularly at low pH. We show that these phenotypes may be due to an increased cation uptake resulting from a hyperpolarization of the plasma membrane. The loss of Pmp3p function can be replaced by the expression of a plant homologue. This result confirms the suitability of the yeast system to study the molecular mechanisms underlying halotolerance in higher plants, as previously described (see recent reviews by Schachtman and Liu, 1999; Serrano et al., 1999).
Results
Isolation and sequence of the PMP3 gene
Microsequencing of the chloroform–methanol extract of the yeast plasma membranes has led to the identification of two small (38 residues) and highly similar polypeptides, Pmp1p and Pmp2p (Navarre et al., 1992, 1994). A close examination of these preparations revealed the presence of a third hydrophobic polypeptide beginning with the sequence Met-Asp-Ser-Ala-Lys. This protein, named Pmp3p, was unnoticed initially because it represented <10% of the total protein content of this fraction (Figure 1A). The first 25 residues of Pmp3p were then determined unambiguously, using the organic solvent extract prepared from the mutant strain CN12, containing a deletion of both PMP1 and PMP2 genes (see Table I). A search for identity between this amino acid sequence and yeast expressed sequence tags (ESTs) revealed a match between EST 101712 (accession No. T36719) and the C-terminal end of the microsequenced Pmp3p.
Fig. 1. Pmp3p is a novel 55 amino acid proteolipid of the yeast plasma membrane. (A) Yields (in pmol) of phenylthiohydantoin derivatives recovered after each cycle of Edman degradation for the proteolipid fraction extract derived from S.cerevisiae W303-1B plasma membranes. (B) Alignment of Pmp3p with RCI2A (A.thaliana), RCI2B (A.thaliana), ESI3 (L.elongatum) and BLT101 (Hordeum vulgare): amino acids identical in Pmp3p and one of the plant 54 amino acid polypeptides are boxed. (C) Silver-stained Tricine–SDS–polyacrylamide gels of purified plasma membrane preparations (50 µg/gel lane). Lane 1, molecular weight markers; lane 2, PMP3 wild-type strain W303-1B; lane 3, pmp3Δ mutant strain CN3.
Table I. Yeast strains used.
| Strain | Genotype | Reference |
|---|---|---|
| W303-1B | MATα ade 2-1 can 1-100 his3-11,15 leu 2-3,112 trp1-1 ura3-1 | Wallis et al. (1989) |
| CN3 | W303-1B Δpmp3::TRP1 | this work |
| CN12 | W303-1B Δpmp1::URA3 Δpmp2::HIS3 | Navarre et al. (1994) |
| CN123 | W303-1B Δpmp1::URA3 Δpmp2::HIS3 Δpmp3::TRP1 | this work |
| YR93 | MATa ade2 his3-Δ200 leu2-3,112 lys2-Δ201 ura3-52 gal2 Δpmr2-2::HIS3 | Wieland et al. (1995) |
| YR93-3 | YR93 Δpmp3::URA3 | this work |
| YR93-1 | YR93 Δnha1::LEU2 | this work |
| YR93-31 | YR93 Δpmp3::URA3 Δnha1::LEU2 | this work |
| YR93-31-RCI2A | YR93 Δpmp3::PMP3PROM-RCI2A-PMP3STOP Δnha1::LEU2 | this work |
| CY162 | MATα ura3-52 trk1Δ his 4-15 trk2Δ1::pCK64 | Ko and Gaber (1991) |
| CY162-3 | CY162 Δpmp3::URA3 | this work |
A PCR product of PMP3 was amplified from yeast DNA by using a degenerate primer encoding the N-terminal sequence of Pmp3p and a second primer found in the 3′-non-coding region of EST 101712. The PCR product was labelled and used as a probe to screen an EcoRI genomic DNA library by hybridization. Positive clones containing the PMP3 gene in a 2 kb EcoRI fragment were subcloned and sequenced. The deduced open reading frame (ORF) was found to encode a highly hydrophobic 55 amino acid protein (Mr = 6109), which is predicted to contain two membrane-spanning segments. Unlike Pmp1p and Pmp2p (Navarre et al., 1992, 1994), Pmp3p conserves its first N-terminal methionine residue, as indicated by microsequencing. Searching for homologues of Pmp3p in protein databases revealed 40% sequence identity with several 54 residue plant polypeptides whose expression is induced by high salinity or low temperature (Figure 1B). For instance, ESI3 is encoded by a gene that belongs to a group of 11 members referred to as ESI (for early salt stress-induced) because their expression is induced in the roots of the salt-tolerant wheatgrass Lophopyrum elongatum upon addition of 250 mM NaCl (Gulick and Dvorak, 1992; Galvez et al., 1993). ESI3 (Gulick et al., 1994) is 100% identical to the polypeptide encoded by the blt101 gene of winter barley. Transcription of blt101 is induced in all tissue types tested (shoot meristem, mature leaves and roots) by low temperature treatment (Goddard et al., 1993). Pmp3p, moreover, is also highly similar to two related 54 amino acid Arabidopsis thaliana polypeptides, RCI2A and RCI2B, whose synthesis is induced transiently by low temperatures (Capel et al., 1997).
Once the complete sequence of the S.cerevisiae genome was available, we were able to locate the PMP3 gene on the right arm of chromosome IV (YDR276c), between genes YDR275w and MTH1 (YDR277c), from position 1 013 467 to position 1 013 634. Northern blot analysis of total RNA showed the PMP3 transcript to be ∼0.5 kb long and fairly abundant (data not shown), in agreement with the presence of a perfect TATA box (TATAATA) located 54 bp upstream from the AUG codon, a high codon adaptation index value (0.37) and the analysis of the yeast transcriptome (Velculescu et al., 1997) indicating the presence of 56 mRNA copies per cell.
NaCl sensitivity associated with deletion of PMP3
To determine the physiological function of Pmp3p in yeast, the chromosomal copy of PMP3 was disrupted by homologous gene recombination. Haploid strains CN3 (pmp3Δ) and CN123 (pmp1Δ pmp2Δ pmp3Δ) were obtained, indicating that Pmp3p is not essential under normal growth conditions. Figure 1C (Tricine–SDS–PAGE) shows that plasma membranes isolated from the CN3 (pmp3Δ) mutant lack a silver-stainable band of 6.2 kDa, corresponding exactly to the predicted molecular mass of Pmp3p.
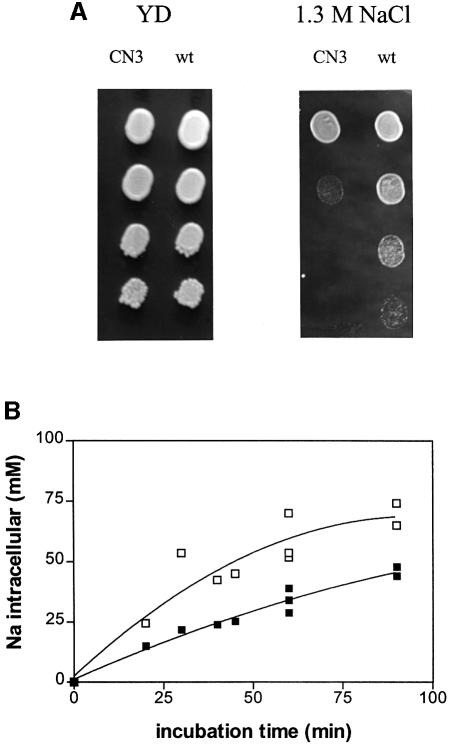
The CN3 strain was more sensitive to high concentrations of NaCl (>1 M) than the PMP3 wild-type strain (W303-1B) (Figures 2A and 3). The NaCl sensitivity phenotype is not explained by an osmotic effect, since the CN3 strain cells grew as well as the wild type on 2.0 and 2.5 M sorbitol (data not shown). The wild-type and mutant strains were incubated with 1 M NaCl and the intracellular Na+ concentrations were determined at various time intervals. At each sampling time, the intracellular Na+ concentration was nearly twice as high in CN3 (pmp3Δ) as in W303-1B (PMP3) (Figure 2B), indicating that the defect of pmp3Δ cells in NaCl tolerance results from a higher intracellular Na+ concentration.
Fig. 2. Effect of PMP3 deletion on NaCl tolerance. (A) Increased sensitivity to NaCl of a pmp3-deleted strain. Serial 10-fold dilutions of saturated cultures of wild-type W303-1B (PMP3) and CN3 (pmp3Δ) were spotted onto YD plates supplemented with the indicated concentrations of NaCl. The plates were photographed after a 2 day incubation at 30°C. (B) Effect of PMP3 deletion on the time course of intracellular Na+ accumulation during salt stress. Exponentially growing CN3 (pmp3Δ; open squares) and W303-1B (PMP3; closed squares) cells in YD medium were stressed by adding NaCl at time zero to a final concentration of 0.9 M. Aliquots were taken at the times indicated for determination of the intracellular Na+ concentration as described in Materials and methods.
Fig. 3. Deletion of PMP3 exacerbates the Na+ sensitivity phenotype of strains lacking Pmr2p/Enap and Nha1p. Strains W303-1B (PMR2 NHA1 PMP3; closed squares), CN3 (PMR2 NHA1 pmp3Δ; open squares), YR93 (pmr2Δ NHA1 PMP3; closed triangles), YR93-3 (pmr2Δ NHA1 pmp3Δ; open triangles), YR93-1 (pmr2Δ nha1Δ PMP3; closed circles) and YR93-31 (pmr2Δ nha1Δ pmp3Δ; open circles) were grown in YD medium to saturation. Then, 10 µl aliquots were transferred to 3 ml of YD medium supplemented with various NaCl concentrations and grown for 15 h at 30°C. Relative growth is the ratio of the OD600 of a given culture to the value obtained with control cells grown in YD. The experiment was repeated three times with similar results.
To examine further the contribution of Pmp3p to Na+ tolerance, we analysed the effect of the PMP3 deletion on the Na+ sensitivity of a mutant deficient in the Enap/Pmr2p plasma membrane Na+-ATPases. In comparative growth assays, YR93-3 (pmr2Δ pmp3Δ) and YR93 (pmr2Δ PMP3) cells showed half-maximal inhibition at 200 and 300 mM NaCl, respectively (Figure 3). Thus, deletion of PMP3 in the pmr2Δ background markedly increased the sensitivity to Na+.
It has been reported previously that, in a pmr2Δ background, the loss of the plasma membrane Na+/H+ antiporter, Nha1p, renders yeast more sensitive to Na+ (Prior et al., 1996; Bañuelos et al., 1998). We confirmed this observation with strain YR93-1 (pmr2Δ nha1Δ PMP3), which showed half-maximal growth inhibition at ∼200 mM NaCl (Figure 3). YR93-1 (pmr2Δ nha1Δ PMP3) and YR93-3 (pmr2Δ NHA1 pmp3Δ) thus behave similarly in this respect. To test whether the effects of deleting NHA1 and PMP3 are additive in this sensitivity test, we constructed strain YR93-31 (pmr2Δ nha1Δ pmp3Δ). This strain showed half-maximal inhibition at 125 mM NaCl (Figure 3). The additivity of the NaCl sensitivities conferred by PMP3 deletion and PMR2 and/or NHA1 deletions suggests that Pmp3p does not interfere with either of the two plasma membrane Na+ efflux transporters and that the NaCl sensitivity phenotype of pmp3Δ cells might be correlated with an increase in Na+ uptake.
Potassium transport associated with PMP3 deletion
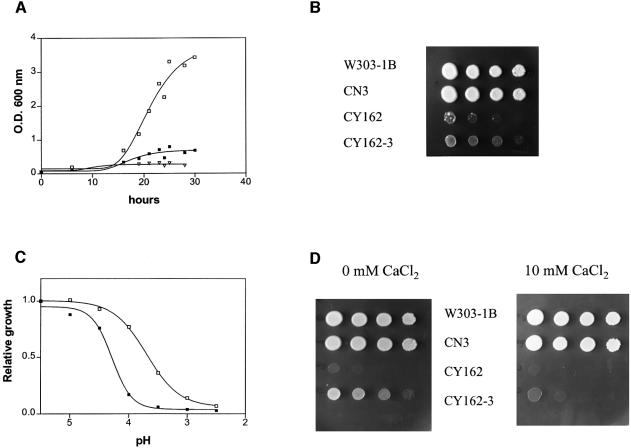
To examine further the role of the PMP3 deletion in cation uptake, we analysed the effect of deleting PMP3 in a strain lacking the Trk1p and Trk2p K+ transporters, and which grows only on media supplemented with high K+ concentrations (Ko and Gaber, 1991). Growth curves of CY162 (trk1Δ trk2Δ PMP3) and CY162-3 (trk1Δ trk2Δ pmp3Δ) in YD medium without any added KCl showed that deletion of PMP3 considerably enhanced the growth of the trk1Δ trk2Δ cells (Figure 4A). As a matter of fact, the CY162 (trk1Δ trk2Δ PMP3) cells started to grow after a lag phase of ∼48 h, the culture eventually reaching a higher cell density than that of CY162-3 (trk1Δ trk2Δ pmp3Δ). It is likely that spontaneous suppressor mutations were selected that restored the ability to grow on low potassium medium, as observed previously with glucose and amino acid transporter mutants (Ko et al., 1993; Wright et al., 1997; Liang et al., 1998; Madrid et al., 1998). Isolated colonies were indeed detected after 3 days when the CY162 strain was plated on YD medium (Figure 4B).
Fig. 4. Growth assays of trk1Δ trk2Δ and trk1Δ trk2Δ pmp3Δ. (A) Growth of CY162 (trk1Δ trk2Δ PMP3) (closed symbols) and CY162-3 (trk1Δ trk2Δ pmp3Δ) (open symbols) in YD medium without any potassium added supplemented with 1 mM EGTA (squares) or with 10 mM CaCl2 (triangles). (B) Serial 10-fold dilutions of overnight cultures of W303-1B (TRK1 TRK2 PMP3), CN3 (TRK1 TRK2 pmp3Δ), CY162 (trk1Δ trk2Δ PMP3) and CY162-3 (trk1Δ trk2Δ pmp3Δ) in YD medium containing 100 mM KCl were spotted onto YD plates without any KCl added. The plates were photographed after a 3 day incubation at 30°C. (C) Strains CY162 (trk1Δ trk2Δ PMP3; closed squares) and CY162-3 (trk1Δ trk2Δ pmp3Δ; open squares) were grown to saturation in YD medium containing 100 mM KCl. Then, 10 µl aliquots were transferred to 3 ml of YD containing 100 mM KCl at the indicated pH, and the OD600 was measured after 15 h at 30°C. Relative growth is expressed as the ratio of the OD600 of a given culture at the indicated pH to the OD600 of the control culture at pH 5.5. The experiment was repeated three times with similar results. (D) Serial 10-fold dilutions of overnight cultures of W303-1B (TRK1 TRK2 PMP3), CN3 (TRK1 TRK2 pmp3Δ), CY162 (trk1Δ trk2Δ PMP3) and CY162-3 (trk1Δ trk2Δ pmp3Δ) in YD medium containing 100 mM KCl were spotted onto YD plates containing 100 mM KCl at pH 3.0 and 10 mM CaCl2 when indicated. The plates were photographed after a 2 day incubation at 30°C.
Deletion of TRK1,2 confers hypersensitivity to low pH, even in the presence of a high concentration of K+ (Ko and Gaber, 1991). In our assay, the growth defect of the trk1Δ trk2Δ PMP3 (CY162) was observed at a pH <4.0. Interestingly, deletion of PMP3 in the trk1Δ trk2Δ background (strain CY162-3) restored growth at pH 3.0–4.5 (Figure 4C).
The phenotypes obtained with the trk1,2Δ cells could be explained by an increase in K+ uptake caused by PMP3 deletion.
Sensitivity to toxic cations and membrane hyperpolarization associated with PMP3 deletion
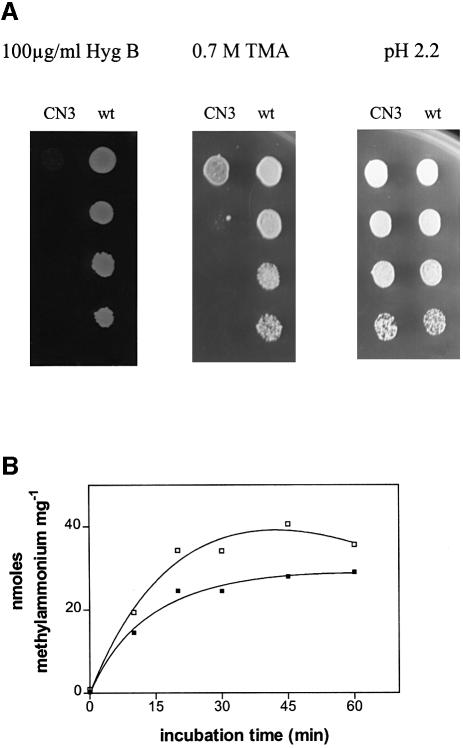
Deletion of PMP3 confers sensitivity to toxic cations other than Na+, such as hygromycin B and tetramethylammonium (TMA). Indeed, pmp3Δ (CN3) cells failed to grow on media containing 100 µg/ml hygromycin B or 700 mM TMA, whereas the growth of the PMP3 (W303-1B) cells was not modified under the same conditions (Figure 5A). The plasma membrane electric potential has been reported to be a major determinant of toxic cation tolerance. It is therefore possible that the PMP3 deletion causes a modification of the membrane potential, which might increase the rate of cation uptake into the cell and, consequently, the cell’s sensitivity to toxic cations. To compare the membrane potential of the W303-1B (PMP3) and CN3 (pmp3Δ) strains, we measured [14C]methylammonium uptake as an indicator of plasma membrane potential (Vallejo et Serrano, 1989; Mulet et al., 1999). Uptake of methylammonium was increased in CN3 (pmp3Δ) when compared with W303-1B (PMP3) (Figure 5B), consistent with a membrane potential hyperpolarization in pmp3Δ cells.
Fig. 5. Effect of PMP3 deletion on membrane potential indicators. (A) Increased sensitivity to toxic cations of pmp3-deleted strains. Serial 10-fold dilutions of saturated cultures of wild-type W303-1B (PMP3) and CN3 (pmp3Δ) were spotted onto YD plates supplemented with the indicated concentrations of hygromycin B, TMA or H+. The plates were photographed after a 2 day incubation at 30°C. (B) Increased methylammonium uptake caused by deletion of PMP3. W303-1B (PMP3; closed squares) and CN3 (pmp3Δ; open squares) cells were grown to exponential phase in MM and washed. The uptake of 2 mM [14C]methylammonium was determined at the times indicated as described in Materials and methods. The experiment was repeated twice with similar results.
The Pmp1p and Pmp2p isoproteolipids are required for maximal activity of Pma1p (Navarre et al., 1994). One can therefore imagine that the hyperpolarization observed in the pmp3Δ strain might result from an activation of Pma1p. This appears unlikely as deletion of PMP3 had no effect on Pma1p activity. Purified plasma membranes from CN3 (pmp3Δ) or W303-1B (PMP3) displayed similar Pma1p-mediated MgATP hydrolysis activity at each MgATP concentration tested (Figure 6A). As a control, plasma membranes were also purified from a pmp1Δ pmp2Δ pmp3Δ (CN123) mutant. As previously reported for CN12 (pmp1Δ pmp2Δ PMP3) (Navarre et al., 1994), the ATPase activity was decreased 2-fold, in comparison with that of CN3 or W303-1B (Figure 6A). Asolectin vesicles were reconstituted from plasma membranes prepared from W303-1B (PMP3) and CN3 (pmp3Δ). No difference in MgATP-dependent H+ pumping was observed between the two strains: proton pumping, expressed as a percentage of ACMA (9-amino-6-chloro-2-methoxy-acridine) quenching, showed comparable dependence on specific ATPase activity (Figure 6B). It seems likely therefore that, in the pmp3Δ mutants (CN3), the increasing Na+, K+ and hygromycin B uptake is associated with membrane hyperpolarization through a mechanism that does not require the function of Pma1p. Note, however, the absence of any ACMA quenching by the membrane vesicles of the pmp3Δ strain at low MgATP concentrations (<0.1 mM), in comparison with the wild type (Figure 6B).
Fig. 6. Deletion of PMP3 has no effect on plasma membrane H+-ATPase activity. (A) ATP dependence of ATPase activity of plasma membranes from W303-1B (PMP1 PMP2 PMP3; closed squares), CN3 (PMP1 PMP2 pmp3Δ; open squares) and CN123 (pmp1Δ pmp2Δ pmp3Δ; open triangles). Each curve represents the average of three independent preprations. (B) ATP-dependent fluorescence quenching of ACMA versus ATP hydrolysis. The initial rate of fluorescence quenching in sealed plasma membranes from W303-1B (PMP3; closed squares) and CN3 (pmp3Δ; open squares) was determined in the presence of varying MgATP concentrations (from 0.05 to 4 mM) and plotted as a function of the ATP hydrolysis activity measured under the same conditions.
Calcium reverses the phenotypes of pmp3-deleted strains
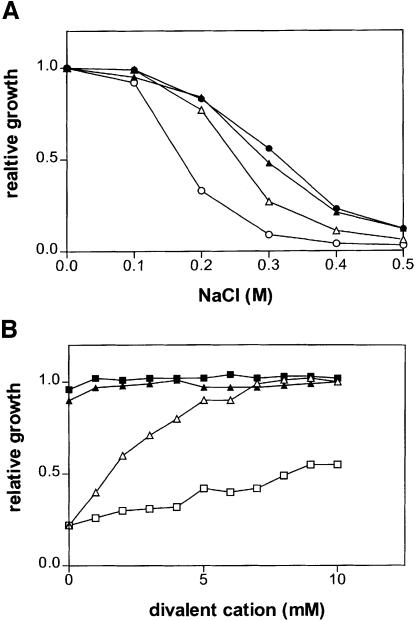
We tested whether the increased uptake of Na+ and K+ in pmp3Δ mutants is inhibited by divalent cations. In YD medium without any added potassium, the growth of CY162-3 (trk1Δ trk2Δ pmp3Δ) was impaired in the presence of 10 mM CaCl2, similarly to that of CY162 (trk1Δ trk2Δ PMP3) in the absence of CaCl2 (Figure 4A). Similarly, the growth of CY162-3 (trk1Δ trk2Δ pmp3Δ) in a pH 3.0 medium containing 100 mM KCl was totally inhibited by 10 mM CaCl2 (Figure 4D). To investigate how calcium affects the Na+ sensitivity caused by the PMP3 deletion, the Na+ sensitivity of YR93 (pmr2Δ PMP3) and YR93-3 (pmr2Δ pmp3Δ) were compared in the presence of either 10 mM CaCl2 or 1 mM EGTA. At 10 mM CaCl2, YR93-3 (pmr2Δ pmp3Δ) and YR93 (pmr2Δ PMP3) displayed similar sensitivity to NaCl, which is comparable to that seen with YR93 in the absence of added CaCl2 (Figure 7A). To examine further the effect of divalent cations on the Na+ sensitivity of YR93-3 (pmr2Δ pmp3Δ), the cells were grown in YD medium containing 0.2 M NaCl and increasing concentrations of CaCl2 or MgCl2. The NaCl sensitivity phenotype of YR93-3 (pmr2Δ pmp3Δ) decreased continuously with the CaCl2 concentrations ranging from 0 to 7 mM (Figure 7B). Above this concentration, a plateau is reached (Figure 7B). Mg2+ had a lesser protective effect at concentrations up to 10 mM, although millimolar Mg2+ concentrations clearly did improve the strain’s growth (Figure 7B).
Fig. 7. Effect of divalent cations on the NaCl sensitivity due to the PMP3 deletion. (A) Strains YR93 (pmr2Δ PMP3; closed symbols) and YR93-3 (pmr2Δ pmp3Δ; open symbols) were grown in YD medium to saturation, then 10 µl aliquots were transferred to 3 ml of YD medium containing either 1 mM EGTA (circles) or 10 mM CaCl2 (triangles) supplemented with the indicated concentration of NaCl. After 15 h at 30°C, the OD600 of all cultures was measured. Relative growth is the ratio between the OD600 obtained for a given NaCl concentration and the OD600 of a control culture in YD. The experiment was repeated twice with similar results. (B) Strains YR93 (pmr2Δ PMP3; closed symbols) and YR93-3 (pmr2Δ pmp3Δ; open symbols) were grown in YD medium to saturation, then 10 µl aliquots of each culture were transferred to 3 ml of YD medium containing 0.2 M NaCl and either MgCl2 (squares) or CaCl2 (triangles) at the indicated concentration. Relative growth is the ratio between the OD600 obtained for a given divalent ion concentration and the OD600 of the corresponding culture containing 10 mM CaCl2. The experiment was repeated twice with similar results.
Expression of Arabidopsis thaliana RCI2A in yeast
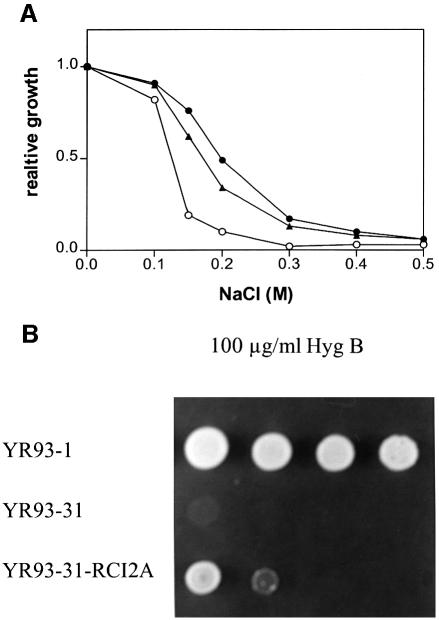
To determine whether the 54 residue plant polypeptides are functional homologues of the yeast Pmp3p, we placed the A.thaliana RCI2A cDNA clone under the control of the PMP3 transcription promoter and introduced it by homologous recombination into the yeast chromosomal PMP3 locus. The resulting yeast strain, YR93-31-RCI2A, expresses the A.thaliana RCI2A gene instead of the S.cerevisiae PMP3 gene. Na+ sensitivity of YR93-1 (pmr2Δ nha1Δ PMP3), YR93-31 (pmr2Δ nha1Δ pmp3Δ) and YR93-31-RCI2A (pmr2Δ nha1Δ pmp3Δ RCI2A) cells was tested in liquid medium containing increasing concentrations of NaCl. Expression of the plant RCI2A gene in the pmr2Δ nha1Δ background conferred the same sodium sensitivity as expression of the yeast PMP3 gene in the same background (Figure 8A), demonstrating that the yeast Pmp3p and the plant RCI2A are functionally interchangeable with regard to Na+ tolerance. Moreover, expression of the plant RCI2A gene is capable of partially suppressing the hygromycin B sensitivity of the pmp3 mutant (Figure 8B), suggesting that the plant RCI2A abolishes the hyperpolarization resulting from PMP3 deletion.
Fig. 8. The A.thaliana cDNA T20621 complements the PMP3 deletion with regard to NaCl and hygromycin B sensitivity. (A) Strains YR93-1 (pmr2Δ nha1Δ PMP3; closed circles), YR93-31 (pmr2Δ nha1Δ pmp3Δ; open circles) and YR93-31-RCI2A (pmr2Δ nha1Δ pmp3Δ RCI2A; closed triangles) were grown in YD medium to saturation, then 10 µl aliquots were transferred to 3 ml of YD supplemented with NaCl at the indicated concentration. After 15 h at 30°C, the OD600 of all cultures was measured. Relative growth is the ratio of the OD600 of a given culture to the OD600 of a control culture in YD. The experiment was repeated twice with similar results. (B) Serial 10-fold dilutions of saturated cultures of YR93-1 (pmr2Δ nha1Δ PMP3), YR93-31 (pmr2Δ nha1Δ pmp3Δ) and YR93-31-RCI2A (pmr2Δ nha1Δ pmp3Δ RCI2A) were spotted onto YD plates supplemented with 100 µg/ml hygromycin B. The plate was photographed after a 2 day incubation at 30°C.
Discussion
Sequence comparison of yeast Pmp3p, a novel and small proteolipid of plasma membrane, shows high homology with Escherichia coli P77240, with Caenorhabditis elegans P34655, Q20516, Q22702, Q22701 and Q22700 putative proteins and with several 54 residue plant polypeptides. All these proteins belong to the upf0057 family (Milton Saier, personal communication) and may share a common function, since at least one of them, the product of the A.thaliana RCI2A gene, can functionally replace Pmp3p in yeast. In the plant L.elongatum, the expression level of ESI3 mRNA, encoding a Pmp3p homologue, increases 3-fold after only 6 h of treatment with 250 mM NaCl or KCl. Then, it gradually declines to the basal level, even though the plant is kept on a high-salt medium (Galvez et al., 1993). In A.thaliana, an increase in RCI2 transcripts is detectable after 12 h of exposure to low temperature (4°C). Maximal induction (up to 10 times the basal level) occurs after 24 h and then begins to decrease (Capel et al., 1997). In barley, the blt101 transcript level rises after 24 h of low-temperature treatment and peaks after 7 days. If the plant is transferred back to the control temperature (20/15°C), the transcript level decreases rapidly to the basal level within 24 h (Goddard et al., 1993; Dunn et al., 1994; Phillips et al., 1997). The rapid but transient rise in the corresponding transcripts under conditions of high salinity or low temperature suggests that the plant homologues of Pmp3p mediate an early minimal protection against sudden stress, before an overall and more permanent response is initiated. Gene replacement of yeast PMP3 by A.thaliana RCI2A indicates that the two genes are functionally interchangeable, and therefore share a conserved function. However, we failed to detect an induction of PMP3 transcription during a 150 min incubation of pmr2Δ (YR93) and pmr2Δ nha1Δ (YR93-1) cells in the presence of 0.15–0.3 M NaCl (data not shown), probably because the basal level is already very high in non-stressed cells.
In yeast, deletion of PMP3 increases Na+ sensitivity in a strain deficient in the major plasma membrane Na+ efflux systems: the Pmr2p/Enap Na+-ATPases and Nha1p Na+/H+ antiporter. Loss of PMP3 also partially suppresses the K+ requirement of the trk1Δ trk2Δ CY162 mutant and reduces its hypersensitivity to low pH. We showed that pmp3Δ strains were sensitive to toxic cations such as hygromycin B or TMA. Methylammonium uptake is increased in the pmp3Δ cells, consistent with a hyperpolarization of the plasma membrane potential. Taken together, these results indicate that the absence of Pmp3p facilitates a non-specific monovalent cation uptake through an increase in the membrane potential, which is mediated by an uptake system different from Trk1,2p.
The plasma membrane potential is a major determinant of toxic cation tolerance. Mutations in the electrogenic H+-ATPase, Pma1p, lead to increased Na+ resistance, partly because of the reduced Na+ uptake linked to a reduction in the membrane potential (Nass et al., 1997; Withee et al., 1998). pma1 mutants having reduced Pma1p activity exhibit increased tolerance to hygromycin B (McCusker et al., 1987), as a consequence of their membrane potential defect (Perlin et al., 1988). The membrane hyperpolarization seen in pmp3 mutants could not be explained by an up-regulated plasma membrane H+-ATPase activity. Deletion of PMP3 indeed has no effect on ATP hydrolysis, ATP-dependent H+ pumping in asolectin vesicles reconstituted from plasma membranes and medium acidification by intact cells (data not shown).
In addition to the plasma membrane H+-ATPase, inactivation of Trk1p and Trk2p has been reported to affect membrane hyperpolarization (Madrid et al., 1998), resulting in increased sensitivity not only to Na+ and H+, but also to hygromycin B and TMA (Madrid et al., 1998; Mulet et al., 1999). Deletion of TRK1,2 (Madrid et al., 1998) or HAL4,5, genes for two isokinases regulating the K+ transporters (Mulet et al., 1999), results in membrane hyperpolarization that would subsequently activate low-affinity cation uptake pathways (Madrid et al., 1998; Mulet et al., 1999), including several permeases involved in glucose, amino acid, inositol or choline uptake. This hypothesis is supported by the ability of mutated permeases to suppress the phenotype of trk1Δ trk2Δ mutants on K+-limiting medium (Ko et al., 1993; Wright et al., 1997; Liang et al., 1998; Madrid et al., 1998). However, our results indicate that the membrane potential defect in the pmp3 mutants is independent of the Trk1,2p K+ transporter activity, since deletion of PMP3 and TRK1,2 had additive phenotypes.
Patch–clamp experiments on trk1Δ trk2Δ spheroplasts have confirmed the existence of a low-affinity inward K+ current that is sensitive to divalent cations (Bihler et al., 1998; Roberts et al., 1999). This current, named NSC1, is detected at extracellular Ca2+ concentrations <0.1 mM. Interestingly, extracellular K+ could be replaced by Na+ with only a slight modification of the magnitude of the Ca2+-sensitive low-affinity current (Roberts et al., 1999), suggesting that the uptake systems apparently can also take up Na+. The presumptive monovalent cation transport system that is triggered through the membrane hyperpolarization caused by the loss of Pmp3p shares several properties with the NSC1 system, as millimolar concentrations of Ca2+ suppress the increased Na+ and K+ uptake observed in Pmp3p-deficient strains. Alternatively, it is possible that high Ca2+ concentrations act independently of the NSC1 system by dissipating the membrane hyperpolarization mediated by PMP3 deletion.
The PMP3 deletion suppresses the growth defect of the trk1Δ trk2Δ mutant at low pH, although the pmp3Δ deletion alone has no effect on cell tolerance to acidic pH (Figure 5A), despite the hyperpolarized state of the pmp3Δ cells. This observation could be explained if trk1Δ trk2Δ pmp3Δ CY162-3 show decreased proton permeability in comparison with trk1Δ trk2Δ PMP3 CY162 membranes. Preliminary measurements of intracellular pH after the cells were incubated for 3 h at pH 3.0 showed increased acidification of CY162 cells’ interior when compared with CY162-3 cells (data not shown), as if the deletion of PMP3 prevents acidification of the cell’s interior under conditions of low external pH.
In conclusion, the loss of Pmp3p induces a hyperpolarization of the membrane potential. It is possible that Pmp3p activates a depolarizing cation leak (different from Trk1,2p) or mediates the cation leak itself. The growth of trk1Δ trk2Δ pmp3Δ cells on acidic medium is consistent with a decrease of plasma membrane proton permeability, suggesting that Pmp3p possibly could mediate a proton leak. If Pmp3p encodes a proton leak, the similarity between the growth of W303-1B and CN3 in acidic medium probably results from the non-hyperpolarized state of wild-type cells that correlates with a lesser sensitivity to low pH. Further analysis of the proton permeability of pmp3 mutants will be necessary to understand the contribution of Pmp3p in the regulation of membrane potential, a conserved function that could be of crucial importance in halotolerance.
Materials and methods
Media and growth conditions
Yeast strains were grown at 30°C either in rich medium (YD) containing 2% glucose and 2% yeast extract (KAT) or in minimal medium (MM) containing 2% glucose, 0.7% yeast nitrogen base without amino acids (Difco) and the required amino acids. Solid media additionally contained 2% agar (Sigma). NaCl, KCl, CaCl2 and tetramethylammonium chloride were added as indicated. Hygromycin B was added to the medium after autoclaving. Solid YD medium at pH 3.0 or 2.2 was prepared by mixing a 2-fold concentrated YD medium adjusted to the desired pH with HCl to a concentrated agar solution after autoclaving them separately. The ability of yeast strains to grow in various liquid conditions was tested by adding equal amounts of cells from overnight cultures in YD to 3 ml of YD supplemented with 100 mM KCl adjusted to the desired pH with HCl or with various amounts of NaCl in the presence of EGTA, CaCl2 or MgCl2, as indicated. After 15 h at 30°C, the OD600 was measured for each culture. The relative growth of a strain in a given medium was expressed as the ratio of the OD600 obtained with this strain to the OD600 of a control culture.
Isolation and sequencing of the PMP3 gene
A 60 ng aliquot of genomic DNA was amplified using a degenerate 23mer oligonucleotide corresponding to the N-terminal amino acid sequence Met-Asp-Ser-Ala-Lys-Ile-Ile-Asn of Pmp3p and the oligonucleo tide 5′-GAGGGTGACCCGAACATGTTGATACCG-3′, found in the 3′-non-coding region of the EST 101712 clone. The amplification reaction was carried out in the presence of 100 pmol of each oligonucleotide primer, 100 µM dATP, 100 µM dGTP, 100 µM dTTP, 20 µM dCTP and 8 µl of [α-32P]dCTP for 25 cycles (denaturation at 93°C for 30 s, annealing at 55°C for 40 s and extension at 72°C for 3 min). The radiolabelled PCR product was used as a probe to screen a library (2 × 104 clones) by DNA hybridization. The library consists of EcoRI fragments of DNA from W303-1B that were ligated into pTZ19U. Positive clones containing PMP3 in a 2 kb EcoRI fragment were subcloned and sequenced.
Yeast strains
The yeast strains used in this study are listed in Table I. They are derived from the haploid strains W303-1B (Wallis et al., 1989), YR93 (Wieland et al., 1995) and CY162 (Ko and Gaber, 1991).
The promoter and 3′ end regions of PMP3 were amplified by PCR and cloned into the pTZ vector as HindIII–BamHI and BamHI–EcoRI fragments, respectively. The resulting plasmid pTZ::PMP3PROM-PMP3STOP was then cut with BamHI and ligated to a BglII fragment containing either the TRP1 or the URA3 gene, respectively, from the pFL39 or pFL34 vector (François Lacroute, Gif-sur-Yvette, France). The promoter and 3′ end regions of NHA1 were amplified by PCR on genomic DNA and cloned, respectively, into the EcoRI–BamHI and BamHI–XbaI sites of the pSK vector. A BamHI fragment containing the LEU2 gene was isolated from the YDpL vector (Berben et al., 1991) and cloned into the BamHI site of the resulting plasmid. The plasmids containing the null allele of PMP3 and NHA1 were restricted with EcoRI–KpnI and EcoRI–XbaI, respectively, and the generated DNA was transformed into yeast cells. Gene deletion was checked by PCR screening as described by Decottignies et al. (1998).
RCI2A DNA was prepared as follows. The 0.4 kb A.thaliana cDNA clone 88O14T7 (a kind gift from the Arabidopsis Biological Resource Center) was cut with HindIII and EcoRI. The resulting 369 bp fragment starts 41 bp upstream from the initiation ATG and extends 183 bp beyond the stop codon of the RCI2A ORF. This fragment was blunted with Klenow and cloned into the BamHI site (blunted with Klenow) of pTZ::PMP3PROM-PMP3STOP. Correct integration of RCI2A was checked by SspI–EcoRV restriction mapping. The resulting pTZ::PMP3PROM-RCI2A-PMP3STOP plasmid was cut with EcoRI to generate a 2.3 kb fragment. This fragment was used to transform YR93-31, where PMP3 is disrupted by the URA3 marker. Loss of the URA3 marker was monitored by plating one-tenth of an overnight culture in YD on solid YD medium containing 1 g/l of 5-fluoroorotic acid. The resistant cells were screened by PCR to make sure that RCI2A had replaced the URA3 marker at the chromosomal PMP3 locus.
Measurement of the intracellular sodium concentration
Yeast were grown in rich medium (2% yeast extract KAT, 2% glucose) and harvested in the exponential growth phase (final OD600 = 0.8). Cells (0.5 mg/ml) were incubated for 1 h at 4°C in H2O and transferred to a solution containing 10 mM MES buffer pH 6.0, 50 mM glucose and 1 M NaCl. After incubation at 28°C, 50 mg of cells were harvested by centrifugation for 3 min at 8000 g, resuspended twice in a cold solution containing 20 mM MgCl2 and 1.5 M sorbitol, centrifuged as described above and resuspended in 2 mM CTAB (cetyltrimethylammonium bromide). After 10 min at room temperature, the supernatant was diluted 10 times in H2O and analysed for Na with an atomic emission spectrometer (ICP model JY48, Jobin Yvon).
Measurement of methylammonium uptake
Cells were grown in MM at low cell density (final OD600 = 0.8), washed twice, resuspended with water and stored on ice. For uptake measurements, the cells were diluted to a final OD600 of 5 in a reaction medium containing 50 mM glucose and 10 mM MES adjusted to pH 6.0 with KOH. After a 5 min incubation, [14C]methylamine hydrochloride (2.0 mM and 2.5 µCi/ml final concentration) was added. At the indicated times, 100 µl samples were withdrawn and diluted into 10 ml of ice-cold 20 mM MgCl2 to stop the transport reaction, filtrated onto a 0.45 µm nitrocellulose filter (Millipore HA) and washed three times with 10 ml of the same solution. Moist filters were transferred to a scintillation cocktail and the radioactivity was measured using a Beckman LS 1701 liquid scintillation counter.
Other methods
Plasma membranes were prepared as described by de Kerchove d’Exaerde et al. (1995) and the proteolipid fraction was extracted with chloroform–methanol as described by Navarre et al. (1992). The amino acid sequence of Pmp3p was determined with an Applied Biosystems sequenator (model 477A) equipped with an on-line phenylthiohydantoin derivative analyser (model 120A). The protein content was measured as described by Lowry et al. (1951) with bovine serum albumin as the standard. The protein samples were electrophoresed on Tricine gels (Schägger and von Jagow, 1987) and silver stained. ATPase assays were performed at pH 6.0, using a free Mg2+ concentration of 1 mM and an ATP-regenerating system (1 mM phosphoenolpyruvate and 200 µg/ml pyruvate kinase) (Supply et al., 1993). The ATP concentration was varied from 0.15 to 5.2 mM. The preparation of sealed plasma membranes and the measurement of ATP-dependent transport were performed as described by Morsomme et al. (1996), except that the pH was adjusted to 6.0 for vesicle reconstitution and ACMA quenching measurements.
Accession number
PMP3 sequence data have been submitted to the DDBJ/EMBL/GenBank database under accession No. X91499.
Acknowledgments
Acknowledgements
We are grateful to Dr Rick Gaber (Northwestern University, Evanston, IL) and Dr Hans Rudolph (Stuttgart University, Germany) for the CY162 cells and YR93 cells, respectively; the Arabidopsis Biological Resource Center (Drs Keith Davis and Doreen Ware) and Dr Thomas Newman for the gift of the A.thaliana EST T20621; Dr Keith Weinstock of TIGR for additional information on the S.cerevisiae EST 101712; Louis Gerlache (Unité des Eaux et Forêts, Université Catholique de Louvain, Belgium) for the use of his atomic emission facilities; and Dr André Tonon (Ludwig Institute, Belgium) for the use of FACScan facilities even though we do not present the fluorescence results. In our laboratory, we are indebted to Bénédicte Purnelle for her cheerful help with nucleotide sequencing, Hervé Degand for Pmp3p microsequencing, Joseph Nader for technical assistance, Anabelle Decottignies for stimulating discussions, and Marc Boutry for his constant interest. Finally, we are very pleased to thank Michel Ghislain for critical and helpful readings of this manuscript. C.N. was supported by the Fonds National de la Recherche Scientifique (FNRS, Belgium). This work was funded by the Interuniversity Poles of Attraction Program–Belgian State, Prime Minister’s Office–Federal Office for Scientific, Technical and Cultural Affairs.
References
- Bañuelos M.-A., Sychrova,H., Bleykasten-Grosshans,M., Souciet,J.-L. and Potier,S. (1998) The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology, 144, 2749–2758. [DOI] [PubMed] [Google Scholar]
- Berben G., Dumont,J., Gilliquet,V., Bolle,P.-A. and Hilger,F. (1991) The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast, 7, 475–477. [DOI] [PubMed] [Google Scholar]
- Bihler H., Slayman,C. and Bertl,A. (1998) NSC1: a novel high-current for cations in the plasma membrane of Saccharomyces cerevisiae. FEBS Lett., 432, 59–64. [DOI] [PubMed] [Google Scholar]
- Capel J., Jarillo,J., Salinas,J. and Martinez-Zapater,J. (1997) Two homologous low-temperature genes from Arabidopsis encode highly hydrophobic proteins. Plant Physiol., 115, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decottignies A., Grant,A., Nichols,W., de Wet,H., McIntosh,D. and Goffeau,A. (1998) ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem., 273, 12612–12622. [DOI] [PubMed] [Google Scholar]
- de Kerchove d’Exaerde A., Supply,P., Dufour,J.-P., Bogaerts,P., Thinès,D., Goffeau,A. and Boutry,M. (1995) Functional complementation of a null mutation of the yeast Saccharomyces cerevisiae plasma membrane H+-ATPase by a plant H+-ATPase gene. J. Biol. Chem., 270, 23828–23837. [DOI] [PubMed] [Google Scholar]
- Dunn M., Goddard,N., Zhang,L., Pearce,R. and Hughes,M. (1994) Low-temperature-responsive barley genes have different control mechanisms. Plant Mol. Biol., 24, 879–888. [DOI] [PubMed] [Google Scholar]
- Folch J. and Lees,M. (1951) Proteolipids, a new type of tissue lipoproteins. J. Biol. Chem., 191, 807–817. [PubMed] [Google Scholar]
- Gaber R. (1992) Molecular genetics of yeast ion transport. Int. Rev. Cytol., 137, 299–353. [DOI] [PubMed] [Google Scholar]
- Gaber R., Styles,C. and Fink,G. (1988) TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol., 8, 2848–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gulick,P. and Dvorák,J. (1993) Characterization of the early stages of genetic salt-stress responses in salt-tolerant Lophopyrum elongatum, salt-sensitive wheat and their amphiploid. Plant Physiol., 103, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblas B., Rubio,F., Quintero,F., Bañuelos,M., Haro,R. and Rodriguez-Navarro,A. (1993) Differential expression of two genes encoding isoforms of ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol. Gen. Genet., 236, 363–368. [DOI] [PubMed] [Google Scholar]
- Goddard N., Dunn,M., Zhang,L., White,A., Jack,P. and Hughes,M. (1993) Molecular analysis and spatial expression pattern of a low-temperature-specific barley gene, blt101. Plant Mol. Biol., 23, 871–879. [DOI] [PubMed] [Google Scholar]
- Gulick P. and Dvorák,J. (1992) Coordinate gene response to salt stress in Lophopyrum elongatum. Plant Physiol., 100, 1384–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick P., Shen,W. and An,H. (1994) ESI3, a stress-induced gene from Lophopyrum elongatum. Plant Physiol., 104, 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R., Garciadeblas,B. and Rodriguez-Navarro,A. (1991) A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett., 291, 189–191. [DOI] [PubMed] [Google Scholar]
- Haro R., Bañuelos,M., Quintero,F., Rubio,F. and Rodriguez-Navarro,A. (1993) Genetics basis of sodium exclusion and sodium tolerance in yeast: a model for plants. Physiol. Plant, 89, 868–874. [Google Scholar]
- Ko C. and Gaber,R. (1991) TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol., 11, 4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C., Liang,H. and Gaber,R. (1993) Roles of multiple glucose transporters in Saccharomyces cerevisiae. Mol. Cell. Biol., 13, 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Ko,C., Herman,T. and Gaber,R. (1998) Trinucleotide insertions, deletions and point mutations in glucose transporters confer K+ uptake in Saccharomyces cerevisiae. Mol. Cell. Biol., 18, 926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O., Rosebrough,N., Farr,L. and Randall,R. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193, 265–275. [PubMed] [Google Scholar]
- Madrid R., Gomez,M., Ramos,J. and Rodriguez-Navarro,A. (1998) Ectopic potassium uptake in a trk1trk2 mutant of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem., 273, 14838–14844. [DOI] [PubMed] [Google Scholar]
- McCusker J., Perlin,D. and Haber,J. (1987) Pleiotropic plasma membrane ATPase mutations of Saccharomyces cerevisiae. Mol. Cell. Biol., 7, 4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza I., Rubio,F., Rodriguez-Navarro,A. and Pardo,J. (1994) The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem., 269, 8792–8796. [PubMed] [Google Scholar]
- Miosga T., Witzel,A. and Zimmerman,F. (1994) Sequence and function analysis of a 9.46 kb fragment of Saccharomyces cerevisiae chromosome X. Yeast, 10, 965–973. [DOI] [PubMed] [Google Scholar]
- Morsomme P., de Kerchove d’Exaerde,A., De Meester,S., Thines,D., Goffeau,A. and Boutry,M. (1996) Single point mutations in various domains of a plant plasma membrane H+-ATPase expressed in Saccharomyces cerevisiae increase H+-pumping and permit yeast growth at low pH. EMBO J., 15, 5513–5526. [PMC free article] [PubMed] [Google Scholar]
- Mulet J., Leube,M., Kron,S., Rios,G., Fink,G. and Serrano,R. (1999) A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol., 19, 3328–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R. and Rao,R. (1998) Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. J. Biol. Chem., 273, 21054–21060. [DOI] [PubMed] [Google Scholar]
- Nass R., Cunningham,K. and Rao,R. (1997) Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. J. Biol. Chem., 272, 26145–26152. [DOI] [PubMed] [Google Scholar]
- Navarre C., Ghislain,M., Leterme,S., Ferroud,C., Dufour,J.-P. and Goffeau,A. (1992) Purification and complete sequence of a small proteolipid associated with the plasma membrane H+-ATPase of Saccharomyces cerevisiae. J. Biol. Chem., 267, 6425–6428. [PubMed] [Google Scholar]
- Navarre C., Catty,P., Leterme,S., Dietrich,F. and Goffeau,A. (1994) Two distinct genes encode small isoproteolipids affecting the plasma membrane H+-ATPase of Saccharomyces cerevisiae. J. Biol. Chem., 269, 21262–21268. [PubMed] [Google Scholar]
- Niu X., Bressan,R., Hasegawa,P. and Pardo,J. (1995) Ion homeostasis in NaCl stress environments. Plant Physiol., 109, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata M., Petrecca,K., Lake,N. and Orlowski,J. (1998) Identification of a mitochondrial Na+/H+ exchanger. J. Biol. Chem., 273, 6951–6959. [DOI] [PubMed] [Google Scholar]
- Perlin D., Brown,C. and Haber,J. (1988) Membrane potential defect in hygromycin B-resistant pma1 mutants of Saccharomyces cerevisiae. J. Biol. Chem., 263, 18118–18122. [PubMed] [Google Scholar]
- Phillips J., Dunn,M. and Hughes,M. (1997) mRNA stability and localisation of the low-temperature barley gene family blt14. Plant Mol. Biol., 33, 1013–1023. [DOI] [PubMed] [Google Scholar]
- Prior C., Potier,S., Souciet,J.-L. and Sychrova,H. (1996) Characterization of the NHA1 gene encoding a Na+/H+ antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett., 387, 89–93. [DOI] [PubMed] [Google Scholar]
- Ramirez J., Ramirez,O., Saldana,C., Coria,R. and Peña,A. (1998) A Saccharomyces cerevisiae mutant lacking a K+/H+ exchanger. J. Bacteriol., 180, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J., Haro,R. and Rodriguez-Navarro,A. (1994) TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae.J. Bacteriol., 176, 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Fischer,M., Dixon,G. and Sanders,D. (1999) Divalent cation block of inward currents and low-affinity K+ uptake in Saccharomyces cerevisiae. J. Bacteriol., 181, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro A., Quintero,F. and Garciadeblas,B. (1994) Na+-ATPases and Na+/H+ antiporters in fungi. Biochim. Biophys. Acta, 1187, 203–205. [DOI] [PubMed] [Google Scholar]
- Schachtman D. and Liu,W. (1999) Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci., 4, 281–287. [DOI] [PubMed] [Google Scholar]
- Schägger H. and von Jagow,G. (1987) Tricine–sodium dodecyl sulfate–polyacrylamide electrophoresis for the separation of proteins in range from 1 to 100 kDa. Anal. Biochem., 166, 368–379. [DOI] [PubMed] [Google Scholar]
- Serrano R. (1996) Salt tolerance in plants and microorganisms. Int. Rev. Cytol., 165, 368–379. [DOI] [PubMed] [Google Scholar]
- Serrano R. et al. (1999) A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot., 50, 1023–1036. [Google Scholar]
- Supply P., Wach,A. and Goffeau,A. (1993) Enzymatic properties of the PMA2 plasma membrane-bound H+-ATPase of Saccharomyces cerevisiae. J. Biol. Chem., 268, 19753–19759. [PubMed] [Google Scholar]
- Vallejo C. and Serrano,R. (1989) Physiology of mutants with reduced expression of plasma membrane H+-ATPase. Yeast, 5, 307–319. [DOI] [PubMed] [Google Scholar]
- Velculescu V., Zhang,L., Zhou,W., Vogelstein,J., Basrai,M., Bassett,D., Hieter,P., Vogelstein,B. and Kinzler,K. (1997) Characterization of the yeast transcriptome. Cell, 88, 243–251. [DOI] [PubMed] [Google Scholar]
- Wallis J., Chrebet,G., Brodsky,G., Rolfe,M. and Rothstein,R. (1989) A hyper-recombination mutation in S.cerevisiae identifies a novel eukaryotic topoisomerase. Cell, 58, 409–419. [DOI] [PubMed] [Google Scholar]
- Wieland J., Nitsche,A., Strayle,J., Steiner,H. and Rudolph,H. (1995) The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J., 14, 3870–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withee J., Sen,R. and Cyert,M. (1998) Ion tolerance of Saccharomyces cerevisiae lacking the Ca2+/CaM-dependent phosphatase (calcineurin) is improved by mutations in URE2 or PMA2. Genetics, 149, 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Ramos,J., Gomez,M., Moulder,K., Scherrer,M., Munson,G. and Gaber,R. (1997) Potassium transport by amino acid permeases in Saccharomyces cerevisiae. J. Biol. Chem., 272, 13647–13652. [DOI] [PubMed] [Google Scholar]