Abstract
The columnar organization of the mammalian neocortex is based on radially oriented axon collaterals which precisely link cells from distinct cortical layers. During development, these interlaminar connections are specific from their initial outgrowth: collaterals form only in the target layers and there are no transient axonal collaterals in the nontarget layers. To examine whether positional cues within individual cortical layers regulate the laminar specificity of collateral formation, explants of cells destined for different cortical layers were cultured on membranes prepared from target and nontarget layers. Axonal growth and branching were examined on homogeneous membrane substrates and on alternating stripes of membranes from different layers. Results show that axons branch preferentially on membrane substrates from those layers that they would target in vivo. In addition, when cortical axons were given a choice to grow on membranes from either their target or their nontarget layer, they exhibited a clear preference for the target layers. This indicates that membrane-associated cues confined to individual layers regulate the formation of collaterals of cortical axons and restrict their growth to their target layers. Heat inactivation of membranes from target layers resulted in reduced axonal branching. The same manipulation of membranes from nontarget layers increased axonal branching for one population of cortical neurons. Taken together, these results suggest that membrane-associated molecules confined to individual layers induce and prevent the formation of axon collaterals in distinct populations of cortical neurons. Thus, the expression of layer-specific cues provides important constraints for the remodeling of local circuits during cortical development.
A prominent component of the intrinsic circuitry of the neocortex are collateral connections of axons from pyramidal and spiny stellate cells. Within one cortical layer, tangentially oriented collaterals form regular clusters of axonal branches (1–3) and interconnect cells with similar functional specificities (4–6). In the vertical domain, pyramidal neurons have a main axon that projects out of the cortex and makes local connections within some of the 6 cortical layers (7–11) For instance, the axons of pyramidal cells in layers 2/3 give rise to tangentially oriented collaterals in layers 2/3 and layer 5 that branch profusely within these layers but do not extend into the adjacent layers 4 or 6 (see Fig. 1A). On the other hand, many pyramidal cells in layer 6 send their efferent axon back to the thalamus. These axons emit collaterals in layer 6, which ascend to branch in the overlaying layers 4 and 3. As axon collaterals of layer 6 cells traverse layer 5 they make very few branches in this layer. Physiological studies in primary visual cortex revealed that this precise pattern of interlaminar connections generates characteristic response properties of cortical neurons (12–14).
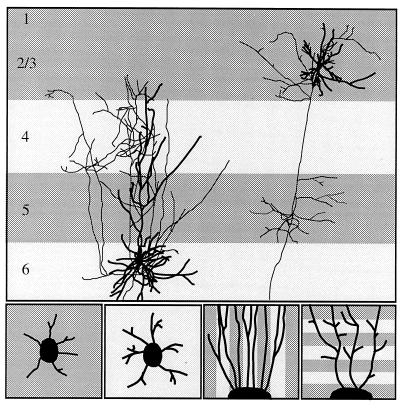
Figure 1.
(Upper) Schematic diagram of interlaminar connections of pyramidal cells in layers 2/3 and in layer 6. [The layer 6 cell is from M. Hübener and J.B., unpublished work; the layers 2/3 cell is reproduced with permission from ref. 31 (copyright 1994, by Cell Press).] Thick processes are dendrites, thin processes, axons. Notice that axon collaterals of the layer 6 cell target lightly shaded laminae (layer 6 and layer 4), whereas axon collaterals of the layers 2/3 cell arborize in the darkly shaded laminae (layers 2/3 and layer 5). (Lower) In vitro experiments designed to investigate the role of membrane-associated molecules on the formation of layer-specific cortical circuits. In the two panels on the left, explants of cortical cells destined either for layers 2/3 or for layer 6 are placed on a homogeneous membrane carpet from target and nontarget layers. In the two panels on the right, axons extending from cortical explants are confronted with alternating lanes of membranes prepared from target and nontarget layers. Axons grow either parallel to the membrane lanes (axonal guidance assay) or perpendicular to the lanes (axonal branching assay).
During development, collateral clusters within a cortical layer emerge from an initially diffuse pattern by elimination of collaterals in inappropriate regions and selective growth in appropriate regions (15–17). There is evidence that this process is under the control of correlated neuronal activity (5, 18). In contrast, the formation of axonal projections between cortical layers is specific from the initial outgrowth; collaterals almost never form in inappropriate cortical layers (19–21). One mechanism which could explain this high degree of laminar specificity is that molecular surface components expressed in different layers, and recognized by specific axonal populations, control laminar branching specificity. To test this hypothesis, we examined the growth and branch formation of cortical axons under different in vitro conditions. For this we purified membranes from single cortical layers of 9- to 11-day-old rats, the time at which intrinsic axonal arbors are being formed (22). These membranes were then offered as substrates for explants from different populations of cortical neurons. Our in vitro assays provided evidence for the existence of positional cues restricted to individual layers that contribute to the laminar specificity of local circuits in the cortex.
MATERIALS AND METHODS
Preparation of Cortical Membrane Substrates.
Blocks of cortex taken from 9- to 11-day-old rats were dissected in Gey’s balanced salt solution supplemented with 6.5 mg/ml glucose and cut with a tissue chopper in 250-μm-thick slices. Under a dissecting microscope with oblique illumination, the laminar borders between some of the cortical layers are visible. In the upper part of the slice, a bright band can be clearly distinguished whose lower border coincides with the boundary between layer 4 and layer 5. It was not possible to identify precisely the border between layers 2/3 and layer 4. However, the boundary between layer 6 and layer 5 was detected as a difference in the opalescence of the tissues; layer 5 appears more transparent than layer 6. Finally, there is a marked change in the transparency between the gray and the white matter. These landmarks were confirmed by Nissl staining of intact and dissected slices from 9- to 11-day-old animals. To isolate tissue from the cortical layers, individual slices were cut along the visible landmarks with a microknife. The tissues from the different layers were collected separately and membranes were prepared as described (23). The optical densities (OD) of the membrane suspensions were measured with a spectrophotometer at 220 nm. In most experiments, OD was adjusted to 0.2 after 15-fold dilution in 2% SDS. When membranes from target and nontarget layers were mixed 1:1, OD was adjusted to 0.2 for the mixed membranes and to 0.1 for the pure membranes. Uniform substrates were prepared by incubating membrane preparations for 2 h at 37°C on laminin-coated glass coverslips. Alternating stripes of membranes were prepared according to the method developed by Bonhoeffer and colleagues (23, 24).
Preparation of Explants.
To isolate different populations of cortical cells we took advantage of the fact that cells destined for cortical layers are produced sequentially in the ventricular zone, from the deep to the upper cortical layers (25–28). Explants of layer 6 cells (200 × 200 × 200 μm) were prepared at embryonic day (E) 15 or E16, the time at which these cells are generated in the rat (27, 28). Subplate neurons, which are also present at these ages, exhibit axonal arborizations very similar to those of layer 6 cells (29). At a later developmental stage, the deep layers cells have migrated out of the ventricular zone, which then contains the cells destined to form the superficial layers. We therefore isolated the ventricular zone from individual cortical slices at E19 to prepare explants of cells destined for layers 2/3. To prepare explants from the hindbrain, a block of tissue was dissected from E16 presumptive cerebellar and pons regions. The explants were kept for 2–4 days in vitro as described in ref. 30.
Analysis of Axonal Growth, Guidance, and Branching.
In several cases, the neuronal origin of the processes extending from the explants was controlled by immunostaining with the neuron-specific marker SMI31 (Sternberger–Meyer, Jarrettsville, MD). Observations were made with a ×20 phase-contrast objective (Zeiss Plan-Neofluar, numerical aperture 0.50) for axonal counts and a ×40 phase-contrast objective (Zeiss Plan-Neofluar, numerical aperture 0.75) for branch analysis. For growth analysis, main axons extending from the explants were counted, collaterals excluded. In several experiments we also determined the average length of the six longest fibers as an estimate of the axonal growth rate. Under the same conditions, collateral formation was assessed qualitatively with a branching index. Each explant that extended at least the average number of axons was examined at high magnification to visualize axonal branches. An index 0 was given to explants from which most axons extended as long, unbranched fibers with less than 5 side branches. An index 2 was given to cultures which developed a dense network of more than 10 axonal collaterals, and an index 1 was given to intermediate branch formation. All Petri dishes were coded so that the type of substrate was not known to the person analyzing the explants. To validate the determination of the branching index, 176 explants from different experimental conditions were scored independently by a second investigator. The difference in the branching indices determined by the two observers was less than 13%.
In the stripe assays in the parallel orientation, a pair of stripes central to the explant was selected and fibers were counted about 400 μm away from the explants to allow them to encounter stripe borders. In the orthogonal orientation, collaterals were counted after fibers crossed at least two membrane stripes. Because fiber outgrowth was very dense close to the explants, this allowed selection of individual axons. Fascicles and short fibers were not taken into account. Particular care was taken with defasciculating fibers and crossing fibers by additional observations at a high magnification (×40 objective and ×1.6 Optovar). As for the measurements on homogeneous substrates, the analysis of the stripe assays was done blindly. In all experiments, statistic analysis of the data was done using a Student t test.
RESULTS
Axonal outgrowth from embryonic cortical explants, comprising neurons destined either for layer 6 or for layers 2/3, was analyzed under various experimental situations which are summarized in Fig. 1. Explants were placed on homogeneous membrane substrates or on alternating stripes of membranes from target and nontarget layers. In the latter case, axons extended parallel and orthogonal to the membrane stripes.
When cortical explants were placed on a uniform membrane substrate prepared from layer 6, layer 5, and layers 1–4, they extended numerous axons. Overall the average outgrowth from layer 6 neurons was higher than from layers 2/3 neurons; however, the number of axons extending from the explants was independent of the layer from which the membranes were prepared (data not shown). To determine how the different membrane substrates influenced the growth rate, we measured the length of axons extending from layer 6 explants (n = 60) and layers 2/3 explants (n = 30). After 3 days in vitro, 50% of axons from layer 6 neurons exceeded a length of 600 μm on layer 6 membranes, whereas only about 30% of axons reached this length on layer 5 or layers 1–4 membranes (P < 0.002). Similarly, 50% of axons from layers 2/3 neurons were longer than 338 μm on layer 6 membranes, but only 30% and 34% on layers 1–4 and 5 membranes, respectively (P < 0.02). Thus, the length of both sets of axons was highest on membranes from layer 6. In contrast, under the same experimental conditions, axons from layer 6 and layers 2/3 neurons branched differentially on the membrane substrates. For layer 6 neurons, the branching index increased from 0.64 (n = 125 explants) on layer 5 membranes to 1.41 (n = 103 explants) and 1.2 (n = 122 explants) on layer 6 and layers 1–4 membranes, respectively (P < 0.0001). For layers 2/3 neurons, the branching index increased from 0.66 (n = 24 explants) on layer 6 membranes to 1.58 (n = 24 explants) on layer 5 membranes (P < 0.0001; Fig. 2 A and B; Fig. 4 A and B). Thus, axons emitted more collaterals on membranes from their target layers than on membranes from their nontarget layers. For neurons from layers 2/3, layers 1–4 membranes are a mixture of target and nontarget layers, but the branching index on these membranes was similar to the branching index observed on layer 5 membranes, a pure target layer for these cells (Fig. 4B). These observations on axonal elongation and branching on membranes from isolated layers suggest that collateral formation and axonal elongation of cortical neurons are regulated independently.
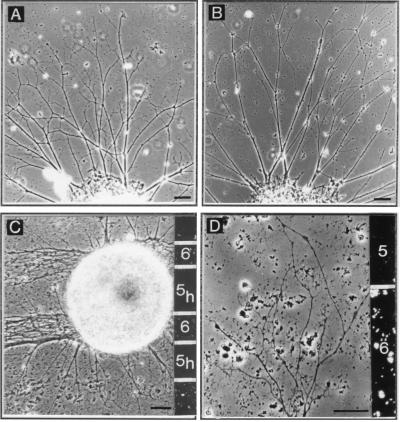
Figure 2.
(A and B) Phase-contrast photomicrographs of layer 6 explants on a homogeneous membrane carpet prepared from layer 6 (target layer; A) and from layer 5 (nontarget layer; B). The two explants extend a comparable number of axons; however, axons form more branches on membranes from target layer than on membranes from nontarget layer. (C and D) Micrographs from membrane stripe assays with layer 6 explants. (C) Stripes of heat-inactivated layer 5 membranes (subscript h) and native layer 6 membranes. When growing parallel to the stripes, axons grow almost exclusively on the native membranes from layer 6. (D) Axons extending perpendicular to alternating stripes of membranes from layer 6 and layer 5 and arborizing preferentially on their target membranes. In each case, membranes isolated from layer 6 are marked with fluorescent beads, as shown on the right of each panel. (Scale bars: 50 μm in A–C and 25 μm in D.)
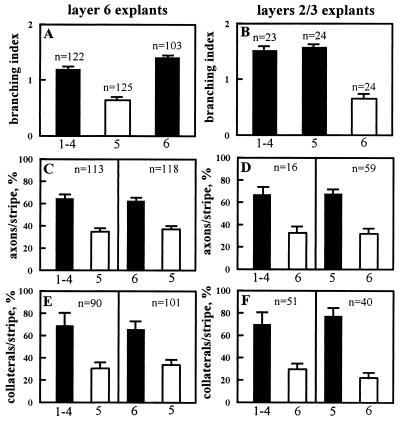
Figure 4.
Quantitative analysis of axonal growth and branch formation under different experimental conditions. (Left) Histograms presenting results from layer 6 explants. (Right) Results from layers 2/3 explants. Filled bars correspond to membrane substrates from the target layers and open bars, substrates from the nontarget layers. (A and B) Axonal branching index on homogeneous membrane substrates prepared from different layers, as indicated on the x axis; n = number of explants scored. (C and D) Stripe assay with axons growing parallel to alternating stripes of membranes from two different cortical layers indicated on the x axis (see Fig. 1D). Bars represent the percentage of axons growing on each set of lanes; n = number of pairs of stripes examined on a total of 144 and 50 explants for layers 6 neurons and layers 2/3 neurons, respectively. (E and F) Stripe assay with axons growing perpendicular to membrane stripes (see Fig. 1E). Bars represent the percentage of axonal branches formed in each set of lanes; n = number of axons examined.
In the stripe assay, with axons extending parallel along alternating membrane stripes prepared from target and nontarget layers, initially fibers emerged from the explants on both types of membranes. However, as axons grew away from the explants and encountered a stripe border, in most cases they continued their growth on stripes prepared from membranes of their target layers. As a result, 400 μm away from the explants, layer 6 axons avoided the stripes from their nontarget layer 5 and grew preferentially on the stripes from layers 1–4 (64%, P < 0.0001) or layer 6 (63%, P < 0.0001; Fig. 4C). Layers 2/3 axons avoided membranes from layer 6 and instead grew on membranes from layers 1–4 (66%, P < 0.0002) or layer 5 (67%, P < 0.0001; Fig. 4D). In contrast, under the same conditions, axons extending approximately perpendicular to the membrane stripes freely crossed the stripe borders, but they emitted more branches when they crossed stripes from the target layer than from the nontarget layer (Fig. 2D; Fig. 3). On 191 fibers examined, 69% and 66% of the axonal branches from layer 6 cells were located in the target membrane stripes prepared from layers 1–4 and 6, respectively (P < 0.0001; Fig. 4E). Again, layers 2/3 axons exhibited more collaterals on both membranes from layers 1–4 (70%, P < 0.0002) and layer 5 (78%, P < 0.0001) than on membranes from the nontarget layer (Fig. 4F). As illustrated in Fig. 3, for 90-μm-wide axonal segments, 1 to 6 branches were observed on target membrane stripes. In control experiments, axons from the hindbrain exhibited the same growth rate and branching index on uniform membrane carpets from the different cortical layers, and these axons showed no growth and branching preference for any cortical layer in the stripe assay (Table 1).
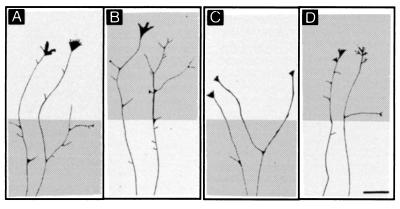
Figure 3.
Camera lucida drawings of representative axons crossing stripes with membranes from target layer (dark gray) and membranes from nontarget layer (light gray). (A and B) Axons from layer 6 neurons. (C and D) Axons from layers 2/3 neurons. In A and C axons encounter first a membrane stripe from target layers and in B and D axons encounter first a stripe with nontarget membranes. In each case, axons branch preferentially on their target membranes. (Scale bar: 25 μm.)
Table 1.
Data from growth, branching, and guidance experiments from layer 6 explants, from layers 2/3 explants, and, as a control, from hindbrain explants
| Explants | Substrate | Stripe | Collaterals or axons† per stripe, % | Branching index | Fibers per explant |
|---|---|---|---|---|---|
| Layer 6 | 1–4 | 1.11 ± 0.12 (n = 40) NS | |||
| 5 | 0.50 ± 0.08 (n = 56)** | ||||
| 6 | 1.15 ± 0.10 (n = 47) NS | ||||
| 1–4 + 5 | 0.95 ± 0.08 (n = 77) NS | ||||
| 6 + 5 | 1.20 ± 0.09 (n = 54) NS | ||||
| 6/5h (n = 30) | 6 | 73.8 ± 8** | |||
| 5h | 26.2 ± 6 | ||||
| 6h/5 (n = 21) | 6h | 46.1 ± 16 NS | |||
| 5 | 53.9 ± 8 | ||||
| 1–4/5h (n = 37) | 1–4 | 76.4 ± 8** | |||
| 5h | 23.6 ± 7 | ||||
| 1–4h/5 (n = 30) | 1–4h | 41.5 ± 9 NS | |||
| 5 | 58.5 ± 8 | ||||
| 6h/6 (n = 45) | 6h | 30.4 ± 6** | |||
| 6 | 69.6 ± 6 | ||||
| 5h/5 (n = 35) | 5h | 51 ± 10 NS | |||
| 5 | 49 ± 6 | ||||
| Layers 2/3 | 6/5h (n = 20) | 6 | 41.7 ± 8 NS | ||
| 5h | 58.3 ± 16 | ||||
| 6h/5 (n = 27) | 6h | 23.8 ± 8** | |||
| 5 | 76.2 ± 7 | ||||
| 6h/6 (n = 26) | 6h | 67.4 ± 10* | |||
| 6 | 32.6 ± 7 | ||||
| 5h/5 (n = 25) | 5h | 30 ± 8* | |||
| 5 | 70 ± 13 | ||||
| Hindbrain | 1–4 | 0.30 ± 0.06 (n = 69) | 9.6 ± 0.9 (n = 153) NS | ||
| 5 | 0.34 ± 0.06 (n = 66) | 10.1 ± 1.4 (n = 102) NS | |||
| 6 | 0.26 ± 0.05 (n = 77) | 10.4 ± 1.4 (n = 129) NS | |||
| 1–4/5 (n = 18) | 1–4 | 50.5 ± 8 NS | |||
| 5 | 49.5 ± 10 | ||||
| 6/5 (n = 43) | 6 | 50.4 ± 6 NS | |||
| 5 | 49.6 ± 7 | ||||
| 1–4/5 (n = 25) | 1–4 | 52.9 ± 5 NS | |||
| 5 | 47.1 ± 6 | ||||
| 6/5 (n = 25) | 6 | 48.3 ± 4 NS | |||
| 5 | 51.6 ± 5 |
Results are mean ± SEM. The suffix h designates membrane substrates that have been heat-inactivated. NS, not significant; ∗, significantly different with P < 0.001; ∗∗, significantly different with P < 0.0001. For branching index, n = number of explants scored; in stripe experiments, n = number of axons examined for collaterals per stripe and n = number of explants for axons per stripe.
Axons per stripe refers to the last four values only.
These differential effects of layer-specific cues on collateral formation of cortical axons could be due to signals present in the target layers that induce and/or stabilize branch formation, to signals in the nontarget layers that inhibit and/or destabilize axonal branches, or to a combination of both. Our observation that layers 2/3 neurons exhibited many branches on membranes from layer 5, a target layer, as well as on membranes from layers 1–4, a mixture of target and nontarget layers, was already an indication for the existence of axonal sprouting factors in the target layers. For layer 6 cells, we mixed membranes from layer 5, their nontarget layer, with membranes from either layers 1–4 or layer 6, their target layers. If membranes from the nontarget layer contain inhibitory signals for branch formation, their addition should decrease the number of axons collaterals. However, comparable amounts of collaterals were found on the mixed substrates and on the target layers alone. As illustrated in the top part of Table 1, statistical analysis of the branching index on membranes from layers 1–4, 5, and 6 and the mixtures 1–4 + 5 and 6 + 5 showed that only the value on membranes from layer 5 was different from the others (P < 0.0001). This experiment suggests that the differential branching is due to molecular signals associated with membranes from the target layers. To further address this issue, we examined the effects of heat inactivation of the target and nontarget membranes on the axonal growth and branch formation. When the explants were cultured on uniform substrates, heat inactivation strongly reduced the growth on membrane preparations from each layer and did not allow analysis of the axonal branching. In contrast, explants cultured on alternating stripes of inactivated and native membranes extended axons. When growing parallel to the lanes, axons from both layer 6 and layers 2/3 neurons exhibited a strong preference for the stripes with native membranes, independent of whether they were derived from target or nontarget layers. However, axons extending perpendicular to the lanes were able to cross the stripes with heat-inactivated membranes (Fig. 2C). When the treatment was applied to the nontarget membranes, axons from both layer 6 and layers 2/3 cells branched preferentially on membranes from their target layers (P < 0.0001 in all combinations; Table 1), as was the case with native membranes. In contrast, in the stripe assays with inactivated target membranes and native nontarget membranes, the branch frequency strongly decreased on the inactivated target membranes. In this situation, there was no longer a significant difference in the number of axon collaterals between the two types of stripes (Table 1). Finally, we compared axonal branching in stripe assays with native and heat-inactivated membranes derived from the same layer. On target membranes, we observed for both layer 6 and layers 2/3 cells a 3-fold reduction of the axonal branching in the inactivated lanes (Table 1). On inactivated and native nontarget membranes, layer 6 neurons emitted a low number of axonal branches, with a equal distribution in each set of lanes. Thus, axons branched similarly on inactivated target membranes, native nontarget membranes, and inactivated nontarget membranes. In contrast, for layers 2/3 cells we observed a 2-fold increase of the branching frequency in the inactivated nontarget lanes, with 67% of the branches emitted on these membranes (Table 1). Thus, axonal branching was similar on inactivated target membranes and native nontarget membranes but increased on both native target membranes and inactivated nontarget membranes. Since axonal branching of layers 2/3 neurons increased when the nontarget membranes were heat inactivated, inhibitory signals also contribute to regulate the branch formation for this set of cortical axons.
DISCUSSION
The results of this study demonstrate that axons of different sets of cortical neurons respond differentially to membranes isolated from individual cortical layers. Axons branched more frequently on membrane substrates prepared from those layers that they contact in vivo than on membranes prepared from their nontarget layers. When given a choice, axons grew preferentially on membranes from their target layers rather than on membranes from their nontarget layers. These observations suggest that membrane-associated molecules regulate the formation of axonal branches in the appropriate cortical layers and prevent axon collaterals growing into inappropriate layers.
In accordance with previous studies, we found that membranes from postnatal cortex provided a good substrate for the growth of cortical axons (30, 32). There was no difference in the number of axons extending on membranes prepared from different cortical layers. Axons growing on membranes from layer 6, however, were longer than those growing on membranes from layer 5 or layers 1–4. One possible explanation for this is that the dissections of layer 6 membranes included portions of the white matter, which is rich in growth-promoting molecules in the extracellular matrix and on the axonal surface (30, 32, 33). These growth promoters, however, do not appear to be responsible for the guidance of axons within the cortical layers. Although axons of layers 2/3 neurons grew longer on uniform membranes derived from layer 6 than on membranes from layer 5, on alternating membrane stripes these axons extended preferentially on the lanes of membranes from layer 5 rather than layer 6. Thus factors that cause an increase in growth rate do not necessarily orient growing axons, even when they are localized in defined tracks.
The present results also indicate that the signals which regulate the branching of axons are different from those which promote the extension of axons from the cell body. The formation of collateral branches was highest on membrane substrates from the target layers and not necessarily on membrane substrates which contained the highest concentration of growth promoters. In vivo, collaterals emerge in the target layers from the main efferent axon after it has grown out of the cortex (21). Sprouting of collateral branches from the primary axon shaft can also occur during the formation of cortical projections to distant targets. The axons of many neurons in layer 5 initially project to the spinal cord and bypass the pons, but later sprout and innervate this target (34–37). Coculture experiments have shown that the pons releases diffusible factors that promote the formation of collateral branches along axons of layer 5 neurons (37). In the present study we found that cortical axons branch in response to membrane-associated factors. However, chemotropic molecules that function as diffusible signals can also bind tightly to cell surfaces (38, 39). It is therefore conceivable that the same molecule may act as a substrate-bound cue that directs the sprouting of axons within the cortical layers and act as a diffusible cue that controls collateral branch formation at distant targets.
There is evidence that both attractive and repulsive stimuli play a role in axon guidance and branch formation during development. One system in which inhibitory mechanisms are well studied is the topographic projections from the retina to the tectum. In the retinotectal system, fibers from the temporal retina are repelled by membranes from the posterior tectum, their nontarget region. In the stripe assay, when these axons extended parallel to the lanes, they preferred to grow on membranes from the anterior tectum, their target region (23), and when they extended across the lanes, they selectively branched on the membranes from the topographically correct tectal region (40). In this system, heat inactivation of membranes from the nontarget region abolished their repellent properties and retinal axons no longer showed a preferential growth in the stripe assay, whereas inactivation of membranes from the target region had no effect (23). In our experiments, heat inactivation of membranes from both target and nontarget layers almost abolished axonal outgrowth, presumably because growth-supporting molecules, which appear to be present in all cortical layers, had been inactivated. In the stripe assay, however, axons were able to grow across heat-inactivated membranes. In this situation, branch formation was strongly reduced on inactivated membranes from target layers. When growing on stripes with native target and inactivated nontarget membranes, no detectable change was observed in the branching preference for the target lanes. However, axons from layers 2/3 cells branched more on inactivated than on native nontarget membranes. Taken together, these observations provide evidence that promoting signals in the target layers play a role in mediating the layer-specific branching of cortical axons and that additional signals prevent collateral formation in the nontarget layers.
The molecular nature of the factors that sculpt local cortical circuits is not known. So far only a few reports have provided evidence for molecular differences between the developing cortical layers. Studies using in situ hybridization revealed that some genes coding for transcription regulators are expressed in the cortex in a layer-specific fashion, but their “downstream” gene products remain unclear (e.g., refs. 41 and 42). Particularly interesting in the present context are some recent findings on laminar differences of growth factors, neurotrophins, and their receptors (reviewed in ref. 43). There is now increasing evidence that these molecules, in addition to their well established action on cell proliferation and cell survival, are also involved in the regulation of neuritic growth and branching. For example, injections of NT-3 into the rat spinal cord enhanced collateral sprouting of corticospinal axons during normal development and after lesions in adult animals (44). Neurotrophins exogenously applied to organotypic slice cultures of developing ferret visual cortex influenced in a laminar-specific fashion the dendritic morphology of neurons from various cortical layers (45). It has been shown that neurotrophic factors can bind to membrane-associated molecules and thereby form a molecular complex that promotes the interaction with their receptors (see, e.g., ref. 46). Thus, if neurotrophic factors regulate the construction of local cortical circuits, then laminar specificity might be the result of the restricted spatial distribution of these factors, their receptors, or membrane-associated molecules that bind and subsequently present these factors to growing axons.
Our study shows that cortical axons in vitro express their lamina-specific branching behavior at very early developmental stages, before the neurons have migrated into their final position in the cortex. This is reminiscent of previous work which indicates that several aspects of the phenotype of cortical neurons are determined at very early developmental stages. For instance, transplantation experiments revealed that cortical progenitor cells, after the final S phase of the cell cycle, are already committed to produce neurons that migrate into a specific layer in the cortex (47). Other studies indicated that the transmitter phenotype of cortical neurons is also specified during the S phase of the cell cycle (48). The fate of cortical neurons appears to be determined by signals from the local environment as the cells are generated. The present results show that explants isolated from the ventricular zone, when placed on membrane substrates from different cortical layers, extend axons that branch and grow preferentially on membranes from those layers that they would target in vivo, after they have migrated to the superficial layers. We do not know whether the laminar-specific branching pattern is already specified at the progenitor cell level or only after the cells have finished their final mitosis. Whatever the case, it seems clear that by the time cortical neurons leave the ventricular zone they “know” the layer in which they will reside, the transmitter they will express, and the connections they will form.
In our experiments axons branched preferentially on membrane substrates from the target layers; however, about 30% of branches were found on membranes from nontarget layers. In the developing cat visual cortex in vivo, it has been reported that axonal branches are almost never observed in inappropriate layers (21). There are several possibilities to explain why the branching specificity in the developing rat cortex in vitro is not as sharp as in cat cortex in vivo. First, there might be species differences. The layer-specific branching of cortical axons in the rat is not as pronounced as in higher mammals (7–11). Second, explants prepared at embryonic stages do not consist of pure populations of neurons. E16 explants, in addition to layer 6 and subplate cells, also include 20–25% layer 5 cells (27, 28), which display different branching behavior. Likewise, in the procedure of isolating the layers for membrane preparation, tissues could be contaminated by fragments of adjacent layers. Third, it has been reported that the branching frequency of cortical axons extending out from explants is higher than in slice preparations, which mimic more closely the in vivo situation (36, 37). This suggests that cortical axons in vitro branch spontaneously and therefore also form some axon collaterals on membranes from inappropriate layers. Finally, in vivo there might be factors which are reduced or absent in our in vitro assay which contribute to sharpening the laminar branching. However, our in vitro experiments do show that axons were able to express a branching preference on membranes from appropriate layers alone.
In conclusion, our results indicate that positional cues restricted to different cortical layers induce collateral branch formation of specific sets of axons. Since different populations of cortical axons reacted differentially to membrane-associated molecules of a given cortical layer, either there are several factors present in each layer influencing axonal branching or the same factors act differentially on different axonal populations. Whatever the case, axons from neurons in various cortical layers must express different receptors for lamina-specific molecules, or their receptors must be coupled to different signaling pathways. Moreover, the layer-specific signals must act very locally on cortical axons, since in the stripe assay the increase in collateral branch formation was restricted to 90-μm axonal segments, the width of the stripes. Thus, the layer-specific expression of membrane-associated signals provides a molecular framework for subsequent activity-dependent mechanisms that control the remodeling of tangential connections between cortical columns.
Acknowledgments
We thank Naura Chounlamountri for excellent technical assistance and Mary Behan and Herb Killacky for helpful comments on the manuscript. This work was supported by the Human Frontiers Science Program.
ABBREVIATION
- Ex
embryonic day x
References
- 1.Fisken R A, Garey L J, Powell T P S. Phil Trans R Soc Lond B. 1975;272:487–536. doi: 10.1098/rstb.1975.0099. [DOI] [PubMed] [Google Scholar]
- 2.Rockland K S, Lund J S, Humphrey A L. J Comp Neurol. 1982;209:41–58. doi: 10.1002/cne.902090105. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C D, Wiesel T N. J Neurosci. 1983;3:1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert C D, Wiesel T N. J Neurosci. 1989;9:2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Löwel S, Singer W. Science. 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 6.Malach R, Amir Y, Harel M, Grinvald A. Proc Natl Acad Sci USA. 1993;90:10469–10473. doi: 10.1073/pnas.90.22.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund J S, Boothe R G. J Comp Neurol. 1975;159:305–334. [Google Scholar]
- 8.Gilbert C D, Wiesel T N. Nature (London) 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- 9.Martin K A C, Whitteridge D. J Physiol. 1984;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhalter A. J Comp Neurol. 1989;279:171–186. doi: 10.1002/cne.902790202. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick D. Cereb Cortex. 1996;6:329–341. doi: 10.1093/cercor/6.3.329. [DOI] [PubMed] [Google Scholar]
- 12.Bolz J, Gilbert C D. Nature (London) 1986;320:362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- 13.Schwark H D, Malpeli J G, Weyand T G, Lee C. J Neurophysiol. 1986;56:1074–1087. doi: 10.1152/jn.1986.56.4.1074. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz C, Bolz J. J Neurosci. 1991;11:2995–3007. doi: 10.1523/JNEUROSCI.11-10-02995.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callaway E M, Katz L C. J Neurosci. 1990;10:1134–1153. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luhmann H J, Singer W, Martínez Millán L. Eur J Neurosci. 1990;2:358–368. doi: 10.1111/j.1460-9568.1990.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 17.Lübke J, Albus K. J Comp Neurol. 1992;323:42–58. doi: 10.1002/cne.903230105. [DOI] [PubMed] [Google Scholar]
- 18.Callaway E M, Katz L C. Proc Natl Acad Sci USA. 1991;88:745–749. doi: 10.1073/pnas.88.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund J S, Boothe R G, Lund R D. J Comp Neurol. 1977;176:149–188. doi: 10.1002/cne.901760203. [DOI] [PubMed] [Google Scholar]
- 20.Meyer G, Ferres-Torres R. J Comp Neurol. 1984;228:226–244. doi: 10.1002/cne.902280209. [DOI] [PubMed] [Google Scholar]
- 21.Katz L C. Eur J Neurosci. 1991;3:1–9. doi: 10.1111/j.1460-9568.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller M W, Peters A. J Comp Neurol. 1981;203:555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- 23.Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Development (Cambridge, UK) 1987;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- 24.Wizenmann A, Thies E, Klostermann S, Bonhoeffer F, Bähr M. Neuron. 1993;11:975–983. doi: 10.1016/0896-6273(93)90126-c. [DOI] [PubMed] [Google Scholar]
- 25.Angevine J B, Sidman R L. Nature (London) 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 26.Hicks S P, D’Amato C J. Anat Rec. 1968;160:619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- 27.Bayer S A, Altman J. Neocortical Development. New York: Raven; 1991. [Google Scholar]
- 28.Miller M W. In: Development and Maturation of Cerebral Cortex, Cerebral Cortex. Peters A, Jones E G, editors. Vol. 7. New York: Plenum; 1988. pp. 133–175. [Google Scholar]
- 29.Friauf E, McConnell S K, Shatz C J. J Neurosci. 1990;10:2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Götz M, Novak N, Bastmeyer M, Bolz J. Development (Cambridge UK) 1992;116:507–519. [Google Scholar]
- 31.Lo D C, McAllister A K, Katz L C. Neuron. 1994;13:1263–1268. doi: 10.1016/0896-6273(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle R, Schlaggar B L, Braisted J E, O’Leary D D M. J Neurosci. 1995;15:3039–3052. doi: 10.1523/JNEUROSCI.15-04-03039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart G R, Pearlman A L. J Neurosci. 1987;7:3325–3333. doi: 10.1523/JNEUROSCI.07-10-03325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Leary D D M, Terashima T. Neuron. 1988;1:901–910. doi: 10.1016/0896-6273(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 35.Kuang R Z, Kalil K. J Comp Neurol. 1994;344:270–282. doi: 10.1002/cne.903440208. [DOI] [PubMed] [Google Scholar]
- 36.Bastmeyer M, O’Leary D D M. J Neurosci. 1996;16:1450–1459. doi: 10.1523/JNEUROSCI.16-04-01450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato M, Lopez-Mascaraque L, Heffner C D, O’Leary D D M. Neuron. 1994;13:791–803. doi: 10.1016/0896-6273(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 38.Serafini T, Kennedy T E, Galko M J, Mirzayan C, Jessell T M, Tessier-Lavigne M. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 39.Luo Y, Raible D, Raper J A. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 40.Roskies A L, O’Leary D D M. Science. 1994;265:799–803. doi: 10.1126/science.8047886. [DOI] [PubMed] [Google Scholar]
- 41.He X, Treacy M N, Simmons D M, Ingaraham H A, Swanson L W, Rosenfeld M G. Nature (London) 1989;340:35–42. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- 42.Frantz G D, Weimann J M, Levin M E, McConnell S K. J Neurosci. 1994;14:5725–5740. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allendoerfer K L, Shatz C J. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 44.Schnell L, Schneider R, Kolbeck R, Barde Y A, Schwab M E. Nature (London) 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 45.McAllister A K, Lo D C, Katz L C. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 46.Yayon A, Klagsbrun M, Esko J D, Leder P, Ornitz D M. Cell. 1991;64:841–849. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 47.McConnell S K, Kaznowski C E. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 48.Götz M, Bolz J. Eur J Neurosci. 1994;6:18–32. doi: 10.1111/j.1460-9568.1994.tb00244.x. [DOI] [PubMed] [Google Scholar]