Abstract
The neurotransmitter glutamate is neurotoxic when it is accumulated in a massive amount in the extracellular fluid. Excessive release of glutamate has been shown to be a major cause of neuronal degeneration after central nervous system injury. Under normal conditions, accumulation of synaptically released glutamate is prevented, at least in part, by a glial uptake system in which the glia-specific enzyme glutamine synthetase (GS) plays a key role. We postulated that glial cells cannot cope with glutamate neurotoxicity because the level of GS is not high enough to catalyze the excessive amounts of glutamate released by damaged neurons. We examined whether elevation of GS expression in glial cells protects against neuronal degeneration in injured retinal tissue. Analysis of lactate dehydrogenase efflux, DNA fragmentation, and histological sections revealed that hormonal induction of the endogenous GS gene in retinal glial cells correlates with a decline in neuronal degeneration, whereas inhibition of GS activity by methionine sulfoximine leads to increased cell death. A supply of purified GS enzyme to the culture medium of retinal explants or directly to the embryo in ovo causes a dose-dependent decline in the extent of cell death. These results show that GS is a potent neuroprotectant and that elevation of GS expression in glial cells activates an endogenous mechanism whereby neurons are protected from the deleterious effects of excess glutamate in extracellular fluid after trauma or ischemia. Our results suggest new approaches to the clinical handling of neuronal degeneration.
Keywords: neuroprotection, glia, ischemia, trauma, glucocorticoids
Glutamate neurotoxicity plays an important role in the process of neuronal degeneration after trauma or focal ischemia (for reviews, see refs. 1 and 2). Glutamate, a neurotransmitter that mediates normal excitatory synaptic transmission by interaction with postsynaptic receptors, is neurotoxic when present in excessive amounts. Injured neurons release massive amounts of glutamate, which induce neuronal cell death by continuous overexcitation of postsynaptic receptors. In this way, the initial trauma is amplified and causes the damage to spread to neighboring cells. Under normal conditions, the synaptically released glutamate is taken up into glial cells, where it is converted into glutamine by the glia-specific enzyme glutamine synthetase [GS; l-glutamate:ammonia ligase (ADP-forming); EC 6.3.1.2]; glutamine reenters the neurons and is hydrolyzed by glutaminase to form glutamate, thus replenishing the neurotransmitter pool (3, 4). This biochemical pathway fails, however, to prevent glutamate neurotoxicity after insult. We hypothesized that GS is a limiting factor in this process and that its level in glial cells is not high enough to catalyze the excessive amounts of glutamate released by damaged cells. If this is the case, an increase in GS expression should have neuroprotective benefits.
To examine the neuroprotective potential of GS, it is necessary to employ an experimental system in which expression of GS can be modulated. The embryonic chicken retina is eminently suitable for this kind of study because the level of GS in this tissue can be closely regulated by glucocorticoids (5–8). Glucocorticoids regulate GS expression at the transcriptional level and induce a marked increase in GS enzyme activity in Muller glial cells (9–13). Retinal tissue is also an established paradigm for glutamate neurotoxicity: (i) glutamate serves as a neurotransmitter in photoreceptors, bipolar cells, and ganglion cells (14); (ii) insult leads to accumulation of relatively high levels of glutamate in the extracellular fluid (15, 16); (iii) administration of glutamate leads to neuronal cell death (17–19); and (iv) glutamate receptor antagonists can protect against neuronal degeneration (20). Here we used the retinal tissue to demonstrate that elevation of GS expression in glial cells by induction of the endogenous gene or exogenous supply of the purified enzyme can protect against neuronal cell degeneration.
MATERIALS AND METHODS
Tissue Preparation and Treatment.
Retinas were isolated under sterile conditions from chicken embryos (White Leghorn) at days 15–17 of embryonic development (E15–E17). The tissue was placed in CMF buffer (137 mM NaCl/2.7 mM KCl/8 mM Na2HPO4/1 mM KH2PO4/5.5 mM glucose) and cut into pieces of about 10 mm2. This process of tissue insult was termed trauma. The tissue pieces were cultured in 25-ml Erlenmeyer flasks, one retina per flask, in 3 ml of Krebs medium (118 mM NaCl/4.8 mM KCl/2.4 mM CaCl2/1.2 mM KH2PO4/1.2 mM MgSO4/1.2 mM glucose/25 mM NaHCO3, pH 7.4). The flasks were gassed with 95% air/5% CO2, sealed, and incubated on a gyratory shaker (65 rpm) at 38°C. To simulate exposure of the tissue to ischemic conditions, the retinal pieces were cultured in glucose-free Krebs medium in Erlenmeyer flasks that were gassed for 50 min with N2. After the first hour of incubation, the Krebs medium in tissues exposed to trauma and to ischemia was replaced by serum-free Bio-MPM medium (Biological Industries, Kibbutz Beit Haemek, Israel). The flasks were gassed with 95% air/5% CO2, sealed, and incubated on a gyratory shaker for a further 3–48 h. After 24 h the medium was changed again. Medium samples were collected for lactate dehydrogenase (LDH) analysis.
To induce GS expression in the organ-cultured retina, cortisol (Sigma) was added to the medium to a final concentration of 0.33 μg/ml. GS expression in ovo was induced by injecting 0.001–2 mg of hydrocortisone 21-phosphate (Sigma), dissolved in 0.1 ml of distilled water, extraembryonally into the amniotic cavity by using a 25-gauge needle that was inserted through the shell at the blunt end of the egg. The shell was sealed with cellophane tape and the eggs were incubated for additional 24 or 48 h before the retina was excised. In some experiments, l-methionine sulfoximine (MSO; Sigma), a specific GS inhibitor, or dl-buthionine-[S,R]-sulfoximine (Sigma), a close derivative that was used as a control, were coinjected (1 mg in 0.1 ml of distilled water per egg).
Supply of Purified GS Enzyme.
GS, purified from sheep brain (Sigma), was stabilized in a lipid environment before use. Briefly, l-α-phosphatidylcholine Type IIS from soybean (Sigma) was dispersed in distilled water at an initial concentration of 20 mg/ml, briefly sonicated, and subjected to centrifugation at 10,000 × g for 5 min. The supernatant was collected and used to prepare stock solutions of the GS enzyme for addition to the culture medium or injection into fertilized eggs at a dilution of 1:10. The enzyme (100 μl) was injected extraembryonally into the amniotic cavity by inserting a 25-gauge needle through the shell at the blunt end of the egg. To inject the enzyme behind the eyeball, a window 25 mm in diameter was cut in the shell at the blunt end, and the enzyme was injected (50 μl) using a 25-gauge needle. Control eggs were injected with carrier. The shell was sealed with cellophane tape and the eggs were incubated for additional 24 or 48 h before the retina was excised.
LDH and GS Assays.
Neuronal degeneration was quantitatively assessed by measurement of LDH release into the culture medium as described (21). Briefly, samples of culture medium (25 μl) were added to freshly prepared LDH substrate buffer (0.76 mM sodium pyruvate/85 μM NADH in 0.1M KH2PO4 buffer, pH 7.5) at room temperature. Absorbance of the reaction mixture at 340 nm was measured with a spectrophotometer for 1 min, and the amount of LDH released by a single retina (108 cells) was calculated in units from the slope of the absorbance curve. One unit is the amount of LDH that will cause a decrease of 0.001/min in the absorbance of a 1-ml reaction mixture. Because the culture medium was changed after the first hour of incubation, the amount of LDH measured in the discarded medium was added to the amount of LDH release at later time points. GS activity was measured in tissue sonicates as described (22). Its specific activity was expressed as micromoles of γ-glutamylhydroxamate per hour per milligram of protein.
Quantitation of DNA Fragmentation.
DNA fragmentation was assayed as reported (23). Retinal tissue was washed in CMF buffer and homogenized by 10 strokes with pestle A and 10 strokes with pestle B in a Dounce homogenizer in cold lysis buffer [20 mM EDTA/0.5% (vol/vol) Triton X-100/5 mM Tris⋅Cl, pH 7.4]. The homogenate was incubated for 15 min on ice before centrifugation for 15 min at 27,000 × g to separate intact chromatin (pellet) from DNA fragments (supernatant). Fragmented DNA was extracted twice with phenol chloroform, precipitated overnight with 2 volumes of ice-cold ethanol at −20°C, washed with 70% ethanol, air-dried, and resuspended in TE buffer (10 mM Tris⋅HCl/1 mM EDTA, pH 8.0). DNA samples, each prepared from an equal number of cells, were fractionated by electrophoresis in 1.8% agarose gels. The gels were stained with ethidium bromide, visualized by UV light, and photographed. DNA fragmentation was quantitated by densitometric scanning of the pictures.
Histology and Immunohistochemistry.
E17 retinas excised from treated or untreated eggs were fixed with Carnoy’s fixative and embedded in paraffin either immediately after excision or after being cultured for 4 h. Paraffin sections (5 μm) were stained either with hematoxylin/eosin or by the indirect immunofluorescence method. For immunofluorescence, sections were stained with rabbit anti-GS antiserum (kindly provided by A. A. Moscona, University of Chicago, Chicago) and then with fluorescein-conjugated anti-rabbit antibodies (Sigma). Antibody binding was detected by immunofluorescence with epi-illumination from a UV source.
RESULTS
Induction of GS Expression by Glucocorticoids Protects Against Neuronal Degeneration.
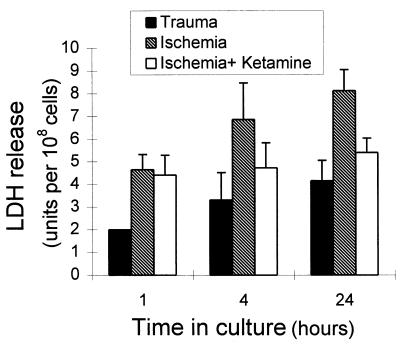
Excision of retinal tissue from E17 embryos and subsequent cutting of the tissue into pieces injured many cells and caused substantial cell death. This trauma was manifested by a massive and progressive release of LDH, which is a quantitative measure of the extent of cell degeneration (ref. 21; Fig. 1). LDH efflux increased even further upon exposure of the tissue to ischemic conditions, and this increase was largely prevented by the addition of ketamine, an N-methyl-d-aspartate-receptor antagonist. This finding is in agreement with previous observations suggesting that insult-induced retinal cell degeneration is mediated by glutamate (20). All of the experiments described below were carried out with retinal tissues exposed to both trauma and ischemia. Because the results were quite similar, we present here only those obtained with traumatized tissue.
Figure 1.
LDH release by retinal tissue exposed to trauma or ischemia. Retinal tissue from E17 embryos was exposed to trauma by the process of excising the tissue and subsequently cutting it into pieces. The tissue was organ-cultured for 24 h, and LDH release was measured in samples of the culture medium 1, 4, and 24 h after tissue excision (solid bars). E17 retina was exposed to ischemia by organ-culturing the tissue pieces for 50 min in glucose-free medium in flasks that were gassed with 95% N2/5% CO2 (hatched bars). In some experiments the culture medium of the ischemic tissue contained the glutamate antagonist ketamine (10 mM/ml) (open bars). The levels of LDH were measured in medium samples 1, 4, and 24 h after tissue excision. Each bar represents the mean ± SD of three separate experiments, each performed in triplicate (n = 9).
Supplying cortisol in ovo to the embryo or to retinal explants did not affect the level of LDH expression (data not shown) but induced a progressive increase in the level of GS. The increase in GS correlated inversely with the level of LDH released by the injured tissue (Fig. 2A). Supplying cortisol to the embryo 48 h before insult resulted in a 15-fold increase in GS activity and a 40% decline in LDH release, whereas supplying cortisol 24 h before insult induced a 7-fold increase in GS activity and a 20% decline in LDH release. These effects were dose-dependent; at low levels of cortisol, GS activity was low and LDH release was high, whereas at higher levels of cortisol, GS activity was high and LDH release was low (Fig. 2B). The inverse correlation between LDH release and GS activity suggests that GS might be functionally implicated in protection against neuronal degeneration.
Figure 2.
Inverse correlation between GS induction and LDH release. (A) Cortisol (1 mg per egg) was injected into E15, E16, or E17 eggs, which were then incubated for an additional 48, 24, or 4 h, respectively. Retinal tissue was excised, cut into pieces, and organ-cultured for 4 h. The levels of GS in the tissue and of LDH in the culture medium were measured. (B) E15 eggs were injected with cortisol in the indicated amounts and incubated for an additional 48 h. Retinal tissues were excised, cut into pieces, and organ-cultured for 4 h. The level of GS in the tissue and of LDH in the culture medium was measured. (C) E15 eggs were coinjected with cortisol (1 mg per egg) and MSO (1 mg per egg), buthionine sulfoximine (BSO; 1 mg per egg), or carrier. The eggs were incubated for an additional 48 h before the retina was excised. Retinal explants were cut into pieces and organ-cultured for 4 h. The levels of GS in the tissue and of LDH in the culture medium were measured. The levels of GS and LDH obtain after coinjection of cortisol and carrier were given the arbitrary value of 100. Results are means ± SD of three independent experiments, each performed in duplicate (n = 6).
To demonstrate the causative role of GS in this process, we examined the effect of MSO, an inhibitor of GS activity (24), on the extent of neuronal degeneration (Fig. 2C). Buthionine sulfoximine, a close derivative that does not inhibit GS activity, was used as a control. As expected, MSO caused a dramatic decline in GS activity, whereas buthionine sulfoximine did not affect it. MSO-induced inhibition of GS activity caused a 50% increase in the extent of LDH release. These results strongly suggest that the protective activity of cortisol is mediated, at least in part, by GS.
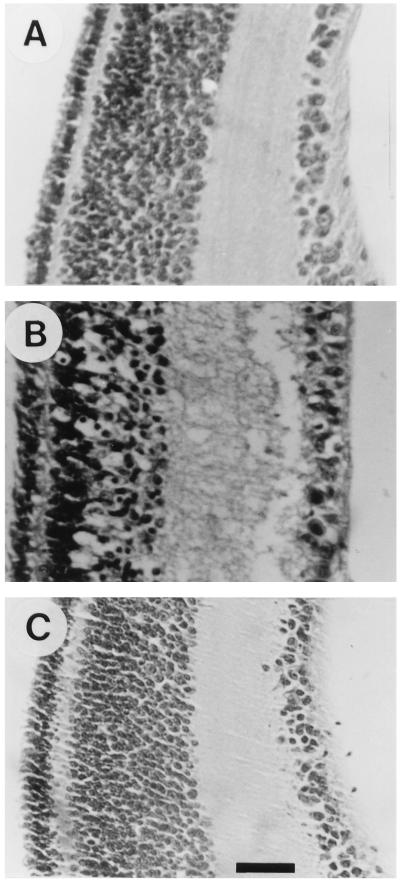
The protective effect of cortisol was also evaluated by analysis of histological sections (Fig. 3). Traumatized tissue, 4 h after insult, differed conspicuously in several respects from the normal appearance of uncultured retina. The inner region of the inner nuclear layer, the inner plexiform layer, and the ganglion cell layer appeared edematous and contained numerous cells with pyknotic nuclei. Addition of cortisol 48 h before tissue excision prevented these pathological changes, although mild cytopathology could be observed in some of the sections. Several studies have suggested the involvement of apoptosis in insult-induced neuronal degeneration (25–28). We therefore examined whether the protective effect of cortisol could also be demonstrated by analysis of internucleosomal DNA fragmentation, a phenomenon associated with apoptosis (Fig. 4). Internucleosomal DNA fragmentation, identified by gel electrophoresis, increased markedly upon insult. Addition of cortisol 48 h before tissue excision caused a 40% decline in the extent of DNA fragmentation. Thus, both qualitative and quantitative assessment of neuronal degeneration, by analysis of LDH efflux, histological sections, and DNA fragmentation, all indicated that a cortisol-induced increase in GS expression is correlated with a decline in cell degeneration.
Figure 3.
Morphological evidence for cortisol protection against neuronal degeneration. E15 eggs were injected with cortisol (1 mg per egg) (A) or carrier (B) and incubated for an additional 48 h before the retina was excised. Retinal explants were cut into pieces, organ-cultured for 4 h, and then embedded in paraffin. Untreated E17 retina was embedded immediately after excision (C). Paraffin sections were stained with hematoxylin/eosin. (Bar = 20 μm.)
Figure 4.
Internucleosomal DNA fragmentation is attenuated by cortisol. E15 eggs were injected with cortisol (1 mg per egg; lane 4) or carrier (lane 3) and incubated for an additional 48 h before the retina was excised. Retinal explants were cut into pieces and organ-cultured for 24 h. DNA was extracted, and samples from an equal numbers of cells (5 × 107 cells per lane) were analyzed by gel electrophorsis and visualized with the fluorescent intercalating dye ethidium bromide. DNA was extracted from untreated E17 retina immediately after tissue excision (lane 2). Molecular weight markers are from a HaeIII digest of ØX174 phage DNA (lane 1).
Purified GS Can Protect Against Neuronal Degeneration.
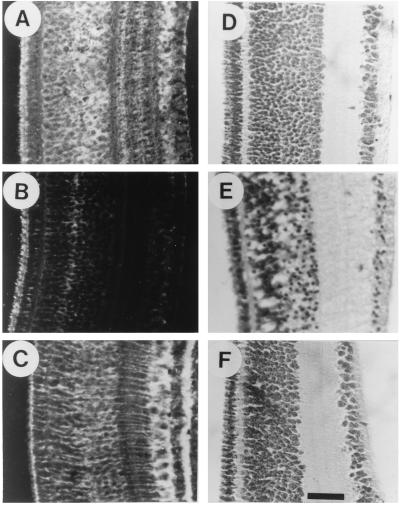
In attempting to demonstrate the protective effect of GS, the most direct approach is to use the purified enzyme itself. We supplied increasing amounts of GS from sheep brain to the culture medium of retinal explants and measured the levels of LDH release. GS caused a dose-dependent decline in LDH release (Fig. 5A). At 10 units per retina, GS provided 50% protection against neuronal degeneration. The effect was even more dramatic when GS was supplied in ovo directly to the embryo (Fig. 5B). Immunohistochemical staining of retinal sections with anti-GS antibodies (Fig. 6A) indicated that the enzyme, injected extraembryonally into the amniotic cavity or intraembryonally behind the eyeball, diffused into the retinal tissue within 1 h. Judging from the staining intensities, GS level in retinas from enzyme-injected embryos were similar to those in cortisol-injected ones (Fig. 6C). However, staining efficiency might depend on the cellular localization of the enzyme, which is intracellular in the case of hormonal induction and extracellular when the enzyme is supplied directly. Injection of GS caused a dose-dependent decline in LDH release, whereas injection of ovalbumin or of heat-inactivated GS had no effect (Fig. 5B). GS could substantially reduce the extent of LDH release if supplied 24 h before insult but lost its protective activity between 24 and 48 h of injection (Fig. 5C). The protective effect of the injected GS could also be demonstrated by inspection of histological sections. As in the case of cortisol (Fig. 3), injection of GS 1 h before insult prevented the pathological changes observed in traumatized tissue (Fig. 3 D–F), although mild cytopathology could be observed in some sections. These results clearly demonstrated that GS is an efficient neuroprotectant that can reach the retinal tissue when injected into the egg and can also exert its protective activity in the extracellular fluid.
Figure 5.
Supply of purified GS enzyme in vitro or in ovo reduces the extent of LDH release. (A) E17 retina was excised, cut into pieces, and organ-cultured in the absence (−) or presence of purified GS enzyme in the indicated unit amounts. The level of LDH in the culture medium was measured after 4 h. Each bar represents the mean ± SD of two separate experiments, each performed in triplicate (n = 6). (B) E17 eggs were injected with carrier (−), with purified GS enzyme in the indicated unit amounts (1 to 8 units per egg), with ovalbumin (20 μg per egg; OVA), or with heat-inactivated GS enzyme (2 units per egg; IGS). The injected eggs were incubated for 2 h before the retina was excised. Retinal explants were cut into pieces and organ-cultured for 4 h, and the level of LDH in the culture medium was then measured. Each bar represents the mean ± SD of two separate experiments, each performed in quadruplicate (n = 8). (C) E15, E16, and E17 eggs were injected with purified GS enzyme (2 units per egg). Retinas were excised from the injected E17 eggs immediately (−) or after 2 or 4 h of incubation. Injected E16 and E15 eggs were incubated for an additional 24 or 48 h, respectively, before the retinal tissues were excised. Tissue explants were cut into pieces and organ-cultured for 4 h, and the level of LDH in the culture medium was then measured. Each bar represents the mean ± SD of two separate experiments, each performed in quadruplicate (n = 8).
Figure 6.
Morphological evidence for protective effects of the purified GS enzyme. E17 eggs were injected with purified GS enzyme (2 units per egg; A and D) or with carrier (B and E) and incubated for 2 h before the retinas were excised. Retinal explants were embedded immediately after excision (A and B) or were cut into pieces, organ-cultured for 4 h, and then embedded in paraffin (D and E). Paraffin sections were stained with rabbit anti-GS-specific antiserum and with goat anti-rabbit IgG (A and B) or with hematoxylin/eosin (D and E). E15 eggs were injected with cortisol (1 mg per egg) and incubated for an additional 48 h before the retina was excised. The excised tissue was embedded in paraffin, and histological sections were stained with rabbit anti-GS-specific antiserum and with goat anti-rabbit IgG (C). Untreated E17 retina was embedded immediately after excision and stained with hematoxylin/eosin (F). (Bar = 25 μm.)
DISCUSSION
The results of this study demonstrate that GS is a potent neuroprotectant and that elevation of GS expression in glial cells activates an endogenous neuroprotective mechanism that markedly reduces the extent of neuronal degeneration in retinal tissue after its exposure to trauma or ischemia. Trauma or ischemia caused extensive cell degeneration in the retinal tissue, which could be assessed in terms of LDH efflux, DNA fragmentation, and histological appearance. The protective effect of ketamine, as well as of other glutamate antagonists (20), points to the involvement of glutamate neurotoxicity in this process. Elevation of GS expression in Muller glial cells by hormonal induction of the endogenous gene protects against neuronal degeneration. The beneficial effect of elevated GS appears to be related to the ability of the enzyme to catalyze the amidation of glutamate to the nontoxic glutamine because inhibition of GS activity by MSO caused an increase in cell death.
Under normal conditions, amidation of glutamate to glutamine occurs in glial cells, which are the only cells in the retinal tissue that express GS (12, 29, 30). This could also be the case in injured tissue. Glutamate is transported into glia and is converted by GS to glutamine, which is subsequently exported from the cells. The increase in GS expression as a consequence of hormonal induction might cause an accelerated transport of postsynaptic glutamate into glia and thus protect against neurotoxicity. Alternatively, in injured tissue GS might catalyze the conversion of glutamate to glutamine not only intracellularly but also, or perhaps predominantly, outside the cells. Trauma or ischemia might damage glial cells and trigger the release of active GS into the extracellular fluid so that the enzyme can act directly on postsynaptic glutamate. Hormonal induction of GS expression might result in more abundant availability of GS in the extracellular space, providing better protection against glutamate neurotoxicity. This latter scenario seems quite plausible, in view of our finding that purified GS can exert its protective activity in the extracellular fluid. The purified enzyme, which consists of eight subunits and has a molecular mass of about 500,000 Da (31), probably remains in the extracellular milieu when injected into a fertilized egg or added to the culture medium of retinal tissue. Nevertheless, its protective activity is as efficient as that of the endogenously induced enzyme. Also of relevance is our recent finding that GS activity can be detected in the culture medium of injured retina (unpublished data). The level of GS in the culture medium is proportional (about 2%) to that found in the tissue, suggesting that after injury the damaged glial cells release GS into the extracellular fluid. These findings support the notion that, in injured tissue, GS might also exert its protective activity in the extracellular space. Interestingly, this enzyme can be detected in the cerebrospinal fluid of patients in advanced stages of Alzheimer disease (32). In some in vivo injury paradigms, neuronal degeneration is accompanied by an increase in astrocyte GS (33–36). This increase has been attributed to reactive astrocytosis that occurs in response to neural insults and might be part of an endogenous mechanism for neuroprotection.
The ability of glucocorticoids, such as cortisol, to induce GS expression and protect against neuronal degeneration might be directly related to findings obtained in a clinical trial. It was demonstrated that treatment with high doses of glucocorticoid (methylprednisolone) improves neurological recovery of patients with acute spinal cord injury (37). Early treatment (within the first 8 h of injury) was essential for the beneficial effect. Similar results were also obtained in animal models of spinal cord and brain injury (38, 39), as well as after retinal photic injury (40, 41). The molecular mechanism underlying these effects is not known. However, on the basis of the results presented in this study, we propose that neurological recovery might be due, at least in part, to induction of GS expression in the injured tissue. Our results highlight some potential weaknesses of glucocorticoid treatment. Induction of GS expression by glucocorticoids is a time-consuming process. Glucocorticoids regulate GS expression by stimulating transcription of the gene, and it takes about 24 h to obtain a significant increase in the enzyme itself. This might explain why glucocorticoids must be supplied early to achieve a beneficial effect and suggests that, in planned neurological interventions, prophylactic administration of glucocorticoids might be advisable. Hormonal induction of GS expression might also depend on particular physiological conditions. We and others (9, 42, 43) have demonstrated, for example, that GS induction depends on cell-to-cell contacts between glia and neurons. Thus, the extent of GS induction in an injured tissue might depend on the severity of the insult and on its anatomical location. The finding that the purified enzyme can protect against neuronal degeneration offers a way to circumvent these obstacles and suggest new approaches to clinical handling of glutamate-mediated neuronal degeneration.
Acknowledgments
We thank M. Wollberg for her help with the histological sections. This work was supported by research grants from the Israeli Ministery of Health and the Adams Super-center for Brain Studies at the Tel Aviv University.
ABBREVIATIONS
- GS
glutamine synthetase
- E
embryonic day
- LDH
lactate dehydrogenase
- MSO
l-methionine sulfoximine
References
- 1.Rothman S M, Olney J W. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 2.Choi D W, Rothman S M. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 3.Van der Berg C J, Garfinkel D. Biochem J. 1971;123:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy A J, Voaden M J, Marshall J. Nature (London) 1974;252:50–52. doi: 10.1038/252050a0. [DOI] [PubMed] [Google Scholar]
- 5.Moscona A A. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Vol. 2. Oxford: Permagon; 1983. pp. 111–135. [Google Scholar]
- 6.Vardimon L, Fox L E, Moscona A A. Proc Natl Acad Sci USA. 1986;83:9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patejunas G, Young A P. J Cell Biochem. 1987;35:205–216. doi: 10.1002/jcb.240350304. [DOI] [PubMed] [Google Scholar]
- 8.Berko Flint Y, Levkowitz G, Vardimon L. EMBO J. 1994;13:646–654. doi: 10.1002/j.1460-2075.1994.tb06303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardimon L, Fox L L, Degenstein L, Moscona A A. Proc Natl Acad Sci USA. 1988;85:5981–5985. doi: 10.1073/pnas.85.16.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H Y, Young A P. J Biol Chem. 1991;266:24332–24338. [PubMed] [Google Scholar]
- 11.Ben Dror I, Havazelet N, Vardimon L. Proc Natl Acad Sci USA. 1993;90:1117–1121. doi: 10.1073/pnas.90.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossman R, Fox L E, Gorovits R, Ben Dror I, Reisfeld S, Vardimon L. Brain Res Mol Brain Res. 1994;21:312–320. doi: 10.1016/0169-328x(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 13.Gorovits R, Yakir A, Fox E F, Vardimon L. Brain Res Mol Brain Res. 1996;43:321–329. doi: 10.1016/s0169-328x(96)00213-6. [DOI] [PubMed] [Google Scholar]
- 14.Massey S C. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Vol. 9. Oxford: Permagon; 1990. pp. 399–426. [Google Scholar]
- 15.Louzada P, Jr, Dias J J, Santos W F, Lachat J J, Bradford H F, Coutinho Netto J. J Neurochem. 1992;59:358–363. doi: 10.1111/j.1471-4159.1992.tb08912.x. [DOI] [PubMed] [Google Scholar]
- 16.Neal M J, Cunningham J R, Hutson P H, Hogg J. J Neurochem. 1994;62:1025–1033. doi: 10.1046/j.1471-4159.1994.62031025.x. [DOI] [PubMed] [Google Scholar]
- 17.David P, Lusky M, Teichberg V I. Exp Eye Res. 1988;46:657–662. doi: 10.1016/s0014-4835(88)80054-x. [DOI] [PubMed] [Google Scholar]
- 18.Zeevalk G D, Hyndman A G, Nicklas W J. J Neurochem. 1989;53:1610–1619. doi: 10.1111/j.1471-4159.1989.tb08559.x. [DOI] [PubMed] [Google Scholar]
- 19.Zeevalk G D, Nicklas W J. J Pharmacol Exp Ther. 1990;253:1285–1292. [PubMed] [Google Scholar]
- 20.Mosinger J L, Price M T, Bai H Y, Xiao H, Wozniak D F, Olney J W. Exp Neurol. 1991;113:10–17. doi: 10.1016/0014-4886(91)90140-8. [DOI] [PubMed] [Google Scholar]
- 21.Koh J Y, Choi D W. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 22.Moscona A A, Moscona M H, Saenz N. Proc Natl Acad Sci USA. 1968;61:160–167. doi: 10.1073/pnas.61.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brune B, Hartzell P, Nicotera P, Orrenius S. Exp Cell Res. 1991;195:323–329. doi: 10.1016/0014-4827(91)90380-d. [DOI] [PubMed] [Google Scholar]
- 24.Ronzio R A, Rowe B W, Meister A. Biochemistry. 1969;8:1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- 25.Kure S, Tominaga T, Yoshimoto T, Tada K, Narisawa K. Biochem Biophys Res Commun. 1991;179:39–45. doi: 10.1016/0006-291x(91)91330-f. [DOI] [PubMed] [Google Scholar]
- 26.Linnik M D, Zobrist R H, Hatfield M D. Stroke. 1993;24:2002–2009. doi: 10.1161/01.str.24.12.2002. [DOI] [PubMed] [Google Scholar]
- 27.MacManus J P, Buchan A M, Hill I E, Rasquinha I, Preston E. Neurosci Lett. 1993;164:89–92. doi: 10.1016/0304-3940(93)90864-h. [DOI] [PubMed] [Google Scholar]
- 28.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S A. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linser P, Moscona A A. Proc Natl Acad Sci USA. 1979;76:6476–6480. doi: 10.1073/pnas.76.12.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorovits R, Ben Dror I, Fox L E, Westphal H M, Vardimon L. Proc Natl Acad Sci USA. 1994;91:4786–4790. doi: 10.1073/pnas.91.11.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe W B, Ronzio R A, Wellner V P, Meister A. In: Methods in Enzymology. Tabor H, Tabor C W, editors. 17A. New York: Academic; 1970. pp. 900–910. [Google Scholar]
- 32.Gunnersen D, Haley B. Proc Natl Acad Sci USA. 1992;89:11949–11953. doi: 10.1073/pnas.89.24.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norenberg M D. J Neuropathol Exp Neurol. 1982;41:347. doi: 10.1097/00005072-198201000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Sandberg M, Ward H K, Bradford H F. J Neurochem. 1985;44:42–47. doi: 10.1111/j.1471-4159.1985.tb07110.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Araki M, Masuzawa T. Exp Neurol. 1992;116:264–274. doi: 10.1016/0014-4886(92)90006-c. [DOI] [PubMed] [Google Scholar]
- 36.Petito C K, Chung M C, Verkhovsky L M, Cooper A J. Brain Res. 1992;569:275–280. doi: 10.1016/0006-8993(92)90639-q. [DOI] [PubMed] [Google Scholar]
- 37.Bracken M B, Shepard M J, Collins W F, Holford T R, Young W, Baskin D S, Eisenberg H M, Flamm E, Leo Summers L, Maroon J, Marshall L F, Perot P L, Piepmeier J, Sonntag V K H, Franklin C W, Wilberger J E, Winn H R. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 38.Hall E D. J Neurosurg. 1985;62:882–887. doi: 10.3171/jns.1985.62.6.0882. [DOI] [PubMed] [Google Scholar]
- 39.Braughler J M, Hall E D, Means E D, Waters T R, Anderson D K. J Neurosurg. 1987;67:102–105. doi: 10.3171/jns.1987.67.1.0102. [DOI] [PubMed] [Google Scholar]
- 40.Rosner M, Lam T T, Fu J, Tso M O. Arch Ophthalmol. 1992;110:857–861. doi: 10.1001/archopht.1992.01080180129040. [DOI] [PubMed] [Google Scholar]
- 41.Rosner M, Lam T T, Tso M O. Res Commun Chem Pathol Pharmacol. 1992;77:299–311. [PubMed] [Google Scholar]
- 42.Linser P, Moscona A A. Dev Biol. 1983;96:529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- 43.Reisfeld S, Vardimon L. Mol Endocrinol. 1994;8:1224–1233. doi: 10.1210/mend.8.9.7838155. [DOI] [PubMed] [Google Scholar]