Abstract
The Hand proteins of the bHLH family of transcriptional factors play critical roles in vertebrate cardiogenesis. In Drosophila, the single orthologous Hand gene is expressed in the developing embryonic dorsal vessel (heart), lymph glands, circular visceral musculature, and a subset of CNS cells. We demonstrate that the absence of Hand activity causes semilethality during the early larval instars. The dorsal vessel and midgut musculature are unaffected in null mutant embryos, but in a large fraction the lymph glands are missing. However, homozygous adult flies lacking Hand possess morphologically abnormal dorsal vessels characterized by a disorganized myofibrillar structure, reduced systolic and diastolic diameter, abnormal heartbeat contractions, and suffer from premature lethality. In addition, their midguts are highly deformed; in the most severe cases, there is midgut blockage and a massive excess of ectopic peritrophic membrane tubules exiting a rupture in an anterior midgut bulge. Nevertheless, the visceral musculature appears to be relatively normal. Based on these phenotypes, we conclude that the expression of the Drosophila Hand gene in the dorsal vessel and circular visceral muscles is mainly required during pupal stages, when Hand participates in the proper hormone-dependent remodeling of the larval aorta into the adult heart and in the normal morphogenesis of the adult midgut endoderm during metamorphosis.
Keywords: Drosophila, bHLH factor, heart, midgut, metamorphosis
Introduction
The basic helix-loop-helix (bHLH) proteins are transcriptional regulators that play key roles in developmental processes such as myogenesis and neurogenesis (for reviews see Hjalt, 2004; Massari and Murre, 2000). One prominent class of bHLH proteins are the Hand proteins, which in vertebrates regulate cardiogenesis, branchial arch development, limb bud outgrowth, neurogenesis, and trophoblast development (for a review see Firulli, 2003). Higher vertebrates possess two paralogs of the Hand gene: Hand1 and Hand2, which are both expressed in the developing heart, lateral plate mesoderm, and a group of neural crest cells. In the developing heart, expression of both Hand genes is initially overlapping in the cardiac crescent. Subsequently, Hand2 is expressed throughout the linear heart tube while Hand1 is restricted to the region corresponding to the prospective left ventricle (Srivastava et al., 1995; Srivastava et al., 1997; Thomas et al., 1998). During cardiac looping, Hand2 then becomes restricted to regions of the heart that will give rise to the right ventricle. The importance of the Hand genes in cardiogenesis has been demonstrated by the phenotypes of knockout mutations in the mouse (Firulli et al., 1998; Riley et al., 1998; Srivastava et al., 1997). In particular, double-mutant mice for both Hand1 and Hand2 (homozygous for a cardiac-specific Hand1 null allele of and a Hand2 null allele) are early embryonic lethal and exhibit severe ventricular hypoplasia and other cardiac defects (McFadden et al., 2005).
Given the difficulties in interpretating Hand gene function due to genetic redundancy and the requirement for conditional alleles of Hand1 for studying cardiac development, another approach is to analyze conserved orthologs in genetic model organisms that possess a single Hand paralog. In zebrafish, a mutation in the single Hand gene, hands off, causes an almost complete absence of ventricular precursors, suggesting that Hand is critical for myocardial specification (Yelon et al., 2000). In Drosophila melanogaster, the analysis of the gene regulatory networks governing cardiogenesis in Drosophila melanogaster has proved very fruitful in elucidating the corresponding, evolutionarily conserved processes in vertebrates. The Drosophila Hand ortholog was discovered during a genome-wide survey for new bHLH genes (Moore et al., 2000) and its embryonic expression has been described (Kölsch and Paululat, 2002). It is expressed in the developing embryonic dorsal vessel (heart), lymph glands, circular visceral musculature, and a subset of CNS cells. Cardiac expression of Drosophila Hand initiates during mid-cardiogenesis within both myocardial and pericardial cells and is apparently directly regulated by the cardiogenic regulators Tinman (an NK-homeodomain protein), Pannier (a GATA factor), and Tailup (a LIM homeodomain factor)(Han and Olson, 2005; Tao et al., 2007). In order to define the function of Drosophila Hand in the development of the cardiac and visceral muscle tissues, as well as in blood-forming organs, we have generated a null mutation in this gene. In addition to the early larval lymph glands, the resulting mutant phenotype is primarily manifested in the dorsal vessel and gut of the adult stages and reveals an important role of this gene in the hormone-dependent remodeling of these organs during metamorphosis.
Materials and Methods
Drosophila strains and genetics
The EP insertion line GE10558 was from Genexel, Inc. (South Korea). The Df(2L)J1 Tft/CyO and w1118; Df(2L)Exel7046/CyO lines were from the Bloomington Stock Center. The w1118; HandKO/CyO knockout lines (alleles KO1-KO4) and Hand-GFP line were gifts from Zhe Han (UT Southwestern Med. Ctr., Dallas). Since complementation results for HandKO2 and HandKO3 alleles were similar, only those for HandKO2 are discussed. A non-lethal recombinant chromosome containing HandKO2 derived from the original chromosome was obtained by crossing w1118; HandKO2/CyO flies with yw flies and allowing the w; HandKO2/+ progeny to cross inter se for several generations. The HandKO2 allele was followed by the whs gene within the knocked-out Hand gene (Han et al., 2006) which results in eyes that are brick red (without an eyespot) in the original w1118; HandKO2/CyO line. Flies with a different eye color (bright red with an eyespot), which were possible homozygotes of a HandKO2 chromosome that had recombined away a linked lethal mutation, were individually outcrossed to yw flies. The progeny from one candidate fly were all brick red without an eyespot, confirming that the parent was homozygous for the HandKO2 knockout mutation. Ten progeny flies heterozygous for this recombined chromosome were individually balanced over CyO and these flies were then self-crossed to verify that the HandKO2 chromosome was homozygous viable.
Generation and characterization of the Hand173 null mutation
The EP insertion of GE10558 is 158 bp upstream of the Hand gene ORF. P-element-mediated imprecise excisions of this EP insertion were generated with P transposase derived from TM3, Sb, Δ2-3 (99B) and screened for lethality or semilethality over the deficiency Df(2L)J1 [31B1--31D11] which uncovers the Hand gene. Genomic DNA prepared from homozygous candidate deletion allele embryos was analyzed by PCR to determine the direction and extent of the deletion of genomic DNA flanking the EP insertion site. A 1.7 kb genomic DNA PCR product spanning the excision deletion of allele #173 was sequenced to identify its deletion endpoints.
Analysis of embryonic phenotypes
Immunohistochemistry and whole-mount in situ hybridization were performed as previously described (Knirr and Frasch, 2001; Lo and Frasch, 2001). Tyramide signal amplification (TSA) (PerkinElmer) was used when necessary with biotinylated secondary antibodies and VectaStain ABC components. Embryos were stained with the following primary antibodies: rabbit anti-Mef2 (1:750; gift from Hanh Nguyen, Albert Einstein College, New York, USA), guinea pig anti-Doc2+3 (1:200), rabbit anti-Tin (1:700), rat anti-Odd skipped (1:500, TSA), rabbit anti-β3-Tubulin (1:400, TSA; gift from Renate Renkawitz-Pohl, Philipps University Marburg, Germany), mouse monoclonal anti-Pericardin (1:25, TSA), rabbit anti-Serpent (1:2000, TSA; gift of Deborah Hoshizaki, University of Nevada, Las Vegas, USA), rabbit anti-β-galactosidase (1:1500; Promega), rabbit anti-GFP (1:7000), and mouse monoclonal anti-β-galactosidase 40-1a (1:60, TSA).
Analysis of adult dorsal vessel morphology
The isolated abdomen of an adult female (<2 days post-eclosion) was pinned onto a small Sylgard plate (on ice) through the posterior tip with its ventral side up under a 50 μl drop of PBT (PBS + 0.1% Tween; all solutions used were ice-cold). The ventral epithelium was removed and the gut, ovaries, and fat body were dissected out. The abdomen was fixed in 70 μl of 3.7% formaldehyde in PBS (ice-cold) for 20 min., washed 2X 5 min. with PBT, and blocked in 10% BSA in PBT (room temp.) for at least 30 min. For antibody staining of cardioblast nuclei, anti-Tin or anti-Mef2 primary antibody was used as described above. The abdomens were incubated overnight in primary antibody at 4° C overnight, washed 3X 30 min in PBT, incubated with Cy3-anti-rabbit secondary antibody for 2 hr at room temp., and washed 3X 30 min in PBT. To visualize the dorsal vessel myofibrils, the antibody-stained abdomens were washed 2X 5 min. with PBS, incubated with Alexa488-conjugated phalloidin (1:100 in PBS; Molecular Probes) for 20 min., washed 2X 5 min. with PBS, and mounted in SlowFade Light (Molecular Probes) anti-fade reagent.
Analysis of adult gut morphology
Adult females were decapitated and pinned onto a small Sylgard plate (on ice) through the neckhole and the posterior tip of the abdomen with its ventral side up under a 70 μl drop of PBT (all solutions used were ice-cold). A longitudinal incision was then made from the neckhole to the posterior end of the abdomen and the viscera from the proventriculus to the rectum was teased out and separated from the carcass. Visualization of the visceral musculature myofibrils was done as described above for the dorsal vessel.
Heartbeat Rate Measurements and Analysis of Cardiac Function
Adult flies were maintained at 25°C starting 1 hr before measurement, and individuals were anesthetized with FlyNap (Carolina Biological) for examination. Heartbeat rate was determined by visual inspection and counted twice for 30 sec each at 10 and 15 min after anesthesia with a Leica MZ6 binocular microscope. The two measurements were added to obtain the heartbeat rate in beats per minute (bpm) and then these were averaged for five different individuals to obtain the values shown for each genotype.
For the measurement of diastolic and systolic widths, the indicated genotypes of flies plus the Hand-GFP construct (on the 3rd chromosome) which expresses GFP in the heart (Han and Olson, 2005) were obtained through the appropriate crosses. Live dissected adults were maintained at 25°C in Schneider’s insect medium (Invitrogen Co.) and live video images of the beating hearts expressing GFP (emission wavelength at 488 nm) were recorded with a high-resolution video camera (CoolSNAP HQ Monochrome, Roper Scientific, Inc.) at a sampling frequency of 27 frames per second on a SteREO Lumar .V12 (Zeiss) light microscope with a 1.5X objective and a 100 or 150X magnification. Data acquisition and analyses were performed with the Metamorph/Metafluor software (Universal Imaging). Video images were used to determine cardiac parameters and derived by averaging a minimum of two cardiac cycles during normal regular rhythm. Cardiac chamber dimensions were measured in the A3-A6 abdominal segments of four different individuals for each genotype. Dimensions were obtained from the edge of the superior and inferior walls during diastole and systole.
Results
Generation of a Hand null allele
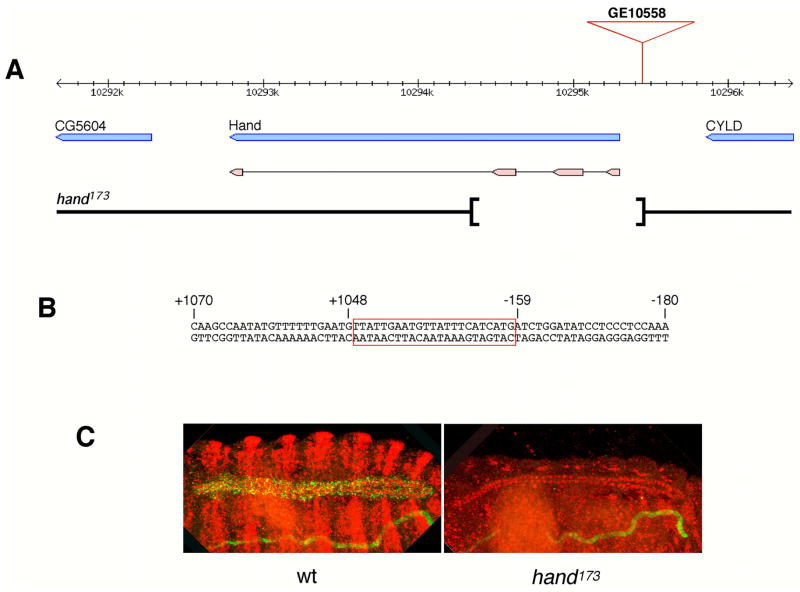
In order to obtain a mutation in the Hand gene, the EP insertion GE10558 immediately upstream of the gene (Fig. 1A) was utilized in a P-element excision screen to generate deletions of Hand sequences. PCR analysis of the resulting eight alleles that were lethal and the four alleles that were semi-lethal over the deficiency Df(2L)J1, which uncovers the Hand gene, identified the semilethal allele #173 as the most useful one for the phenotypic analysis of Hand. The imprecise excision deletion of allele #173 extends from the EP insertion site into the Hand gene without reaching the gene CG5604 immediately downstream of Hand, and also does not extend in the opposite direction into the upstream gene CYLD. PCR analysis and sequencing of the Hand173 allele indicated that 1.2 kb of genomic DNA is deleted from the EP insertion site into the Hand gene, which would remove the first three exons that together encode the first 148 a.a. of the 174 a.a.-long Hand protein (Fig. 1A,B). Since this deletion encompasses the bHLH domain of the Hand protein as well as the known intronic regulatory sequences, and leaves an open reading frame of only 27 a.a. from the C-terminus of Hand, which are unlikely to be expressed, this allele should be a null mutation. This was further confirmed by in situ hybridizations with a genomic probe encompassing the 3′ end of the Hand gene, which demonstrated the absence of Hand mRNA in the dorsal vessel and lymph glands of Hand173 homozygous embryos (Fig. 1C).
Figure 1.
Molecular characterization of the Hand173 deletion mutation. A) Genomic map of the Hand gene region. Genes are shown in blue and the exons of the Hand gene are shown in pink underneath. The location of the insertion of the GE10558 EP element is indicated at the top, and the endpoints of the imprecise excision deletion of Hand173 are denoted by the brackets at the bottom. B) Nucleotide sequence of the genomic DNA bordering the deletion of Hand173. The 23 bp sequence which replaces 1.2 kb of Hand sequences is highlighted by the red box. Sequence numbering is relative to the adenosine of the ATG start codon of the Hand open reading frame. C) Dorsal views of a control (left) and Hand173 mutant (right) embryo stained for Hand mRNA in situ (green) and anti-MEF2 and anti-β-gal (red). The Hand173/CyO control embryo expresses striped wg-lacZ from the balancer chromosome.
Lethal phase analysis of the Hand173 mutation
The Hand173 allele is semilethal over the deficiency Df(2L)J1 and in the homozygous condition. Lethal phase analysis of Hand173 homozygotes indicated that 99% (192/194) of embryos hatch, 59% (118/200) of 1st instar larvae pupate, 94% (47/50) of late 2nd instar and 3rd instar larvae pupate, and 95% (157/165) of pupae eclose. Hence, the semilethal phase appears to be during the 1st and 2nd larval instars and there is neither embryonic nor pupal lethality. Adult Hand173 flies are viable and fertile, allowing the establishment of a homozygous stock. This lethal phase analysis differs significantly from that of Han et al. (2006), as their Hand knock-out null allele (HandKO) generated by the ends-out homologous recombination technique is described as being homozygous lethal, with 40% of homozygous mutant HandKO embryos failing to hatch while the remaining embryos die within 24 hours as 1st instar larvae.
Characterization of Hand mutant embryonic phenotypes
Embryos mutant for Hand (both Hand173 and Hand173/Df(2L)J1) were examined by various molecular markers for possible defects in the tissues that express Hand, namely the dorsal vessel, lymph gland, and visceral musculature.
Expression of regulatory and differentiation genes in the two major components of the embryonic dorsal vessel, the cardioblasts and pericardial cells, was examined in late stage embryos. Several regulatory factors, including MEF2 (Bour et al., 1995; Lilly et al., 1995) in all the cardioblasts and Dorsocross (Reim and Frasch, 2005) in the Svp+ cardioblasts (Fig. 2A,B), Tinman (Azpiazu and Frasch, 1993; Bodmer, 1993) in the Tin+ cardioblasts and Tin+ pericardial cells (Fig. 2C,D), and Odd skipped (Ward and Skeath, 2000) in the Odd+ pericardial cells (Fig. 2K,L), appear to be normally expressed. Dorsal vessel differentiation gene products such as β3-tubulin (Kremser et al., 1999)(Fig. 2E,F) and Sur (Nasonkin et al., 1999)(Fig. 2G,H) in the Tin+ cardioblasts, and Pericardin (Chartier et al., 2002) in the pericardial cells (Fig. 2I,J), are also unaffected. The lack of any obvious defects in gene expression and morphology of the dorsal vessel appears to indicate that Hand is not required for the proper determination and differentiation of the dorsal vessel, although we cannot exclude the possibility of functional defects or subtle structural alterations. Our results differ notably from the phenotypes reported for the Hand knock-out null allele (HandKO) in Han et al. (2006), who reported that 20% of homozygous HandKO embryos possessed a range of morphological defects in the dorsal vessel when examined by MEF2, Pericardin, and Odd expression, with 3% of the mutant embryos exhibiting severe disruptions. We have observed no such perturbations in the structure of the dorsal vessel of more than 100 Hand173 homozygous embryos examined.
Figure 2.
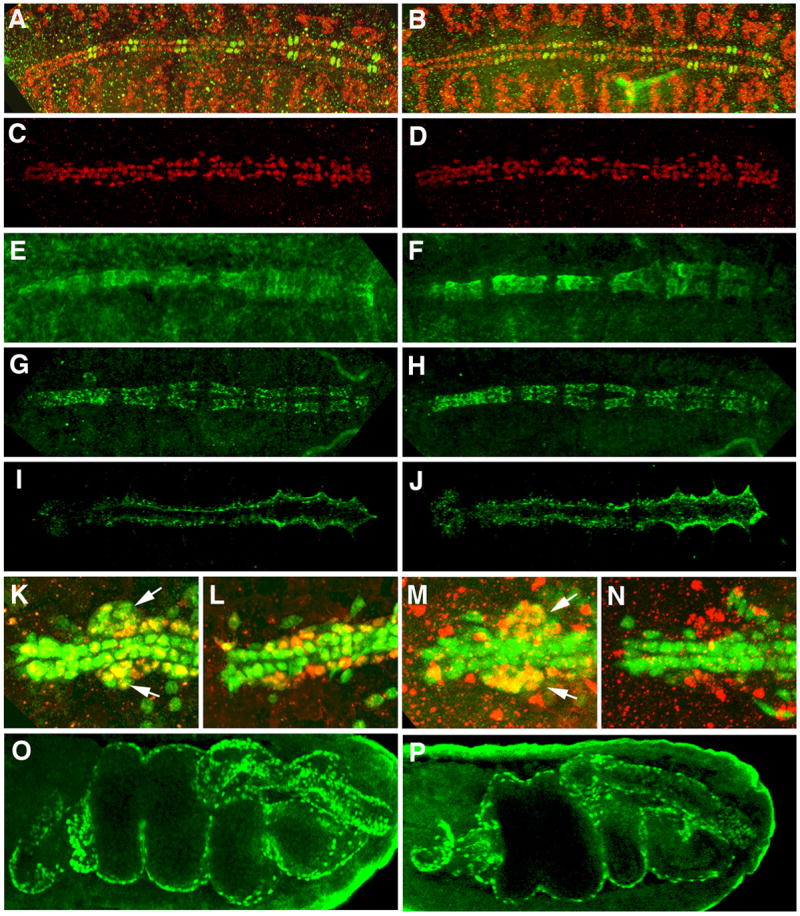
Embryonic expression of molecular markers in Hand mutants. Staining for markers of the dorsal vessel, lymph glands, and circular visceral musculature in homozygous Hand173 (B, D, F, H, J, P) and Hand173/Df(2L)J1;Hand-GFP/+ (L, N) stage 16 embryos was compared to wildtype control embryos of the same stage (A, C, E, G, I, K, M, O). A, B) Dorsocross (green) and MEF2 (red) expression in cardioblasts. C, D) Tinman expression in cardioblasts and pericardial cells. E, F) β3-tubulin expression in cardioblasts. G, H) Sur (sulfonylurea receptor) mRNA expression in cardioblasts. I, J) Pericardin expression by the pericardial cells. K, L) Odd skipped expression (red) in the lymph glands (arrows) and pericardial cells, and Hand-GFP expression (green) in the cardioblasts, pericardial cells, and lymph glands. M, N) Serpent expression (red) in the lymph glands, and Hand-GFP expression (green) in the cardioblasts, pericardial cells, and lymph glands. O, P) Biniou expression in the circular visceral musculature.
In contrast to the dorsal vessel, however, the lymph gland is either absent or reduced in a large proportion of Hand173 embryos, as determined by the expression of the lymph gland-specific molecular markers Odd skipped (Fig. 2K,L) and Serpent (Rehorn et al., 1996) (Fig. 2M,N), as well as with a Hand-GFP construct which is expressed in the lymph glands and dorsal vessel (Han and Olson, 2005). Han et al. (2006) also observed that lymph glands were missing in a large fraction of homozygous HandKO embryos, as shown by Odd skipped and Hand-GFP expression.
The visceral musculature of late stage Hand173 homozygous embryos and larvae does not appear to be grossly altered, as demonstrated by the normal embryonic staining pattern of Biniou, a key regulator of visceral mesoderm development (Zaffran et al., 2001), by the normal morphology of the embryonic gut constrictions and loops that are dependent on the visceral musculature, and by the normal gut muscle pattern in 3rd instar larvae as analyzed by phalloidin staining (Fig. 2O,P and data not shown).
Hand173 adults die prematurely
The Hand173 homozygous stock grows poorly, with a significant proportion of the adult flies apparently dying prematurely. In order to verify this, the viability of Hand173 flies as compared to yw flies was measured by collecting daily cohorts of newly eclosed flies, which were then counted daily for the number of remaining viable flies. A graph of post-eclosion adult viability (Fig. S1 in the supplementary material) confirms that Hand173 homozygous adults suffer a high rate of premature mortality, with the most precipitous decline in viability occurring from 3 to 4 days post-eclosion such that there are 66% dead (vs. 4% for yw control flies) by 5 days post-eclosion, increasing to 84% dead (vs. 11% for yw) by 10 days post-eclosion. Transheterozygous Hand173/Df(2L)J1 adults also show a high rate of premature mortality (5 day mortality of 83%) that is roughly equivalent to that of Hand173 adults.
Given the discrepancy between the viability of Hand173 null mutant flies and the reported lethality of the HandKO null allele of Han et al. (2006), we tested for complementation of the HandKO allele with Hand173 and Df(2L)J1 to ascertain if the embryonic/early larval lethality of HandKO could be rescued. Indeed, the HandKO/Hand173 and HandKO/Df(2L)J1 flies are semilethal, with viable adults that have 5 day mortality rates of 49% and 88%, respectively, which is similar to the rates for Hand173 and Hand173/Df(2L)J1 flies. Complementation of the lethality of HandKO by both Hand173 and Df(2L)J1 clearly demonstrates that the lethality attributed by Han et al. (2006) to the HandKO allele is actually due to an unrelated, linked lethal mutation that lies outside of Df(2L)J1. The failure of HandKO to complement the semilethality and premature adult mortality of Hand173 indicates that this is the correct phenotype for both of these Hand null alleles. Since the HandKO allele was initially identified by its lethality over Df(2L)Exel7046 (Han et al., 2006), which is shorter than Df(2L)J1, we also tested the complementation of both HandKO and Hand173 over this deficiency. Transheterozygotes of either HandKO or Hand173 over Df(2L)Exel7046 are semilethal, with adults that had 5 day mortality rates of 34% and 49%, respectively, confirming that the initial report of lethality for HandKO/Df(2L)Exel7046 is incorrect. The final proof that the HandKO allele is linked to an unknown lethal mutation is provided by our isolation of a derivative of the original HandKO chromosome which has recombined away this lethal mutation from HandKO. This HandKO recombinant is homozygous semilethal and fertile, as are heterozygotes of this HandKO recombinant over Hand173 (these adults suffer a 5 day mortality rate of about 50% and 34%, respectively). The second site mutation may also have segregated during the crosses for the genomic rescue experiments reported by Han et al. (2006).
In light of the above complementation results, it appears that the occasional embryonic dorsal vessel defects observed by Han et al. (2006) for HandKO but not seen by us for Hand173 are attributable to a linked lethal mutation on the chromosome containing the HandKO allele and not to the loss of Hand function.
Adult Hand173 homozygous flies exhibit dorsal vessel abnormalities
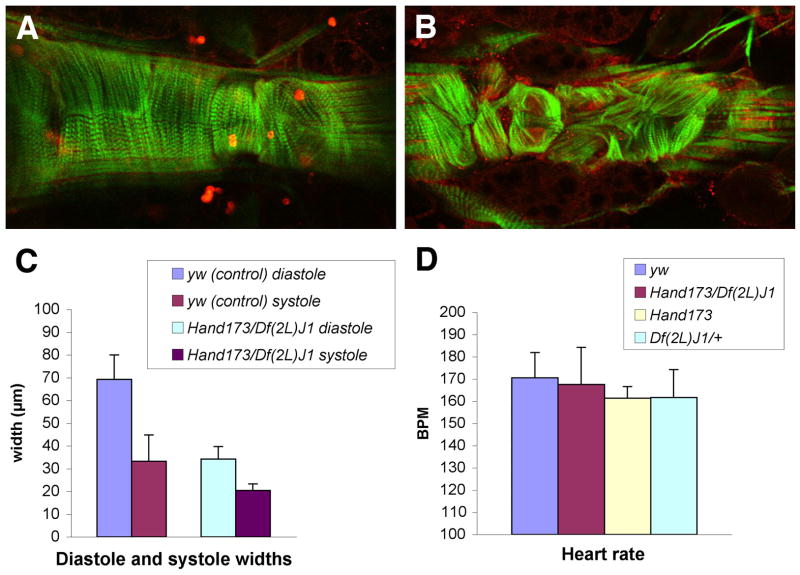
Since it has recently been demonstrated through the use of Hand-GFP constructs that Hand expression in the dorsal vessel and circular visceral muscles is maintained post-embryonically through the larval and pupal stages into adulthood (Han and Olson, 2005; Sellin et al., 2006), these tissues were examined in Hand mutant adults for possible morphological defects that may cause the premature adult mortality seen in these animals. Dorsal vessels of adult Hand173 homozygous flies were examined by staining with phalloidin to visualize the myofibrils of the cardioblasts and with either anti-Tinman or anti-Mef2 to identify the cardioblast nuclei. In a wildtype dorsal vessel the cardioblast myofibrils are characteristically arranged in a spiral fashion around the tubule (Fig. 3A). By contrast, in all Hand mutant flies examined, the dorsal vessels are clearly disorganized, with the myofibrils bent around the cortex of each cardioblast outside of the nucleus (Fig. 3B; see also Movie 1 in the supplementary material). In addition, both the diastolic and systolic widths of the Hand mutant adult dorsal vessel are significantly reduced compared to control (Fig. 3C), as is the ratio of these widths (from 2.1 to 1.7), which would result in a large decrease in the stroke volume of the mutant hearts. Despite these defects in heart morphology, the heartbeat rates of the mutant adult dorsal vessels are similar to the controls (Fig. 3D); however, while the heartbeat contractions still propagate anteriorly, they appear to originate anywhere along the length of the heart, in contrast to normal hearts where they initiate in the posterior. Since the adult dorsal vessel is formed during metamorphosis by remodeling of the aorta of the larval dorsal vessel (Molina and Cripps, 2001; Monier et al., 2005; Sellin et al., 2006), it is possible that the morphological defects observed for the Hand mutant adult dorsal are due to an abnormal larval dorsal vessel. However, since similar stainings of 3rd instar larval dorsal vessels are normal, as are those aspects of cardiac function described above (data not shown), this implies that the defects in Hand mutant adult dorsal vessels arise during metamorphosis.
Figure 3.
Adult dorsal vessel phenotypes in Hand mutants. Anterior is left. A, B) Confocal scan of anti-Tinman (red) and phalloidin (green) staining of the anterior halves of an adult wildtype (A) and Hand173 mutant (B) dorsal vessel. These are ventral views of middle sections of the confocal scan stacks with anterior to the left, which can be viewed in their entirety as Movies 1 and 2 of the supplementary material. C) Bar graph of diastolic and systolic widths of yw (control) and Hand173/Df(2L)J1 adult dorsal vessels (n=4 for each genotype). D) Bar graph of heartbeat rates of yw (control), Hand173/Df(2L)J1, Hand173, and Df(2L)J1/+ adult dorsal vessels (n=5 for each genotype).
Another possible cause of premature mortality in Hand mutant adults may be that a large fraction of Hand 3rd instar larvae are lacking lymph glands, which supply the adult hemocytes prior to disappearing during metamorphosis (Holz et al., 2003). However, it appears that most, if not all, of Hand 3rd instar larvae possess lymph glands of some form, as visualized by staining for Serpent (data not shown), though it is unclear whether their morphology is entirely normal. It seems likely, therefore, that Hand adults possess a normal complement of hemocytes, though it is still possible that they may be functionally abnormal.
Adult Hand173 homozygous flies exhibit gut abnormalities
During dissections of the Hand mutant flies, severe morphological abnormalities were observed in the digestive tract, particularly in the midgut. The most commonly seen deformities were a pronounced bulging and occasional rupturing of the anterior midgut immediately posterior of the proventriculus, coupled with a shortening of the length of the midgut by approximately 25% to 50%, a variable narrowing of the mid- and posterior midgut compared to wildtype, and a distended crop (Fig. 4B). Feeding of the flies with dyed food showed that the distended crop and parts of the anterior midgut of Hand mutant flies are usually filled with food, but unlike in control animals the posterior portions of the digestive tract contain little or no food (Fig. 4B, Fig. S2A, cf. Fig. S2B). In all the Hand mutant guts, the proventriculus, Malpighian tubules, hindgut, and rectum appear normal.
Figure 4.
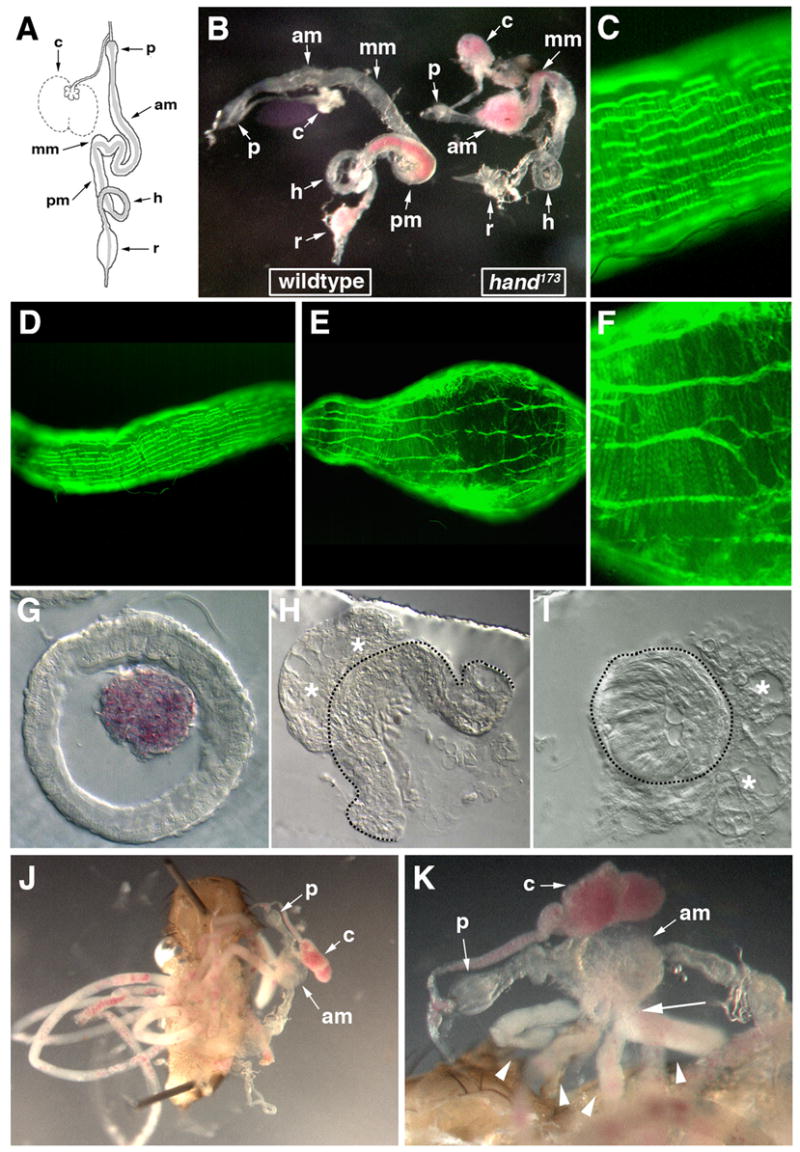
Adult gut phenotypes in Hand173 mutants. A) Schematic diagram of a wildtype adult gut from the proventriculus to the rectum; the peritrophic membrane tubule located within the lumen of the mid- and hindgut is shown in gray and the Malpighian tubules have been omitted. p, proventriculus; am, anterior midgut; mm, mid-midgut; pm, posterior midgut; h, hindgut; r, rectum. The crop is shown in normal (solid line) and distended food storage (dashed line) states. The adult guts in panels B, G–K were from adult flies fed with yeast mixed with red dye, which allows the unambiguous identification of ingested food within the gut. The foregut (anterior to the proventriculus) and the Malpighian tubules have been removed. B) Side-by-side comparison of the dissected guts of a 4.5 day-old wildtype (left) and Hand173 mutant (right) adult with a weak phenotype. C–F) Phalloidin staining (green) of the anterior midgut of a wildtype adult gut (C,D) and the anterior midgut bulge of the Hand173 mutant adult gut (E,F); panels C and F are blow-ups of D and E, respectively. G,H,I) Transverse sections through the posterior midgut of a wildtype adult gut (G), and a ruptured anterior midgut bulge (H) and mid-midgut (I) of Hand173 mutant adult gut. Note the red yeast contained within the peritrophic membrane tubule of the wildtype adult gut (G). Asterisks indicate unidentified tissue attached externally to the Hand173 mutant adult gut and dashed lines show the outer surface of the mutant gut tubes. Gut lumen in (I) is almost completely obstructed. J) Dissected 3 day-old Hand173 mutant adult with a strong phenotype, showing numerous ectopic peritrophic membrane tubule loops (left). The gut with the anterior midgut bulge is positioned to right of the carcass. Note that the crop is distended with red yeast, which is not found elsewhere in the gut proper. Anterior is up. K) Close-up of the midgut in panel J. Note the numerous peritrophic membrane tubule loops (arrowheads) exiting a single orifice (arrow) on one side of the anterior midgut bulge, which are filled with a white non-food material in which bits of red yeast are interspersed.
Examination of the visceral musculature of the adult midgut of the Hand mutant reveals that the pattern of circular and longitudinal visceral muscle fibers is apparently unaffected in the portions of the midgut with a largely normal or narrowed morphology (Fig. S2C in the supplementary material). In the anterior midgut bulge, the longitudinal fibers are stretched lengthwise and separated farther from each other, and the circular fibers are slightly disorganized, although both types of visceral muscles still cover the entire surface of the bulge (Fig. 4E,F, cf. Fig. 4C,D). In examples of Hand mutant adults with more moderate malformations in the midgut, a correspondingly moderate alteration of the midgut visceral musculature was observed (Fig. S2D in the supplementary material). Abnormal arrangement of gut muscle fibers in distended portions of the gut is likely to be secondary to the shape changes of the digestive tract. In order to examine the malformations of the Hand mutant midgut endoderm in greater detail, sectioning of a gut with the severe phenotype was done. In a transverse section of an anterior midgut bulge through a rupture, the thickness of the mutant endoderm in the bulge is not significantly different from wildtype, but it does appear to be abnormal as it is highly vacuolated (Fig. 4G,H). Further posterior, the overall diameter of the mid-midgut is reduced and its lumen is completely occluded, the result of what appears to be abnormally thick endoderm and the narrowing of the midgut at this position (Fig. 4I). Occlusion of the mid- or posterior midgut occurs in a large proportion of but not all Hand mutant adult guts; however, it is generally correlated with prematurely dying or dead adults. This occlusion of the midgut would explain the apparent block in the passage of the food in Hand mutant flies. The expected reduction in nutrient uptake and the resulting starvation is likely to be one explanation for the premature adult mortality of Hand adult mutants. Examination of the guts of older (>30 days) Hand adult survivors reveals that although they have anterior midgut bulges their midguts are not occluded (data not shown), which would presumably allow for the normal uptake of nutrients by the midgut and a normal lifespan.
The most severe phenotype, in addition to the aforementioned deformities, is seen with the peritrophic membrane of the gut. The peritrophic membrane is a linear tube consisting of a chitinous matrix which is produced continuously by specific cells of the proventriculus and lines the interior of the midgut and hindgut (Fig. 4A) (for reviews see Lehane, 1997; Richards and Richards, 1977). In the most severely affected Hand mutant flies, we observe extremely long loops of ectopic peritrophic membrane tubules external to the gut, which are packed into the abdominal cavity (Fig. 4J). These usually exit from a rupture in the side of the anterior midgut bulge and are typically filled with a white material in which some food is dispersed (Fig. 4K). Upon teasing out of these loops it appears that there is actually only one continuous peritrophic membrane tubule, which extends many times the length of a wildtype gut (data not shown). Cross sections of anterior midgut bulges that are not ruptured often show an accumulation of backed-up peritrophic membrane material in these structures (data not shown). We interpret these phenotypes as being a result of the continuous production of peritrophic membrane in the anterior portion of the digestive tract in combination with a block of its passage to the posterior end, where normally it is degraded and, thus, kept at a steady-state length.
Discussion
Of the embryonic tissues that express Hand, only a moderately penetrant loss of the lymph glands was observed in Hand mutant embryos. Neither the dorsal vessel (heart) nor the circular visceral musculature is morphologically affected in these embryos, indicating that Hand is not necessary for the proper embryonic morphogenesis of these tissues, though it is possible that it is required for their normal physiological function and that the loss or reduction of this function may result in the early larval semilethality. The apparent correlation of the fact that a large fraction of late stage Hand embryos lack lymph glands with the high rate of semilethality for early instar larvae, coupled with the observation that surviving 3rd instar Hand173 larvae all appear to possess lymph glands, would seem to imply that the loss of lymph glands is causing larval death due to a deficit in immune function. However, this is unlikely since the hemocytes of the larva arise from the head mesoderm and not the lymph gland, which supplies the hemocytes of the adult (Holz et al., 2003). It may be possible that the early larval semilethality is caused by the loss or functional disruption of the subset of CNS cells that express Hand in the late embryo, which we have not examined, or by the loss of an unknown function of the lymph gland.
The adult phenotypes that we have observed demonstrate a requirement for Hand in the proper morphogenesis of the adult heart and midgut. Adult Hand mutant dorsal vessels are clearly morphologically abnormal, which would reduce cardiac output, and the initiation of heartbeat contraction is also abnormal, which may additionally affect cardiac function. The resulting reduced cardiac output of the Hand adult dorsal vessel is presumed to contribute to the high rate of premature adult mortality seen in Hand mutants. This is supported by the recent demonstration that flies lacking Tinman expression in the dorsal vessel possess morphologically and functionally abnormal adult dorsal vessels and also suffer from a reduced adult lifespan (Zaffran et al., 2006).
Since 3rd instar larval dorsal vessels are morphologically and functionally normal, it appears that the morphological defects seen in Hand mutant adult dorsal vessels only arise during metamorphosis, when the adult heart is remodeled from the larval aorta (Molina and Cripps, 2001; Monier et al., 2005; Sellin et al., 2006). Hence, we conclude that pre-adult Hand expression is required for the proper morphogenesis of the adult heart during metamorphosis but not of the late embryonic/early larval dorsal vessel. We have seen no evidence for a function of Hand in regulating the morphogenesis of the late embryonic/early larval dorsal vessel as reported by Han et al. (2006).
The other major phenotype in Hand adults are malformations of the endoderm of the midgut. Virtually all Hand mutant adults exhibit an anterior midgut bulge, with the remainder of the midgut exhibiting differing degrees of shrinkage and occlusion of the midgut lumen. This occlusion is most relevant to the premature adult mortality, since it would prevent the passage of food through the gut and severely reduce or totally prevent the absorption of nutrients from ingested food, resulting in starvation. This is probably a major factor in the high rate of premature Hand adult mortality.
Hand is not expressed in the embryonic endoderm (Kölsch and Paululat, 2002) and based on the expression of Hand-GFP, which recapitulates the embryonic expression of Hand and is present in the circular visceral musculature through adulthood (Han and Olson, 2005; Sellin et al., 2006), it appears most likely that Hand is also not expressed in the endoderm post-embryonically. As the endoderm of the adult gut is reconstituted during metamorphosis from imaginal precursor cells whereas the larval gut musculature persists and appears normal in Hand mutants (P.L. and M.F., unpublished data), it would follow that normal morphogenesis of the imaginal endoderm is dependent on pre-adult Hand expression in the circular visceral musculature, perhaps through Hand-dependent inductive signals from the muscle to the endoderm.
Our genetic analysis of the function of the Drosophila Hand gene has demonstrated that it is necessary for the proper morphogenesis of the adult dorsal vessel and midgut, which occurs through the remodeling of the corresponding larval organs during metamorphosis. The morphological defects in adult Hand dorsal vessels and the hearts of Hand1 knockout/Hand2 conditional double mutant mice (McFadden et al., 2005) suggest that the role of the Hand gene in regulating cardiac morphogenesis has been conserved to some degree during evolution. Therefore, further analysis of how the Drosophila Hand gene functions molecularly and genetically should prove useful in elucidating the possibly equivalent, evolutionarily conserved functions of the vertebrate Hand genes in cardiac morphogenesis. If the adult midgut phenotype of Drosophila Hand mutants is due to an indirect effect of the loss of Hand function in the morphologically normal circular visceral musculature, this is a phenotype without an equivalent in the vertebrate Hand mutant mice. Like Drosophila Hand, mouse Hand1 is also expressed only in the intestinal smooth muscle layer of the gut during mouse embryogenesis (Cserjesi et al., 1995; Hollenberg et al., 1995); however, any effect of the loss of Hand1 function in the mouse embryonic smooth muscle layer on endoderm development in Hand1 knockout mice may be masked by the early embryonic lethality that is the result of extra-embryonic defects (Firulli et al., 1998; Riley et al., 1998). It would therefore be interesting to observe if tissue-specific loss of Hand1 function in mouse embryonic intestinal smooth muscle utilizing the Hand1 conditional knockout allele would have an effect on mouse embryonic endodermal development.
Supplementary Material
Acknowledgments
We wish to thank Robert Krauss for the use of his binocular microscope and digital camera, and Michel Sémériva for allowing Sébastien Sénatore to carry out research for this paper. Confocal laser scanning microscopy at the MSSM-Microscopy Shared Resource Facility was supported from NIH-NCI shared resources grant (R24 CA095823) and NSF Major Research Instrumentation grant (DBI-9724504). This research was supported by the NIH grants DK59406 from the NIDDK and HD30832 from the NICHD to M.F and by the CNRS to S.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azpiazu N, Frasch M. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Bour BA, et al. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–41. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- Chartier A, et al. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development. 2002;129:3241–3253. doi: 10.1242/dev.129.13.3241. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, et al. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–78. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- Firulli AB. A HANDful of questions: the molecular biology of the heart and neural crest derivatives (HAND)-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- Firulli AB, et al. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–70. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- Han Z, Olson EN. Hand is a direct target of Tinman and GATA factors during Drosophila cardiogenesis and hematopoiesis. Development. 2005;132:3525–36. doi: 10.1242/dev.01899. [DOI] [PubMed] [Google Scholar]
- Han Z, et al. Hand, an evolutionarily conserved bHLH transcription factor required for Drosophila cardiogenesis and hematopoiesis. Development. 2006;133:1175–82. doi: 10.1242/dev.02285. [DOI] [PubMed] [Google Scholar]
- Hjalt T. Basic helix-loop-helix proteins expressed during early embryonic organogenesis. Int Rev Cytol. 2004;236:251–80. doi: 10.1016/S0074-7696(04)36006-7. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, et al. Identification of a new family of tissue-specific basic helix-loop-helix proteins with a two-hybrid system. Mol Cell Biol. 1995;15:3813–22. doi: 10.1128/mcb.15.7.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A, et al. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–62. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- Knirr S, Frasch M. Molecular integration of inductive and mesoderm-intrinsic inputs governs even-skipped enhancer activity in a subset of pericardial and dorsal muscle progenitors. Dev Biol. 2001;238:13–26. doi: 10.1006/dbio.2001.0397. [DOI] [PubMed] [Google Scholar]
- Kölsch V, Paululat A. The highly conserved cardiogenic bHLH factor Hand is specifically expressed in circular visceral muscle progenitor cells and in all cell types of the dorsal vessel during Drosophila embryogenesis. Dev Genes Evol. 2002;212:473–85. doi: 10.1007/s00427-002-0268-6. [DOI] [PubMed] [Google Scholar]
- Kremser T, et al. Tinman regulates the transcription of the beta3 tubulin gene (betaTub60D) in the dorsal vessel of Drosophila. Dev Biol. 1999;216:327–39. doi: 10.1006/dbio.1999.9425. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. Peritrophic matrix structure and function. Annu Rev Entomol. 1997;42:525–50. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- Lilly B, et al. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–93. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- Lo PC, Frasch M. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech Dev. 2001;104:49–60. doi: 10.1016/s0925-4773(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–40. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, et al. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- Molina MR, Cripps RM. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech Dev. 2001;109:51–9. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
- Monier B, et al. Steroid-dependent modification of Hox function drives myocyte reprogramming in the Drosophila heart. Development. 2005;132:5283–93. doi: 10.1242/dev.02091. [DOI] [PubMed] [Google Scholar]
- Moore AW, et al. A genomewide survey of basic helix-loop-helix factors in Drosophila. Proc Natl Acad Sci U S A. 2000;97:10436–41. doi: 10.1073/pnas.170301897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasonkin I, et al. A novel sulfonylurea receptor family member expressed in the embryonic Drosophila dorsal vessel and tracheal system. J Biol Chem. 1999;274:29420–5. doi: 10.1074/jbc.274.41.29420. [DOI] [PubMed] [Google Scholar]
- Rehorn KP, et al. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–31. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- Reim I, Frasch M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development. 2005;132:4911–25. doi: 10.1242/dev.02077. [DOI] [PubMed] [Google Scholar]
- Richards AG, Richards PA. The peritrophic membranes of insects. Annu Rev Entomol. 1977;22:219–40. doi: 10.1146/annurev.en.22.010177.001251. [DOI] [PubMed] [Google Scholar]
- Riley P, et al. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–5. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Sellin J, et al. Dynamics of heart differentiation, visualized utilizing heart enhancer elements of the Drosophila melanogaster bHLH transcription factor Hand. Gene Expr Patterns. 2006;6:360–75. doi: 10.1016/j.modgep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Srivastava D, et al. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–9. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- Srivastava D, et al. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–60. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Tao Y, et al. Mol Cell Biol. 2007 doi: 10.1128/MCB.00093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, et al. The bHLH factors, dHAND and eHAND, specify pulmonary and systemic cardiac ventricles independent of left-right sidedness. Dev Biol. 1998;196:228–36. doi: 10.1006/dbio.1998.8849. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Skeath JB. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development. 2000;127:4959–4969. doi: 10.1242/dev.127.22.4959. [DOI] [PubMed] [Google Scholar]
- Yelon D, et al. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–82. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]
- Zaffran S, et al. biniou (FoxF), a central component in a regulatory network controlling visceral mesoderm development and midgut morphogenesis in Drosophila. Genes Dev. 2001;15:2900–15. doi: 10.1101/gad.917101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffran S, et al. Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development. 2006;133:4073–83. doi: 10.1242/dev.02586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.