Abstract
Immunostimulatory DNA sequences (ISS) containing CpG motifs induce interferon-α (IFN-α) and interferon-γ (IFN-γ) from human peripheral blood mononuclear cells and stimulate human B cells to proliferate and produce IL-6. We studied the motif and structural requirements for both types of activity using novel chimeric immunomodulatory compounds (CICs), which contain multiple heptameric ISS connected by non-nucleoside spacers in both linear and branched configurations. We found that the optimal motifs and structure for IFN-α production versus B cell activation differed. IFN-α production was optimal for CICs containing the sequences 5′-TCGXCGX and 5′-TCGXTCG, where X is any nucleotide. The presentation of multiple copies of these heptameric ISS with free 5′-ends via long, hydrophilic spacers, such as hexaethylene glycol, significantly enhanced the induction of IFN-α. Conversely, human B cell activity was predominately dependent on ISS motif, with 5′-TCGTXXX and 5′-AACGTTC being the most active sequences. Thus, we found CICs could be ‘programmed’ for IFN-α production or B cell activation as independent variables. Additionally, CICs with separate human- and mouse-specific motifs were synthesized and these were used to confirm in vivo activity in mice. CICs may offer unique advantages over conventional ISS because identification of the optimal motifs, spacers and structures for different biological properties allows for the assembly of CICs exhibiting a defined set of activities tailored for specific clinical applications.
INTRODUCTION
Immunostimulatory DNA sequence oligodeoxynucleotides (ISS ODNs), generally containing 15–25 bases, are capable of inducing innate immune responses that mimic responses induced by microbial DNA. The basis for host recognition of microbial, but not mammalian, DNA is the high frequency of non-methylated CpG dinucleotides present in the DNA of most microorganisms (1). Stimulation with ISS initiates a cascade of cytokine induction and cellular changes that are responsible for the potent and varied activities of ISS, including promotion of increased resistance to a variety of infectious microorganisms, the development of greatly enhanced T cell and antibody responses, and preferential induction of T cells of the Th1 type (2–5). Clinical trials with ISS, including our lead compound, 1018 ISS, are underway for the treatment of ragweed allergy (6), asthma and cancer, as well as in combination with hepatitis B surface antigen for use as an improved hepatitis B vaccine (7).
Recognition of ISS is through Toll-like receptor 9 (TLR9), one of a family of receptors that recognize microbial and viral molecules and trigger rapid innate immune responses (8). TLR9 expression and ISS-responsiveness are restricted to only a few cell types, primarily cells with the ability to take up and present antigens to T cells. Murine ISS-responsive cells include monocytes, B cells and subsets of dendritic cells (9–12). Human cells show a more limited pattern of TLR9 expression; in blood, plasmacytoid dendritic cells and B cells, but not monocytes, express high levels of TLR9 and respond in vitro to ISS (13–15).
Primary in vitro assays widely used to test biological activity of ISS measure the induction of cytokine secretion and the proliferation of B cells in human peripheral blood mononuclear cells (PBMCs) or mouse spleen cells. The cytokines that show the highest degree of ISS-specificity in human PBMC are interferon-α (IFN-α) and interferon-γ (IFN-γ) (16,17), while in the mouse, IFN-γ, IL-12p40 and IL-6 are commonly used (18). This partial disparity in ISS-induced cytokines in human versus mouse is accounted for by the fact that the mouse splenocyte response to ISS is dominated by monocytes (a non-responder cell in humans), which produce large amounts of IL-12 and IL-6. Additionally, we have not found significant IFN-α induction by different sequences in naïve mouse spleen cultures (J.D.Marshall, unpublished results).
Although the presence of at least one CpG dinucleotide is required for an ISS to be active, the residues flanking the CpG(s) contribute significantly to the biological activities of the molecule (19). In addition, the sequence requirements for optimum mouse and human activity differ substantially. The originally defined active ‘CpG motif’, consisting of PuPuCGPyPy, where Pu = purine and Py = pyrimidine (20), appears to be the optimum motif for interaction with murine TLR9 (21). Recent work from our laboratory using very short ODNs (5–7 bases) has shown clearly that a minimal human CpG motif for the potent production of IFN-α and IFN-γ is TCGXX, where X is any nucleotide. However, short ISS containing this motif required adsorption to the surface of cationic poly(D,L-lactide-co-glycolide) microparticles for activity (22). This formulation greatly enhanced the uptake of short ISS into cells, and the activity may also have been increased by the multimeric presentation of the ISS to the TLR9 receptor. As a continuation of this study, we evaluated other methods to present the ISS in a multimeric fashion and increase the uptake of short ISS into cells. As such, we prepared and tested a series of chimeric immunomodulatory compounds (CICs) containing multiple 7mer ISS connected with non-nucleoside spacers. Described herein are the results of studies that identify the optimal CpG motifs, spacers and structures for IFN-α and IFN-γ induction from human PBMCs and for human B cell activation. CICs with human and mouse cross-reactivity were also identified and used to confirm in vivo activity.
MATERIALS AND METHODS
ISS and CIC synthesis
Phosphorothioate ISS and CICs were synthesized on a 1 or 15 µmol scale using a Perseptive Biosystems Expedite 8909 automated DNA synthesizer and manufacturer’s protocols, except where noted. The 3′-β-cyanoethylphosphoramidite nucleoside monomers, 4,4′-O-dimethoxytrityl-hexaethylene glycol-O-(N,N-diisopropyl) 2-cyanoethylphosphoramidite, 4,4′-O-dimethoxytrityl-triethylene glycol-O-(N,N-diisopropyl) 2-cyanoethylphosphoramidite, 1-(4,4′-O-dimethoxytrityloxy)-propanediol-3-O-(N,N-diisopropyl) 2-cyanoethylphos phoramidite and 1,3-bis(4,4′-O-dimethoxytrityl)-glycerol-2-O-(N,N-diisopropyl) 2-cyanoethylphosphoramidite (symmetrical branching phosphoramidite) were obtained from ChemGenes. The trebler phosphoramidite was obtained from Glen Research. All synthesis was conducted in the 3′ to 5′ direction. Sulfurization of the internucleotide phosphite triester linkages was achieved using 0.02 M xanthane hydride (TCI America) in 9:1 acetonitrile:pyridine for 2 min (23). For the branched CICs, the synthesis protocol was modified such that after the addition of the branching phosphoramidite, each reagent delivery was doubled due to the synthesis of two or more nucleic acid chains simultaneously. At the completion of ISS or CIC assembly, the cyanoethyl groups were selectively cleaved by a 5 min treatment with 2 M diethylamine in acetonitrile (5 ml), followed by thorough washing with acetonitrile (2× 20 ml). The ‘trityl-on’ compounds were cleaved from the controlled-pore glass and the bases were deprotected with concentrated aqueous ammonia at 58°C for 16 h. The compounds were purified by RP-HPLC on a PLRP-S column (7.5 × 300 mm; 8 µm; Polymer Labs) using an increasing gradient of acetonitrile in 0.1 M triethylammonium acetate. The purified compounds were concentrated to dryness, the 4,4′-dimethoxytrityl groups were removed with 80% aqueous acetic acid, and then the compounds were precipitated twice from 0.6 M aqueous sodium acetate/pH 5.3 with 2.5 vol of 95% ethanol. The compounds were dissolved in MilliQ water and the yields were determined from the absorbance at 260 nm.
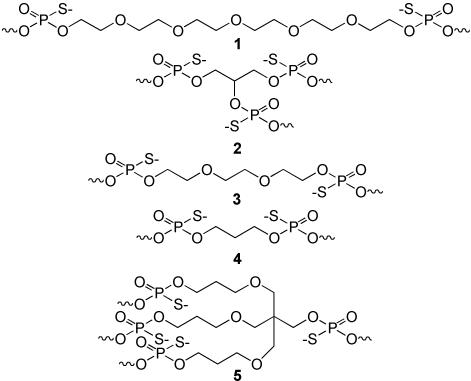
The sequences and structures for 1018 ISS and the CICs, which are denoted by a code number, C, are shown in Table 1. Figure 1 shows the structure of the non-nucleoside spacers after incorporation into the CICs, where 1 is hexaethylene glycol (HEG), 2 is glycerol, 3 is triethylene glycol (TEG), 4 is propanediol (C3) and 5 is trebler. All compounds were characterized by capillary gel electrophoresis, electrospray mass spectrometry (HT Laboratories), and RP-HPLC to confirm composition (Table 1) and purity (>90%). An endotoxin content assay (LAL assay, Northview Laboratories) was also conducted, showing endotoxin levels were <5 EU/mg compound.
Table 1. Phosphorothioate CICs and 1018 ISS and their corresponding molecular weights.
| Code no. | Sequence | Molecular weight (Da) | |
|---|---|---|---|
| Calculated | Found | ||
| ISS: | |||
| 1018 | 5′-TGACTGTGAACGTTCGAGATGA | 7152 | 7152 |
| Linear CICs and control: | |||
| C01 | 5′-TCGATTT-HEG-5′-TCGATTT-HEG-5′-TCGATTT | 7425 | 7425 |
| C02 | 5′-TTCGATT-HEG-5′-TTCGATT-HEG-5′-TTCGATT | 7425 | 7425 |
| C03 | 5′-TTTCGAT-HEG-5′-TTTCGAT-HEG-5′-TTTCGAT | 7425 | 7425 |
| C04 | 5′-TTTTCGA-HEG-5′-TTTTCGA-HEG-5′-TTTTCGA | 7425 | 7424 |
| C05 | 5′-ACGTCGA-HEG-5′-ACGTCGA-HEG-5′-ACGTCGA | 7482 | 7482 |
| C06 | 5′-TCGTTTT-HEG-5′-TCGTTTT-HEG-5′-TCGTTTT | 7398 | 7398 |
| C07 | 5′-TCGTCGA-HEG-5′-TCGTCGA-HEG-5′-TCGTCGA | 7455 | 7455 |
| C08 | 5′-TCGACGT-HEG-5′-TCGACGT-HEG-5′-TCGACGT | 7455 | 7454 |
| C09 | 5′-TCCTCCA-HEG-5′-ACCTTAG-HEG-5′-AGATGAT (control) | 7407 | 7408 |
| Branched CICs and control: | |||
| C10 | (5′-TCGTTTT-HEG)2-glycerol-HEG-5′-TCGTTTT | 7927 | 7929 |
| C11 | (5′-TCGTCGA-HEG)2-glycerol-HEG-5′-TCGTCGA | 7984 | 7985 |
| C12 | (5′-TCGACGT-HEG)2-glycerol-HEG-5′-TCGACGT | 7984 | 7985 |
| C13 | (5′-TCGTCGT-HEG)2-glycerol-HEG-5′-TCGTCGT | 7957 | 7958 |
| C14 | (5′-TCGTCGA-TEG)2-glycerol-TEG-5′-TCGTCGA | 7588 | 7588 |
| C15 | (5′-TCGTCGA-C3)2-glycerol-C3-5′-TCGTCGA | 7365 | 7366 |
| C16 | (5′-TCGTCGA)2-glycerol-5′-TCGTCGA | 6903 | 6904 |
| C17 | (5′-TCGATCG-HEG)2-glycerol-HEG-5′-TCGATCG | 7984 | 7985 |
| C18 | (5′-TCGCTCG-HEG)2-glycerol-HEG-5′-TCGCTCG | 7912 | 7912 |
| C19 | (5′-TCGGTCG-HEG)2-glycerol-HEG-5′-TCGGTCG | 8032 | 8033 |
| C20 | (5′-TCGTTCG-HEG)2-glycerol-HEG-5′-TCGTTCG | 7957 | 7958 |
| C21 | (5′-TCGACGT-HEG)3-trebler-HEG-5′-TCGACGT | 10 832 | 10 834 |
| C22 | (5′-TCGACGT-HEG)186-Ficoll400 | n/a | n/a |
| C23 | (5′-TCGTCGA-HEG)2-glycerol-HEG-5′-AACGTTC | 7968 | 7970 |
| C24 | (5′-TCGACGT-HEG)2-glycerol-HEG-5′-AACGTTC | 7968 | 7969 |
| C25 | (5′-TAGTCAT-HEG)2-glycerol-HEG-5′-AACCTTC (control) | 7951 | 7953 |
Figure 1.
Structures of the non-nucleoside spacers after incorporation into CICs. Non-nucleoside spacers are: 1, hexaethylene glycol (HEG) spacer; 2, glycerol spacer; 3, triethylene glycol (TEG) spacer; 4, 1,3-propanediol (C3) spacer; and 5, trebler spacer.
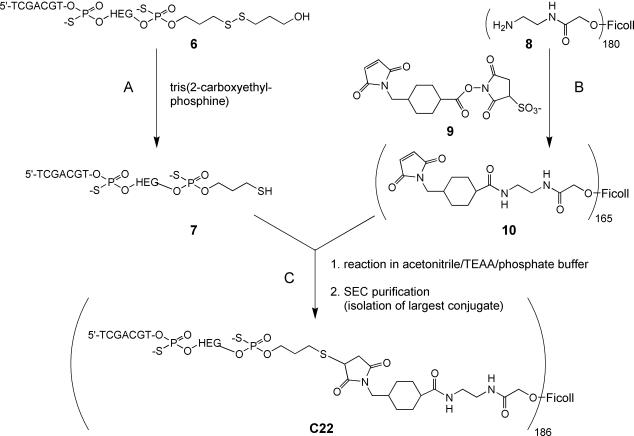
Preparation of (5′-TCGACGT-HEG)186-Ficoll400 (C22)
The scheme for the synthesis of C22 is shown in Figure 2. Phosphorothioate-linked 5′-TCGACGT-HEG-(CH2)3SS (CH2)3OH (6) was synthesized using the 3′-thiol-modifier, C3 S-S CPG (Glen Research), as the solid support and purified as described above. The disulfide, 6, was reduced to the thiol (7) with tris(2-carboxyethylphosphine) hydrochloride as previously described (24), purified by RP-HPLC on a PLRP-S column (4.6 × 250 mm, 5 µm, Polymer Labs) using an increasing gradient of acetonitrile in triethylammonium acetate buffer, and used immediately in the reaction with 10.
Figure 2.
Scheme for the synthesis of (5′-TCGACGT-HEG)186-Ficoll400 (C22). Compounds are: 6, ODN-3′-disulfide; 7, ODN-3′-thiol; 8, (aminoethylcarboxymethyl)180-Ficoll400; 9, sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate; 10, (maleimido)165-Ficoll400. Abbreviations are: TEAA, triethylammonium acetate; SEC, size exclusion chromatography.
Aminoethylcarboxymethyl (AECM)180-Ficoll400 (8) was prepared by the method of Inman (25). On average there were 180 mol of aminoethyl groups per mole of Ficoll (MW = 400 000 Da), determined by an amine assay using Fluoraldehyde™ o-phthalaldehyde Reagent Solution (Pierce Endogen) and following manufacturer’s protocols. (Maleimido)165-Ficoll400 (10) was prepared by reaction of sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (9) with AECM180-Ficoll400 (8) as previously described (26). On average, there were ∼165 mol of maleimide groups per mole of Ficoll, as determined by a maleimide assay (27).
To the (maleimido)165-Ficoll400 (5.5 mg, 0.014 µmol, 10) dissolved in 0.1 M sodium phosphate/pH 6.66 (0.7 ml) was added 7 (6.8 mg, 2.5 µmol) dissolved in ∼30% acetonitrile/triethylammonium acetate buffer/pH 7.0 (3.45 ml). The mixture was put on the shaker at room temperature overnight and the product was purified on a Superdex 200 HR 10/30 column (Amersham Pharmacia) in 10 mM sodium phosphate/150 mM sodium chloride/pH 7.2 buffer. The product eluting at the void volume of the column, which corresponded to the Ficoll conjugate with the highest loading of ODN, was isolated. Calculations using the total weight of the ODN-Ficoll product (C22) and the absorbance value at 260 nm to determine the ODN content showed the product contained, on average, ∼186 mol of ODN per mole of Ficoll.
Human peripheral blood mononuclear cell (PBMC) preparation/cultures
Human PBMCs were isolated as described previously (17) and cultured in RPMI-1640 (Bio-Whitaker) supplemented with 10% heat-inactivated human AB serum (Gemini) plus 50 U/ml penicillin, 50 µg/ml streptomycin, 300 µg/ml glutamine, 1 mM sodium pyruvate and 1× non-essential amino acids (all supplements from Bio-Whitaker). PBMCs were cultured at 0.5 × 106/well (2 × 106/ml) in 96-well flat bottom plates in duplicate with ODNs/CICs at a concentration range of 0.1–20 µg/ml for 20–24 h. Dosing for the CICs (in µg/ml) was calculated using A260 values using a conversion of 39 µg/OD260 and was, therefore, based on the total amount of ODN present in the CIC, disregarding the weight contributed by the spacers. Consequently, all CICs were tested under conditions where an equivalent amount of heptameric ISS was offered. Cell-free supernatants were harvested and cytokine content assayed by ELISA.
Mouse splenocyte preparation/cultures
Spleens from 8–20 week-old BALB/c mice were harvested and the splenocytes isolated using standard teasing and treatment with ACK lysing buffer to remove erythrocytes (Bio-Whittaker). Isolated cells were washed in washing media [RPMI-1640 supplemented with 2% heat-inactivated fetal calf serum (FCS), 50 µM 2-mercaptoethanol, 1% penicillin-streptomycin and 2 mM L-glutamine] and resuspended at 7 × 106 cells/ml in mouse culture media (RPMI-1640 media with 10% heat-inactivated FCS, 50 µM 2-mercaptoethanol, 1% penicillin-streptomycin and 2 mM L-glutamine). Cell cultures were set up in triplicate in 96-well flat-bottom plates in a volume of 100 µl/well and the cells were allowed to rest for at least 1 h after plating. The indicated ODNs and CICs were then added in another 100 µl volume so that the final concentration was 5, 1 or 0.1 µg/ml and the cells were cultured for 24 h. Cell-free supernatants were harvested and assayed for cytokine content by ELISA.
ELISA
Cell-free medium was assayed for cytokine content via commercial kits. hIFN-γ and hIL-6 were assayed with CytoSet antibody pairs (BioSource) and the limits of maximal/minimal detection were 4000/2 pg/ml. hIFN-α was assayed with an ELISA kit (PBL Biomedical Laboratories) and the limit of maximal/minimal detection was 13 000/52 pg/ml. Murine IFN-γ, IL-6 and IL-12p40/p70 were assayed by matched antibody pairs (BD/PharMingen). Lower limits of detection were 54, 36 and 36 pg/ml, respectively. All kits and antibody pairs were used according to manufacturers’ instructions.
B cell purification and functional assays
Human PBMCs were incubated with CD19 MACS beads (Miltenyi Biotec) and passed through a magnet, separating the CD19+ B cells through positive selection (>98% CD19+ as determined by FACS). B cells were cultured at 1 × 105/well (5 × 105/ml) in 96-well round-bottomed plates and incubated in triplicate with 20, 5 or 1 µg/ml ODN/CIC for 96 h. At the end of the culture period, the plates were pulsed with 3H-thymidine (1 µCi/well, Amersham) and incubated for an additional 8 h. Then the plates were harvested and radioactive incorporation determined using standard liquid scintillation techniques, with the data collected in counts per minute (c.p.m.). For IL-6 secretion, supernatants from the assay were harvested and assayed for IL-6 by ELISA.
In vivo treatment of mice with ODNs/CICs
Female BALB/c mice (6–8 weeks) were anesthetized with isofluorine and treated intranasally with 20 µg ODN or CIC in 50 µl pyrogenic-free saline, or with saline only (five mice per treatment group). Six hours after treatment, lungs were harvested, snap-frozen in liquid nitrogen and stored at –80°C.
Gene expression assay and analysis
The protocol for analysis of gene expression in human cells has been detailed elsewhere (17). Briefly, PBMCs were cultured with ODNs or CICs for 10 h and processed for RT–PCR analysis using the GeneAmp 5700 Sequence Detector (PE Biosystems). In other experiments, snap-frozen mouse lungs were homogenized in RLT-buffer using a polytron homogenizer. cDNA derived from mouse lung was diluted 1:100, and PCR was conducted also using the GeneAmp 5700 Sequence Detector. The sequences for synthesized primers for human genes have been previously listed (17). The sequences for synthesized primers for mouse genes are as follows (listed 5′ to 3′): ubiquitin (F: TGG CTATTAATTATTCGGTCTGCAT, R: GCAAGTGGCTA GAGTGCAGAGTAA); IFN-γ (F: CAACAGCAAGGCG AAAAAGG, R: GGACCACTCGGATGAGCTCA); IL-6 (F: ACACATGTTCTCTGGGAAATCGT, R: AAGTGCAT CATCGTTGTTCATACA); IL-12p40 (F: ACAGCACCA GCTTCTTCATCAG, R: TCTTCAAAGGCTTCATCTG CAA); MCP-1 (F: GCTGGAGCATCCACGTGTT, R: ATC TTGCTGGTGAATGAGTAGCA); MCP-3 (F: GGGAAG CTGTTATCTTCAAGACAAA, R: CTCCTCGACCCAC TTCTGATG); TLR9 (F: AGGCTGTCAATGGCTCTCA GTT, R: TGAACGATTTCCAGTGGTACAAGT). Thresh old cycle (CT) values for each gene were normalized to ubiquitin using the formula 1.8(UBQ–GENE)(100 000), where UBQ is the mean CT of triplicate ubiquitin runs, GENE is the mean CT of duplicate runs of the gene of interest, and 100 000 is arbitrarily chosen as a factor to bring all values above 0. Data are expressed as gene/ubiquitin ratio or as fold induction over control. For calculation of the fold induction the negative control for each experiment is assigned a value of 1.
Statistical analysis
Statistical significance for in vitro experiments was calculated using a repeated measures ANOVA with parametric methods, followed by a Dunnett Multiple Comparisons post-test to compute P-values (GraphPad InStat). Data derived from the in vivo mouse experiment were analyzed using a Kruskall Wallis non-parametric ANOVA (GraphPad InStat). Symbols representing significance are: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
RESULTS
Design and preparation of CICs
The use of short ISS ODNs for structure–function studies greatly simplifies the identification of optimal motifs for various ISS activities by distilling the sequence down to the minimal requirements for each activity. However, the cellular uptake of short ODNs is poor relative to longer ODNs (22). Previously, we overcame this limitation by binding short ISS to cationic microparticles; however, this approach does not permit more detailed structural analyses. Herein, we employed a strategy that allowed for the generation of a series of precisely defined immunostimulatory molecules in which these minimal ISS were assembled in various linear and branched configurations with non-nucleoside spacers (Fig. 1). The sequences and structures for the CICs, which are designated by a code number, C, are shown in Table 1. Heptameric sequences containing a 5′-TCG, which had previously been determined to be optimal in their microparticle-complexed form (22), as well as heptamers with a TCG motif located in various positions throughout the sequence were used in order to determine if similar rules for activity apply for CICs as were observed for microparticle-formulated heptameric ISS (C01–C05). The identity of the bases following the 5′-TCG and the inclusion of a second CG dinucleotide were also tested for their effects on activity (C06–C08, C10–C13, C17–C20). CICs containing both human and mouse ISS motifs were prepared to study relative ISS activity in both species and to verify the in vitro results for the novel CICs in an in vivo murine assay (C23–C24). In addition, chimeric compounds were prepared which contained ODNs with no CG dinucleotides in order to demonstrate the specificity of sequence recognition (C09, C25).
CICs were prepared with either linear (C01–C09) or branched structures (C10–C25), with all connections between nucleosides and non-nucleoside spacers consisting of phosphorothioate linkages (Table 1). The non-nucleoside spacer molecules chosen for this study contained one or more 4,4′-dimethoxytrityl (DMT) protecting groups and a β-cyanoethylphosphoramidite reactive group, which allowed for the preparation of the CICs using an automated DNA synthesizer. Linear CICs were synthesized using standard DNA synthesis protocols and non-nucleoside spacers with a single DMT group. Branched CICs were prepared using a non-nucleoside spacer that had two or three DMT-protected reactive sites, thereby allowing the incorporation of two or three identical chains simultaneously. These branched CICs required a two-part synthesis consisting of (i) synthesis of the linear portion of the molecule up to and including the branching spacer from the 3′-end using standard DNA synthesis protocols, followed by (ii) synthesis of two or three chains simultaneously using a protocol that added 2× vol of all reagents.
Figure 1 shows the structure of the non-nucleoside spacers used in this study after incorporation into the CICs. Linear CICs were initially prepared with long, hydrophilic spacers based on hexaethylene glycol (HEG, 1) with the aim of completely isolating the heptamers for identification of the ISS motifs. In order to assess the importance of multiple 5′-ends and the spacing of the individual ISS on activity, a series of branched CICs based on glycerol (2) and containing three copies of the sequence TCGTCGA were prepared with additional HEG (1), triethylene glycol (TEG, 3), or propanediol (C3, 4) spacers or no addition spacers (C11, C14–C16). Additionally, a branched HEG-containing CIC based on the trebler (5) with three free 5′-TCGACGT sequences was synthesized (C21) for comparison with a similar CIC with two free 5′-TCGACGT sequences (C12). All CICs had the expected molecular weight, determined by electrospray mass spectrometry, thereby confirming base composition and incorporation of the spacer molecules (Table 1).
To explore further the range of differences possible with multimeric display of active TCG-containing motifs, a compound containing a large number of 7mer ISS covalently attached to a soluble scaffold was synthesized using a conjugation strategy (Fig. 2). We chose Ficoll with an average molecular weight of 400 000 Da (Ficoll400) as the multimeric scaffold because it is non-toxic and exhibits good water solubility. AECM180-Ficoll400 (8) was reacted with sulfosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (9) to generate (maleimido)165-Ficoll400 (10). Separately, the heptameric disulfide, 6, was reduced to the thiol (7) using tris(2-carboxyethylphosphine). Following purification, 7 was added directly to a solution of maleimide165-Ficoll400 (10). Purification by SEC and selection of the highest loaded fraction, which eluted at the void volume of the column, resulted in a conjugate with an average loading of 186 mol of heptameric ISS per mole of Ficoll400 (C22).
IFN-α induction is affected by ISS sequence, spacer composition and structure of CICs
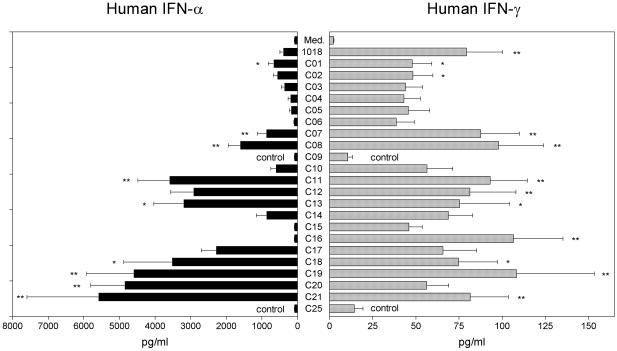
We have previously established that 1018 ISS can stimulate the secretion of IFN-α and IFN-γ from human PBMC in a CpG-dependent manner (17) and have therefore used it as a reference in all assays. Using this assay, we evaluated over 200 linear and branched CICs and determined that the CICs containing heptameric ODNs with a TCG in the 5′ position generally induced the highest amounts of IFN-α (compare C01–C05, Fig. 3). However, not all CICs with sequences containing a 5′-TCG induced IFN-α, demonstrating that the bases following the 5′-TCG were also important (C06). CICs containing a 5′-TCG and a second CG dinucleotide within the heptameric sequence were also evaluated and led to improved IFN-α secretion (compare C06 with C07, C01 with C08, and C10 with C11 and C13). Sequences that did not follow these rules induced significantly less IFN-α, even if they contained multiple CG dinucleotides as in C05 (5′-ACGTCGA, Fig. 3) and in CICs with the sequences 5′-CCGTCGA and 5′-GCGTCGA (data not shown). Interestingly, there were only small and non-significant differences among these compounds in their ability to stimulate IFN-γ production and no evidence for a positive correlation between the levels of IFN-α and IFN-γ induced by these sequences. The CpG specificity of the assay was confirmed by the induction of only background levels of IFN-α and IFN-γ by the chimeric non-CpG control ODNs, C09 and C25.
Figure 3.
Effect of ISS sequence, spacer composition, and structure of CICs on IFN-α and IFN-γ production. PBMCs were isolated from eight donors and stimulated with 20 µg/ml 1018 ISS or CIC for 24 h. Cell-free supernatants were assayed for IFN-γ and IFN-α content by ELISA. Data are shown as means ± SEM. Statistical significance: **, P < 0.01; *, P < 0.05, where 1018 ISS and the linear CICs (C01-C08) were compared to the linear chimeric control ODN, C09, and branched CICs (C10-C21) were compared to the branched chimeric control ODN, C25.
Given the importance of the 5′-TCG for CIC activity, we investigated the activity of branched CICs that contain more than one free 5′-TCG sequence. Over 60 branched CICs containing the most active heptamers derived from the linear CIC study and a variety of spacers were synthesized and evaluated. Figure 3 shows the IFN-α and IFN-γ response from human PBMC to a series of branched CICs containing the same 7mer sequence, but connected to the glycerol branching spacer in different ways (C11, C14–C16). The branched CIC containing HEG spacers between the glycerol branching spacer and the ISS (C11) induced significantly more IFN-α (46-fold) than the branched CICs with either C3 spacers (C15) or no spacers (C16). Interestingly, the CIC containing intermediate length spacers based on TEG (C14) also induced an intermediate amount of IFN-α, further confirming the trend that increased spacing of the individual ISS sequences in the branched CICs leads to higher IFN-α production. However, the incorporation of two HEG spacers between each ISS and the glycerol branching spacer did not improve the IFN-α production further above that observed for C11 (data not shown). The IFN-γ levels exhibited less variation for branched CICs with different spacer composition, or without spacers entirely, with C11 and C16 inducing the highest amounts. Dose titration showed that induction of IFN-γ by all CICs was optimal at 20 µg/ml, with lower, and often only background levels induced at doses of 4 and 0.8 µg/ml (data not shown).
Branched HEG CICs containing two free 5′-ends generated ∼2–4-fold more IFN-α than their linear HEG counterparts (compare C11 with C07 and C12 with C08, Fig. 3). Different heptameric ISS were incorporated into this branched HEG CIC structure and were also found to stimulate high levels of IFN-α from human PBMC (C13, C17–C20). Because of the increase in IFN-α induction by branched HEG CICs with two free 5′-ends compared to linear HEG CICs, we studied whether a HEG CIC with three free 5′-ends would further increase IFN-α synthesis. The branched HEG CIC containing three free 5′-ends, C21, induced moderately higher levels of IFN-α than the corresponding branched HEG CIC containing two free 5′-ends (C12) (Fig. 3). Dose titration of several key CICs in an additional four donors confirmed the general findings that (i) heptameric sequences containing a 5′-TCG plus a second CG are more active than those containing only a 5′-TCG (C01 versus C08), (ii) branched CICs with two free 5′-ends are more active than comparable CICs with only one free 5′-end (C12 versus C08) and (iii) branched CICs containing HEG spacers are more active than those containing shorter spacers (C11 versus C14 and C15, Fig. 4). Notably, the dose titration demonstrates that the 20 µg/ml dose is optimal for differentiation of the IFN-α-inducing activity of simple linear and branched CICs. Additionally, while the magnitude of the IFN-α response is variable from donor to donor, we find that all donors respond to the most active sequences.
Figure 4.
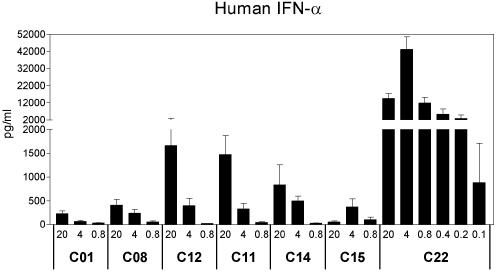
Multimeric presentation of large numbers of free 5′-TCGACGT sequences connected to Ficoll by HEG spacers increases IFN-α induction. PBMCs were isolated from four donors and stimulated with 0.1–20 µg/ml CIC for 24 h. Cell-free supernatants were assayed for IFN-α content by ELISA. Data are shown as means ± SEM.
In order to further study the relationship between IFN-α production and the number of free 5′-TCG-containing motifs in a CIC, a Ficoll-based CIC containing an average of 186 free 5′-TCGACGT sequences linked through HEG spacers (C22) was tested in the human PBMC assay. Dose titration of C22 from 0.1 to 20 µg/ml showed a complex dose–response relationship for IFN-α. Optimum stimulation of IFN-α occurred at ∼2–4 µg/ml, producing a bell-shaped curve over the concentration range tested (Fig. 4). C22 induced ∼26-fold more IFN-α than C12 at one-fifth the dose and is the most active CIC tested to date for IFN-α production. As the CIC concentrations were calculated on the basis of only the ODN content, equivalent concentrations (in µg/ml) of both C22 and C12 contain the same number of heptameric ISS. Thus, highly multimeric presentation of active heptameric ISS with free 5′-ends linked through HEG spacers to a soluble core molecule can significantly increase the amount of IFN-α generated in human PBMC.
CICs are capable of inducing high levels of IFN-inducible genes
We have previously observed that ISS induces a specific pattern of elevated gene expression in human PBMCs (17). This pattern includes IFN-α, IFN-γ and genes inducible by IFN, including the antiviral genes 2,5-oligoadenylate synthetase (2,5-OAS) and interferon-stimulating gene-54K (ISG-54K), and the pro-inflammatory chemokines interferon-inducible protein-10 (IP-10), monokine induced by IFN-γ (MIG) and monocyte chemoattractant protein-2 (MCP-2). As expected, 1018 ISS induced substantial increases in the expression levels of all these genes compared to the negative controls, C09 and C25 (Table 2). Those CICs that had been shown to be more effective inducers of secreted IFN-α than 1018 ISS in Figures 3 and 4 also demonstrated superior ability in the enhancement of IFN-α RNA, inducing ∼6–25-fold higher levels of this gene product. The Ficoll-based CIC, C22, was extremely active, promoting 84-fold higher levels of IFN-α RNA than 1018 ISS, consistent with its vigorous induction of secreted IFN-α. In general, expression of other genes in this panel was similar or somewhat higher compared to 1018 ISS. Additionally, a broad panel of other genes encoding cytokines, chemokines and antiviral products was also checked for expression and no significant differences were found between CICs and 1018 ISS (data not shown). These results indicate that optimally active CICs induce qualitatively similar signals for the transcription of IFN-related genes and qualitatively enhanced signals for the upregulation of the IFN-α gene compared to standard ISS.
Table 2. Profile of gene expression modulated by CICs and ISSa.
| Stimulus | IFN-α | 2,5-OAS | ISG-54K | IFN-γ | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Medium | 1.0 | 0.0 | 1.0 | 0.0 | 1.0 | 0.0 | 1.0 | 0.0 |
| 1018 | 54.0 | 22.9 | 10.6 | 4.0 | 13.3 | 6.8 | 12.1 | 7.0 |
| C07 | 346.8 | 206.8 | 14.6 | 3.6 | 20.1 | 8.4 | 17.3 | 9.0 |
| C11 | 677.9 | 315.2 | 19.4 | 5.1 | 23.0 | 7.6 | 19.9 | 8.0 |
| C08 | 789.8 | 438.4 | 17.6 | 3.7 | 17.5 | 6.4 | 18.5 | 8.1 |
| C12 | 1091.0 | 741.1 | 17.6 | 3.1 | 21.4 | 7.1 | 16.6 | 8.1 |
| C21 | 1389.7 | 884.0 | 19.4 | 4.7 | 24.4 | 9.0 | 22.5 | 9.0 |
| C22 | 4545.8 | 1701.4 | 26.3 | 4.9 | 39.9 | 13.7 | 13.2 | 6.2 |
| C09 | 2.3 | 0.9 | 0.9 | 0.1 | 0.9 | 0.0 | 2.6 | 0.6 |
| C25 |
1.8 |
0.8 |
1.0 |
0.3 |
1.2 |
0.3 |
3.2 |
0.9 |
| Stimulus | IP-10 | MIG | MCP-2 | |||||
| |
Mean |
SEM |
Mean |
SEM |
Mean |
SEM |
|
|
| Medium | 1.0 | 0.0 | 1.0 | 0.0 | 1.0 | 0.0 | ||
| 1018 | 73.7 | 30.2 | 165.2 | 149.1 | 24.8 | 19.0 | ||
| C07 | 127.7 | 59.0 | 198.3 | 177.4 | 29.3 | 12.8 | ||
| C11 | 216.9 | 89.2 | 190.4 | 165.5 | 76.0 | 40.3 | ||
| C08 | 168.9 | 78.3 | 253.8 | 235.3 | 55.3 | 32.3 | ||
| C12 | 178.6 | 82.7 | 131.8 | 115.4 | 60.1 | 30.6 | ||
| C21 | 200.7 | 88.9 | 242.4 | 223.2 | 50.4 | 29.9 | ||
| C22 | 243.8 | 105.0 | 43.2 | 24.6 | 123.0 | 49.3 | ||
| C09 | 1.9 | 0.6 | 9.7 | 7.4 | 1.1 | 0.9 | ||
| C25 | 1.9 | 0.4 | 1.8 | 0.6 | 1.2 | 0.5 | ||
aPBMCs from four donors were cultured for 10 h with 5 µg/ml ISS or CICs and RNA was extracted and analyzed via TaqMan RT–PCR. Gene expression was normalized to ubiquitin expression. Data are presented as the mean of fold induction over medium control (given the value of 1.0) with SEM.
Abbreviations: IFN-α, interferon-α; 2,5-OAS, 2,5-oligoadenylate synthetase; ISG-54K, interferon-stimulating gene-54K; IFN-γ, interferon-γ; IP-10, interferon-inducible protein-10; MIG, monokine induced by IFN-γ; MCP-2, monocyte chemoattractant protein-2.
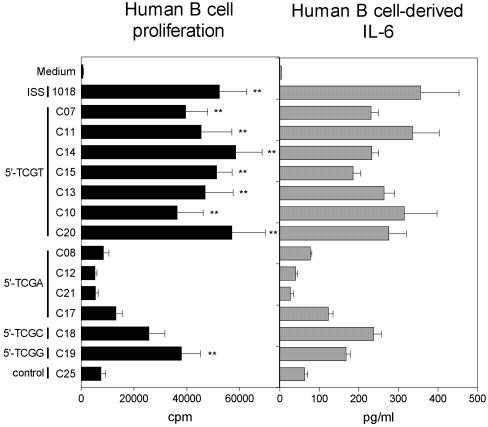
Identification of sequence requirements for activation of human B cells using CICs
Many ISS, including 1018 ISS, are known to directly activate B cells, causing them to proliferate and secrete IL-6 (28–30). Therefore, we tested a variety of CICs on purified peripheral blood B cells in order to study the motif, spacer and structural requirements for B cell proliferation and induction of IL-6. Dose titration was performed in order to determine the optimal concentration for the assay, which was determined to be 5 µg/ml (data not shown). CICs containing heptameric motifs with a 5′-TCGT induced the highest levels of proliferation, while, surprisingly, many CICs containing 5′-TCGA sequences stimulated only low levels or background levels of proliferation (Fig. 5). Comparing CICs containing identical branched CIC structures, spacers and sequences, with the exception of the nucleotide following the 5′-TCG in each motif, confirmed that TCGT sequences were significantly more active than TCGA sequences, with TCGC and TCGG sequences having intermediate activity (compare C13 with C12 and C17–C20). The bases following the 5′-TCGX also had some influence on B cell activity (Fig. 5). We found that C01, containing the ISS motif TCGATTT, induced moderate levels of B cell proliferation, which were intermediate between the low to background levels found for other TCGA-containing CICs and the levels observed for C06, containing the sequence TCGTTTT, and other TCGT- containing CICs (Fig. 5 and data not shown). No significant differences in proliferation were observed for linear versus branched CICs (compare C07 with C11 and C08 with C12) or for CICs containing different types of spacers when compared at the optimal dose (compare C11, C14 and C15), although the CICs containing the TEG (C14) and C3 spacers (C15) maintained somewhat higher activity at suboptimal doses (data not shown). The CIC-induced generation of IL-6 from B cells generally confirmed the observations found from the proliferation data. These results demonstrate that B cell activity is mainly a function of sequence and that multimeric presentation plays little role in the overall activity. They additionally show that CICs can be designed to exhibit different levels of B cell activity, from insignificant up to levels equivalent to 1018 ISS, independently of the amount of IFN-α induced by that CIC.
Figure 5.
The ISS motif in CICs primarily determines the level of human B cell activity. Purified human B cells from four donors for proliferation and two donors for IL-6 production were stimulated with 5 µg/ml 1018 ISS or CIC for 96 h. Proliferation was assessed by 3H-thymidine incorporation and IL-6 was assayed by ELISA. Data are shown as means ± SEM. Statistical significance for proliferation data: **, P < 0.01, where 1018 ISS and the CICs were compared to the branched chimeric control ODN, C25.
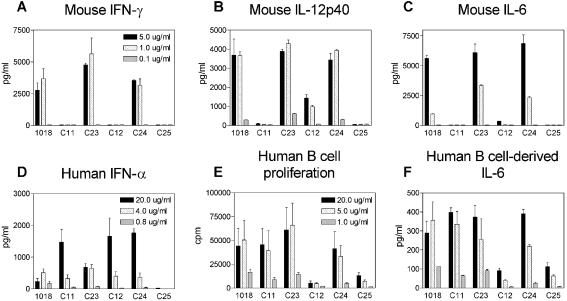
Mouse and human motifs can be independently varied in different segments of a CIC structure
While it is important to optimize ISS and CICs for activity in humans, having corresponding activity in murine systems makes correlation of in vitro and in vivo activity much more accessible and convenient, greatly simplifying drug development. However, studies using CICs containing only human motifs, such as C11 and C12, showed that they had little cytokine-inducing activity in mouse splenocytes (Fig. 6A–C). This was resolved by the replacement of one of the three human ISS in the CIC with a heptameric sequence containing a mouse motif. C23 and C24, each containing a single murine ISS at the 3′-end, AACGTTC, and two human ISS at the 5′-ends of the branched HEG CIC, were able to produce similar or greater levels of IFN-γ, IL-12p40 and IL-6 from mouse splenocytes compared to the positive control 1018 ISS. The consequences of adding the mouse motif to the branched CICs were more complex in terms of the human assays. While the addition of the mouse motif did not affect the amount of IFN-γ secreted in human PBMC for either compound (data not shown), it changed the IFN-α production unpredictably (compare C23 with C11 and C24 with C12, Fig. 6D). Additionally, C23 and C24 were stimulatory for human B cells (Fig. 6E–F). The activity of C24 clearly demonstrated that the sequence AACGTTC is active on human B cells. In summation, C23 and C24 stimulated high levels of IFN-α production and B cell activity in human cells and also maintained ISS in vitro activity in mice, demonstrating that human and mouse ISS motifs can be inserted independently in a CIC in order to obtain activity in both species.
Figure 6.
Mouse and human motifs can be independently varied in different segments of a CIC structure. (A–C) Mouse splenocytes were stimulated with 5 (black bars), 1 (hatched bars) or 0.1 µg/ml (gray bars) 1018 ISS or CIC for 24 h, then cell-free supernatants were assayed for IFN-γ, IL-12p40 and IL-6 content by ELISA. Data are shown as means ± SEM and are representative of two assays. (D) Human PMBC (four donors) and (E and F) B cell (two donors) assays were performed as described in Figures 3 and 5 using ISS/CIC doses of 20 (black bars), 4 (hatched bars) and 0.8 µg/ml (gray bars) for the PMBC assay and 20 (black bars), 5 (hatched bars) and 1 µg/ml (gray bars) for the B cell assay.
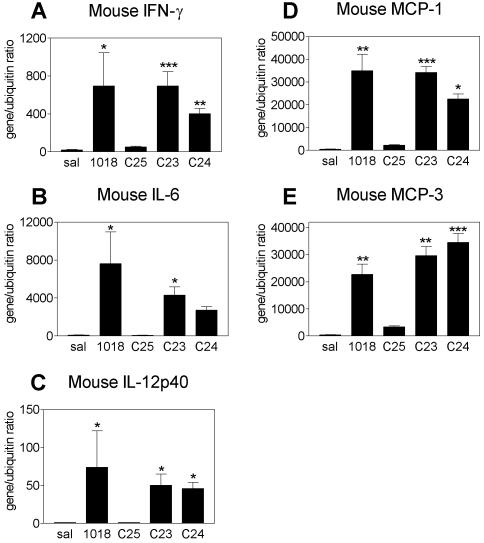
CICs are active in vivo
In order to confirm the in vitro activities observed for CICs correlate with in vivo effects, BALB/c mice were given intranasal treatments of 1018 ISS or CICs. In anesthetized mice, the major portion of intranasally administered solutions is dispersed to the lung (31,32). Treatment with 1018 ISS resulted in high induction of the mRNA expression of IFN-γ, IL-6, IL-12p40 and the chemokines MCP-1 and MCP-3 in mouse lung tissue (Fig. 7). The induction of these genes was at least 20-fold over treatment with saline control. It is noteworthy that the induction of these genes in the lung occurred as early as 3 h after treatment and peaked at 6 h (data not shown), indicative of a rapid response. Intranasal treatment with the negative chimeric control, C25, resulted in poor induction of the mRNA expression of all genes tested, as expected. In sharp contrast, treatment with C23 or C24 upregulated mRNA expression of IFN-γ, IL-6, IL-12p40, MCP-1 and MCP-3, in most cases to a similar degree as 1018 ISS. In conclusion, these findings confirm that 1018 ISS, C23, and C24 demonstrate good ISS activity in vivo in mice.
Figure 7.
Upregulation of ISS-inducible cytokine and chemokine mRNA expression in response to intranasal delivery of CICs and 1018 ISS in mice. Under light anesthesia, mice were intranasally treated with saline, 20 µg chimeric control ODN C25 or 20 µg 1018 ISS or CICs. Lungs were harvested 6 h after treatment (five mice/treatment group). cDNA was prepared and quantitative PCR analysis was performed for the genes shown in (A–E). All genes were normalized to the expression of the housekeeping gene ubiquitin and results are expressed as gene/ubiquitin ratio (mean ± SEM). Statistical significance: ***, P < 0.001; **, P < 0.01; *, P < 0.05 when sequences were compared to saline.
DISCUSSION
ISS have received considerable attention due to their potential for treatment of a wide range of disorders, including asthma, allergy, infectious diseases, and cancer (reviewed in 33). The optimal recognition motif for human cells includes a CG dinucleotide preceded by a T (16,34). We recently extended this finding by demonstrating that the minimal human immunostimulatory CpG motif for the potent induction of IFN-α and IFN-γ from human cells is 5′-TCGXX (22). In order to extend the motif and multimeric presentation studies with short ISS, we designed and tested a series of linear and branched CICs containing heptameric ISS connected with a variety of non-nucleoside spacers all linked through phosphorothioate groups. Similarly to microparticle-formulated short ISS, CICs with a heptameric sequence containing a 5′-TCG gave statistically significant production of IFN-α and IFN-γ from human PBMC relative to the non-CpG controls. Interestingly, while short ISS/microparticle complexes showed no preference for the bases following the 5′-TCG in either IFN-α or IFN-γ induction, CICs containing a 5′-TCG plus a second CpG within the heptameric sequence (5′-TCGXCGX, 5′-TCGXTCG) induced higher levels of IFN-α/IFN-γ than those with only a 5′-TCG (5′-TCGXXXX).
The nature of the non-nucleoside spacers and the overall structure of the CIC substantially influenced the induction of IFN-α, but not IFN-γ, in human PBMC cultures. For branched CICs built on a glycerol scaffold, increasing the length of the spacers between the ISS and the glycerol backbone correlated with augmented IFN-α production, with long, hydrophilic spacers based on HEG being the most potent spacers tested. Additionally, IFN-α production dramatically increased as more free 5′-TCG-containing heptameric ISS were added to the CIC. The Ficoll-based CIC containing 186 free 5′-TCGACGT sequences, C22, was the most active IFN-α-inducing CIC tested and exhibited a bell-shaped dose–response curve. This type of IFN-α dose–response curve was also observed for a new class of conventional ISS identified by our laboratory, called CpG-C, which are characterized by their ability to induce high levels of IFN-α from PBMC and also stimulate B cells (17). It is important to note that despite the enhanced IFN-α-inducing properties of certain branched CICs, they continue to activate the same pattern of gene expression as 1018 ISS, indicating that they are transmitting similar, but enhanced, signals as conventional ISS.
Our laboratory has generated increasing amounts of data supporting the view that multimeric presentation of ISS significantly increases IFN-α induction from PBMC. Presentation of two or more copies of an active ISS via a soluble (glycerol, trebler or Ficoll for CICs) or insoluble (microparticles for heptameric ISS) support results in compounds that strongly induce IFN-α (22). Additionally, members of a class of conventional ISS, called CpG-A, are also known to be high IFN-α inducers (16,35) and form large aggregates due to a combination of their self-complimentary regions and their poly-guanosine motifs, which facilitate the formation of high-order secondary structures (17,36). The specific function of multimeric presentation of active ISS motifs is not yet clear. It is quite possible that TLR9 cross-linking enhances or alters signal transduction, especially the pathways leading to IFN-α expression. Alternatively, the binding of ISS by TLR9 may be weak so that the increased avidity possible with multivalent molecules may be needed for optimum signaling.
Human B lymphocytes are TLR9-positive and directly responsive to ISS and CICs. Our data demonstrate that B cell activity is primarily dependent on the ISS sequence within the CIC, with 5′-TCGTXXX and 5′-AACGTTC exhibiting the most potent activity and 5′-TCGAXXX sequences being the least active. Contrary to findings regarding IFN-α induction, spacer composition and multimeric display of 5′-ISS via branched CICs exhibited less influence on B cell activation. These distinctly different patterns of ISS response suggest that a fundamental difference exists in the manner in which IFN-α-producing cells versus B cells take up and/or recognize ISS. Remarkably, CICs can be uniquely designed such that the B cell activity can vary from 10 to 100% of the activity of 1018 ISS, while maintaining high levels of IFN-α production, a feature that could be highly useful for the treatment of certain diseases that might benefit from an IFN-α-mediated response in the absence of polyclonal B cell stimulation (e.g. asthma).
It is of primary importance to screen and optimize ISS and CICs for activity specifically in human cells because of the differences in sequence motif requirements and TLR9+ cell types in human versus mouse (37). Nevertheless, it can be convenient to introduce mouse activity into an optimized human ISS or CIC in order to simplify in vivo evaluation in non-human systems. Thus, we prepared a series of CICs containing the optimal sequences, spacers and structures for IFN-α/IFN-γ induction from human cells, but replaced one of the human ISS sequences with a mouse-active sequence. These hybrid CICs maintained similar levels of IFN-α and IFN-γ induction as the fully human-active CICs, while also demonstrating good cytokine-inducing activity in mouse splenocytes. The in vitro activity was confirmed by the in vivo study in mice, which showed that CICs induce similar levels of ISS-responsive cytokines and chemokines in lung cells following an intranasal delivery.
Results from studies with longer ISS ODNs connected by spacers have been reported by Agrawal and colleagues (38). Those studies differed from our work by using ISS ODNs containing only mouse-specific motifs and assaying for activity with mouse, not human, cells. The optimal spacers in that system were found to be short, alkyl spacers based on C3 (4) or C4, rather than HEG (1) (38), which we found to be essential in CICs for high IFN-α induction from human cells. In more recent work, this group also demonstrated that short 5′-TCGTT-containing ISS of 5–7 bases in length linked with glycerol and additional C3 spacers induced cytokine production in mouse cultures and modest induction in a human PBMC sample, although IFN-α was not measured (39). In contrast, our studies have concentrated on the activities of branched CICs, with which we demonstrated that in human cells, only IFN-α secretion and IFN-α-related gene expression are increased by compounds with multiple 5′-ends, while other ISS-inducible activities, such as IFN-γ induction, B lymphocyte proliferation and IL-6 production, are unaffected by multimeric delivery. Additionally, another element that appears to be crucial for high IFN-α induction by branched CICs is that the ISS motifs are presented via a long, hydrophilic spacer, such as HEG, possibly because less constrictive spacing allows the heptamers to avoid sterically hindering each other from each associating with a receptor. These findings underscore the importance of sequences, spacers, and structures for deriving optimal ISS activity in the human system.
In conclusion, by using CICs composed of short heptameric CpG DNA sequences linked with non-nucleoside spacers, we have identified optimal motifs for ISS-mediated IFN-α induction from human PBMC, human B cell activation and mouse activity and have demonstrated that some of these activities can be independently modulated. Significantly, identification of the optimal motifs, spacers and structures for these different biological properties allows for the assembly of CICs with a defined set of activities that can be tailored for particular applications. Our major efforts have been directed toward identification of CICs with higher IFN-α-inducing activity, as opposed to high B cell stimulation, because there is little evidence thus far that the clinically important activities of ISS are mediated by polyclonal B cell activation. In fact, for certain indications, including the treatment of allergic asthma and certain cancers, it would be desirable to avoid polyclonal B cell activation, which might result in the potentiation of asthma-mediating B cells or B cell lymphomas. As such, CICs may offer a unique advantage over conventional ISS in clinical applications that require a restricted set of immune-enhancing activities.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Blanca Domingo-Yenes and Jennifer Lizcano for technical assistance, Franck Barrat, Holger Kanzler and Roberto Rodriguez for helpful discussion, Rowena Ng for development of the maleimide assay and Charlene Lee and Sherry Kelly for phlebotomy services.
REFERENCES
- 1.Yamamoto S., Yamamoto,T. and Tokunaga,T. (2000) Oligodeoxyribonucleotides with 5′-ACGT-3′ or 5′-TCGA-3′ sequence induce production of interferons. Curr. Topics Microbiol. Immunol., 247, 23–39. [DOI] [PubMed] [Google Scholar]
- 2.Santeliz J.V., Van Nest,G., Traquina,P., Larsen,E. and Wills-Karp,M. (2002) Amb a 1-linked CpG oligodeoxynucleotides reverse established airway hyperresponsiveness in a murine model of asthma. J. Allergy Clin. Immunol., 109, 455–462. [DOI] [PubMed] [Google Scholar]
- 3.Hafner M., Zawatzky,R., Hirtreiter,C., Buurman,W.A., Echtenacher,B., Hehlgans,T. and Mannel,D.N. (2001) Antimetastatic effect of CpG DNA mediated by type I IFN. Cancer Res., 61, 5523–5528. [PubMed] [Google Scholar]
- 4.Merad M., Sugie,T., Engleman,E.G. and Fong,L. (2002) In vivo manipulation of dendritic cells to induce therapeutic immunity. Blood, 99, 1676–1682. [DOI] [PubMed] [Google Scholar]
- 5.Verthelyi D., Kenney,R.T., Seder,R.A., Gam,A.A., Friedag,B. and Klinman,D.M. (2002) CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol., 168, 1659–1663. [DOI] [PubMed] [Google Scholar]
- 6.Dieudonne F., Vital Durand,D., Eiden,J., Tuck,S., Van Nest,G.E.R., Hamilton,R., Creticos,P.S. and Andre,C. (2001) ISS linked to Amb a 1 allergen (AIC) stimulates IgG response to Amb a 1 by ragweed-allergic humans. J. Allergy Clin. Immunol., 107, 933. [Google Scholar]
- 7.Halperin S.A., Van Nest,G., Smith,B., Abtahi,S., Whiley,H. and Eiden,J.J. (2003) A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimmulatory phosphorothioate oligonucleotide adjuvant. Vaccine, 21, 2461–2467. [DOI] [PubMed] [Google Scholar]
- 8.Hemmi H., Takeuchi,O., Kawai,T., Kaisho,T., Sato,S., Sanjo,H., Matsumoto,M., Hoshino,K., Wagner,H., Takeda,K. et al. (2000) A Toll-like receptor recognizes bacterial DNA. Nature, 408, 740–745. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Orozco E., Kobayashi,H., Van Uden,J., Nguyen,M.D., Kornbluth,R.S. and Raz,E. (1999) Enhancement of antigen-presenting cell surface molecules involved in cognate interactions by immunostimulatory DNA sequences. Int. Immunol., 11, 1111–1118. [DOI] [PubMed] [Google Scholar]
- 10.Chu R.S., Askew,D., Noss,E.H., Tobian,A., Krieg,A.M. and Harding,C.V. (1999) CpG oligodeoxynucleotides down-regulate macrophage class II MHC antigen processing. J. Immunol., 163, 1188–1194. [PubMed] [Google Scholar]
- 11.Gilliet M., Boonstra,A., Paturel,C., Antonenko,S., Xu,X.L., Trinchieri,G., O’Garra,A. and Liu,Y.J. (2002) The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med., 195, 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asselin-Paturel C., Boonstra,A., Dalod,M., Durand,I., Yessaad,N., Dezutter-Dambuyant,C., Vicari,A., O’Garra,A., Biron,C., Briere,F. et al. (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol., 19, 19. [DOI] [PubMed] [Google Scholar]
- 13.Krug A., Towarowski,A., Britsch,S., Rothenfusser,S., Hornung,V., Bals,R., Giese,T., Engelmann,H., Endres,S., Krieg,A.M. et al. (2001) Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol., 31, 3026–3037. [DOI] [PubMed] [Google Scholar]
- 14.Zarember K.A. and Godowski,P.J. (2002) Tissue expression of human Toll-like Receptors and differential regulation of Toll-Like Receptor mRNAs in leukocytes in response to microbes, their products and cytokines. J. Immunol., 168, 554–561. [DOI] [PubMed] [Google Scholar]
- 15.Bauer M., Redecke,V., Ellwart,J.W., Scherer,B., Kremer,J.P., Wagner,H. and Lipford,G.B. (2001) Bacterial CpG-DNA triggers activation and maturation of human CD11c(–), CD123(+) dendritic cells. J. Immunol., 166, 5000–5007. [DOI] [PubMed] [Google Scholar]
- 16.Verthelyi D., Ishii,K.J., Gursel,M., Takeshita,F. and Klinman,D.M. (2001) Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J. Immunol., 166, 2372–2377. [DOI] [PubMed] [Google Scholar]
- 17.Marshall J.D., Fearon,K., Abbate,C., Subramanian,S., Yee,P., Gregorio,J., Coffman,R.L. and Van Nest,G. (2003) Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leuko. Biol., 73, 781–792. [DOI] [PubMed] [Google Scholar]
- 18.Klinman D.M., Yi,A.K., Beaucage,S.L., Conover,J. and Krieg,A.M. (1996) CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12 and interferon gamma. Proc. Natl Acad. Sci. USA, 93, 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuramoto E., Yano,O., Kimura,Y., Baba,M., Makino,T., Yamamoto,S., Yamamoto,T., Kataoka,T. and Tokunaga,T. (1992) Oligonucleotide sequences required for natural killer cell activation. Jpn J. Cancer Res., 83, 1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato Y., Roman,M., Tighe,H., Lee,D., Corr,M., Nguyen,M.D., Silverman,G.J., Lotz,M., Carson,D.A. and Raz,E. (1996) Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science, 273, 352–354. [DOI] [PubMed] [Google Scholar]
- 21.Bauer S., Kirschning,C.J., Hacker,H., Redecke,V., Hausmann,S., Akira,S., Wagner,H. and Lipford,G.B. (2001) Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl Acad. Sci. USA, 24, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fearon K., Marshall,J.D., Abbate,C., Subramanian,S., Yee,P., Gregorio,J., Teshima,G., Ott,G., Tuck,S., Van Nest,G. et al. (2003) A minimal human immunostimulatory CpG motif that potently induces IFN-γ and IFN-α production. Eur. J. Immunol., 33, 2114–2122. [DOI] [PubMed] [Google Scholar]
- 23.Tang J.Y., Han,Y., Tang,J.X. and Zhang,Z. (2000) Large-scale synthesis of oligonucleotide phosphorothioates using 3-amino-1,2,4-dithiazole-5-thione as an efficient sulfur-transfer reagent. Org. Process Res. Dev., 4, 194–198. [Google Scholar]
- 24.Tighe H., Takabayashi,K., Schwartz,D., Van Nest,G., Tuck,S., Eiden,J.J., Kagey-Sobotka,A., Creticos,P.S., Lichtenstein,L.M., Spiegelberg,H.L. et al. (2000) Conjugation of immunostimulatory DNA to the short ragweed allergen Amb a 1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol., 106, 124–134. [DOI] [PubMed] [Google Scholar]
- 25.Inman J.K. (1975) Thymus-independent antigens: the preparation of covalent, hapten-ficoll conjugates. J. Immunol., 114, 704–709. [PubMed] [Google Scholar]
- 26.Lee A.C., Powell,J.E., Tregear,G.W., Niall,H.D. and Stevens,V.C. (1980) A method for preparing beta-hCG COOH peptide-carrier conjugates of predictable composition. Mol. Immunol., 17, 749–756. [DOI] [PubMed] [Google Scholar]
- 27.Singh R. (1994) A sensitive assay for maleimide groups. Bioconjug. Chem., 5, 348–351. [DOI] [PubMed] [Google Scholar]
- 28.Krieg A.M., Yi,A.K., Matson,S., Waldschmidt,T.J., Bishop,G.A., Teasdale,R., Koretzky,G.A. and Klinman,D.M. (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature, 374, 546–549. [DOI] [PubMed] [Google Scholar]
- 29.Jung J., Yi,A.K., Zhang,X., Choe,J., Li,L. and Choi,Y.S. (2002) Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J. Immunol., 169, 2368–2373. [DOI] [PubMed] [Google Scholar]
- 30.Bernasconi N.L., Onai,N. and Lanzavecchia,A. (2003) A role for Toll-like receptors in acquired immunity: upregulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood, 101, 4500–4504. [DOI] [PubMed] [Google Scholar]
- 31.Grunig G., Warnock,M., Wakil,A.E., Venkayya,R., Brombacher,F., Rennick,D.M., Sheppard,D., Mohrs,M., Donaldson,D.D., Locksley,R.M. et al. (1998) Requirement for IL-13 independently of IL-4 in experimental asthma. Science, 282, 2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst S.D., Muchamuel,T., Gorman,D.M., Gilbert,J.M., Clifford,T., Kwan,S., Menon,S., Seymour,B., Jackson,C., Kung,T.T. et al. (2002) New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol., 169, 443–453. [DOI] [PubMed] [Google Scholar]
- 33.Krieg A.M. (2002) CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol., 20, 709–760. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann G. and Krieg,A.M. (2000) Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol., 164, 944–953. [DOI] [PubMed] [Google Scholar]
- 35.Krug A., Rothenfusser,S., Hornung,V., Jahrsdorfer,B., Blackwell,S., Ballas,Z.K., Endres,S., Krieg,A.M. and Hartmann,G. (2001) Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol., 31, 2154–2163. [DOI] [PubMed] [Google Scholar]
- 36.Lee S.W., Song,M.K., Baek,K.H., Park,Y., Kim,J.K., Lee,C.H., Cheong,H.K., Cheong,C. and Sung,Y.C. (2000) Effects of a hexameric deoxyriboguanosine run conjugation into CpG oligodeoxynucleotides on their immunostimulatory potentials. J. Immunol., 165, 3631–3639. [DOI] [PubMed] [Google Scholar]
- 37.Chuang T.H., Lee,J., Kline,L., Mathison,J.C. and Ulevitch,R.J. (2002) Toll-like receptor 9 mediates CpG-DNA signaling. J. Leukoc. Biol., 71, 538–544. [PubMed] [Google Scholar]
- 38.Yu D., Kandimalla,E.R., Bhagat,L., Tang,J.Y., Cong,Y., Tang,J. and Agrawal,S. (2002) ‘Immunomers’-novel 3′-3′-linked CpG oligodeoxyribonucleotides as potent immunomodulatory agents. Nucleic Acids Res., 30, 4460–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhagat L., Zhu,F.G., Yu,D., Tang,J., Wang,H., Kandimalla,E.R., Zhang,R. and Agrawal,S. (2003) CpG penta- and hexadeoxyribonucleotides as potent immunomodulatory agents. Biochem. Biophys. Res. Commun., 300, 853–861. [DOI] [PubMed] [Google Scholar]