Abstract
A combination of social withdrawal and increased aggression is characteristic of several mental disorders. Most previous studies have investigated the neurochemical bases of social behavior and aggression independently, as opposed to how these behaviors are regulated in concert. Neuronal nitric oxide synthase (nNOS) produces gaseous nitric oxide, which functions as a neurotransmitter and is known to affect several types of behavior including mating and aggression. Compared with wild-type mice, we observed that nNOS knockout mice showed reduced behavioral responses to an intruder behind a wire barrier. Similar results were observed in mice treated with the selective nNOS inhibitor 3-bromo-7-nitroindazole (3BrN). In habituation–dishabituation tests, treatment with 3BrN did not block recognition of male urine but did attenuate investigation time compared with oil-treated animals. Finally, nNOS knockout mice and 3BrN treated mice were significantly more aggressive than wild-type and oil-treated males, respectively. In general, these behavioral effects are less pronounced in pair-housed males compared with singly-housed males. Thus, nNOS inhibition results in a phenotype that displays reduced social investigation and increased aggression. These data suggest that further study of nNOS signaling is warranted in mental disorders characterized by social withdrawal and increased aggression.
Keywords: aggression, autism, nitric oxide synthase, serotonin, social investigation
The neurobiological substrates underlying social behavior have received renewed attention in efforts to gain insights into the underpinnings of mental disorders that involve deficits in social behavior (Choleris et al., 2003; DiCicco-Bloom et al., 2006; Insel & Fernald, 2004). In some cases, deficits in social interactions are associated with increased aggression (Anckarsater, 2006; Soderstrom, Sjodin, Carlstedt, & Forsman, 2004). The relationships between mechanisms of social investigation and aggression are poorly understood. The most common test used to study the neurobiological bases of aggression is the rodent resident–intruder test, in which an unfamiliar intruder is introduced into a resident’s’ home cage. Used successfully to identify neural circuits (Newman, 1999) and chemical systems (Nelson & Chiavegatto, 2001) that mediate aggression, the resident–intruder test eliminates the period of social assessment that precedes aggression in naturalistic contexts (Anderson & Hill, 1965).
Mechanisms of social investigation generally have been examined outside of the context of aggressive behavior. A notable exception is a study of the vasopressin receptor 1b (AVPr1b), as selective deletion of AVPr1b results in decreased aggression and decreased investigation of social stimuli (Wersinger, Ginns, O’Carroll, Lolait, & Young, 2002). Importantly, AVPr1b knockout mice retained the ability to detect both male and female urine (Wersinger et al., 2004). Reduced olfactory investigation and aggression is also observed after treatment with an AVPr1b antagonist (Blanchard et al., 2005). In these studies, a loss of social investigation is paired with reduced aggression. In rodents, neural networks activated during aggression are strongly dependent on olfactory input (Choi et al., 2005), so reduced social investigation presumably results in decreased activation of these systems. In support of this hypothesis, selective deletion of the TRP2 gene is associated with reduced neuronal activity in the vomeronasal organ and reduced aggression (but not social investigation; Leypold et al., 2002; Stowers, Holy, Meister, Dulac, & Koentges, 2002).
The enzyme neuronal nitric oxide synthase (nNOS) modulates aggression in mice. Selective deletion of the nNOS gene (nNOS−/−) increases aggression in male mice (Nelson et al., 1995); inhibition of nNOS with a specific nNOS inhibitor (7-nitroindazole) also increases aggression (Demas et al., 1997). Additionally, whereas wild-type mice reduce aggression in response to submissive displays made by intruders, male nNOS−/− mice continue aggressive behavior in spite of submissive displays (Nelson et al., 1995). These data suggest that nitric oxide has broad effects on social interactions. Disruption of nNOS function is also associated with alterations in a variety of affective states (Nelson, Trainor, Chiavegatto, & Demas, 2006), which could affect how an individual reacts in social encounters.
We used several tests to assess the effects of nitric oxide signaling on investigation of intruders in an effort to separate mechanisms of social investigation and aggressive behavior. Mice were tested in a modified version of the sensory contact paradigm (Kudryavtseva, Bondar, & Alekseyenko, 2000; Kudryavtseva, Bondar, & Avgustinovich, 2004), in which residents are exposed to auditory, olfactory, and visual stimuli from an intruder behind a wire barrier. This test was originally designed to assess motivation to engage in aggression. We also examined responses to olfactory cues from a potential intruder using habituation–dishabituation tests. Using nNOS−/− mice and wild-type (WT) mice treated with the selective nNOS inhibitor 3-bromo-7-nitroindazole (3BrN), we examined the effects of nNOS deficiency on both social investigation and aggression. We also compared how social housing conditions affected the behavioral outcomes of nNOS deficiency by testing both singly and pair-housed animals. Our experiments revealed that in domestic mice, motivation to investigate social stimuli can be dissociated from aggressive behavior.
Method
Subjects
Wild-type (WT) mice (B6;129SF1/SImJ, Jackson Stock Number 002633) and nNOS−/− mice (B6;129S4/SvJae-Nos1tm1Plh/J, Jackson Stock Number 101045) were obtained from Jackson Labs (Bar Harbor, ME). The nNOS−/− mutation is on a C57B6 × 129S background. The WT mice are also a C57B6 × 129S cross that is an approximate match to the background of the nNOS−/− line. The 129S backgrounds in the WT and nNOS−/− strains are descended from the same lineage (Simpson et al., 1997). The two strains are a close, but not exact, genetic match. To confirm findings from knockout studies, we conducted additional studies on WT mice treated with an nNOS inhibitor, which provides converging evidence for the role of NO and eliminates the influence of genetic background on behavior. All mice were provided with filtered tap water and Harlan Teklad 8640 food (Madison, WI) ad libitum and maintained in accordance with the recommendations of the National Institutes of Health (1986) Guide for the Care and Use of Laboratory Animals. The light–dark cycle was 16-hr light:8-hr dark, with lights out at 1500. All behavioral tests were conducted between 1400 and 1600 hr under dim red illumination.
Experiment 1: Effect of nNOS Deletion on Social Investigation and Aggression
WT and nNOS−/− mice were randomly assigned to be singly housed or pair-housed with a same sex cagemate of the same genotype (n = 6 per group). For pair-housed animals, 1 male was randomly assigned to be the focal male and the other, the cagemate. The cagemate had a small piece of fur shaved on the flank for identification. All pair-housed animals were assessed for wounding before behavioral tests; we observed no wounds in any pair-housed WT or nNOS−/− mice. After 2 weeks mice were tested in a modified version of the resident–intruder partition test (Kudryavtseva, 2000). In the partition test, a wire screen was placed in a resident’s home cage (30.5 × 17.8 × 14.0 cm) 24 hr before testing to allow for habituation. The screen formed a large compartment (24.1 × 17.8 × 14.0 cm) occupied by the resident and a smaller compartment (6.4 × 17.8 × 14.0 cm) to be occupied by the intruder. Ten minutes before testing, the cagemate of pair-housed animals was removed whereas a sham removal (in which the home cage was opened) was conducted for all singly-housed animals. A WT intruder was placed in the smaller compartment for 10 min, giving residents access to visual, olfactory, and auditory cues. All tests were videotaped and later scored by an observer without knowledge of the treatment groups. The amount of time spent interacting with the barrier by digging underneath or biting the partition was measured. Pretest observations indicated that these behaviors directed at the barrier were largely absent when intruders were not present. Previous studies that expose a resident to a male intruder behind a barrier report increased interaction with the barrier and heightened aggression in resident–intruder tests conducted shortly after partition tests (De Almeida & Miczek, 2002; Fish, Faccidomo, & Miczek, 1999). One hour after each intruder was removed, study subjects were anesthetized with isoflurane and decapitated. Trunk blood was collected and centrifuged for each individual. Plasma was removed and stored at −80 °C for corticosterone and testosterone assays. Brains from WT mice were quickly removed and fixed overnight in 5% acrolein. Brains were then transferred to 30% sucrose for 24 hr and frozen for nNOS immunocytochemistry.
The cagemates from the partition study were tested in resident–intruder aggression tests. WT (n = 6) and nNOS−/− (n = 5) were first singly housed for 2 weeks. Then, a group-housed virgin WT male intruder was placed into each resident’s cage for 10 min under dim red light. During the 10 min test, we quantified the number of bites, bouts of boxing, and attack latency.
Experiment 2: Effect of nNOS Inhibition on Social Investigation and Aggression
WT males were randomly assigned to be singly housed or pair-housed for 2 weeks. Males in each group were then randomly assigned to receive injections of oil or an nNOS inhibitor. The nitric oxide inhibitor 3BrN (Caymen Chemical, Ann Arbor, MI) was dissolved in corn oil to yield a dose of 20 mg/kg in 0.1 mL of oil (Gammie, Olaghere-da Silva, & Nelson, 2000). Subcutaneous injections were administered twice per day between 0800 and 0900 and between 1600 and 1800. All behavioral tests were conducted 30 min following the afternoon injection.
To examine investigation of social odors, we tested mice (n = 10 per group) in habituation–dishabituation tests (Pankevich, Baum, & Cherry, 2004). Urine was collected from adult gonadally intact WT males. Mice were firmly gripped on the scruff of the neck, and urine was collected into centrifuge tubes and frozen at −20 °C. Individual samples were thawed and diluted 1 to 10 before testing. A drop of 10 μl of distilled water was pipetted onto the center of a glass slide, which was then transferred into the cage for a 2-min trial. During each trial the amount of time the mouse spent investigating the drop was recorded. Two more trials with distilled water were then conducted with a 1-min intertrial interval. After the third trial with water, three additional trials were conducted with 10 μl of diluted urine placed on a slide. A fresh slide was used in every trial, and the same urine sample was used for each of the last three trials. To test whether individuals in each group could detect social odors, we used a paired t test to compare time spent investigating the water droplet in the third trial (third water trial, Trial 3) and the urine droplet in the fourth trial (first urine trial, Trial 4). To test for differences between groups in motivation to investigate social odors, we calculated difference scores (Trial 4 time − Trial 3 time) for each individual and analyzed these data with two-way analyses of variance (ANOVA; see Statistical Analyses). Immediately following habituation–dishabituation tests, wire barriers (as described in Experiment 1) were introduced into each home cage. The next day, the cagemate of pair-housed animals was removed, whereas a sham removal (in which the home cage was opened) was conducted for all singly housed animals. After 10 min, the partition test was conducted as described in Experiment I. Immediately after testing, the partition was removed and cagemates were returned. The next day, we tested oil-treated (n = 9) and 3BrN-treated (n = 8) males in resident intruder aggression tests, as described in Experiment 1. A different sexually inexperienced group-housed WT intruder was used in partition and aggression tests. As in partition tests, cagemates were removed (or, in the case of singly housed mice, exposed to sham removal) 10 min before resident–intruder tests.
Radioimmunoassay
Total corticosterone was measured with a 125I corticosterone radioimmunoassay (ICN Biomedicals, Costa Mesa, CA). The intra-assay coefficient of variation (CV) was 10.8%, and the detection limit was 5 ng ml−1. Total testosterone was measured with a 125I testosterone radioimmunoassay (RIA) kit (DSL-4100; Diagnostic Systems Laboratories, Webster, TX). These samples were run in two assays; the intra-assay CV was 5.0%, the inter-assay CV was 8.3%, and the detection limit was 0.1 ng ml−1.
nNOS Immunocytochemistry
Brains were sectioned at 40 μm on a cryostat, and alternate free-floating sections were processed for nNOS immunocytochemistry. Sections were washed three times (5 min each) in phosphate-buffered saline (PBS) and then incubated in 1% sodium borohydride (Fisher, Pittsburgh, PA) in PBS for 10 min. Sections were then rinsed in 20% normal goat serum (Sigma, St. Louis, MO) and 0.3% hydrogen peroxide (Sigma) in PBS for 20 min. Sections were incubated in primary nNOS antibody (No. 23287; ImmunoStar, Hudson, WI; 1:20K) in 1% normal goat serum in 0.5% Triton-X (TX; Sigma) PBS (PBS plus TX) for 48 hr. Next, the sections were rinsed three times (5 min each) in PBS and incubated for 90 min with biotinylated goat–antirabbit antibody (Vector Laboratories, Burlingame, CA) in PBS plus TX. The sections were then rinsed three times (5 min each) in PBS and then incubated for either 30 min in avidin–biotin complex (ABC Elite kit; Vector Laboratories). After three rinses (5 min each) in PBS, the sections were developed in diaminobenzidine in the presence of hydrogen peroxide and nickel (DAB kit; Vector Laboratories) for 90 s. After two rinses (5 min each) in PBS, sections were dipped in distilled water, mounted on gel-coated slides, dehydrated, and coverslipped with Permount (Fisher). Sections containing the ventral lateral septum (vLS), posterior bed nucleus of the stria terminalis (pBNST), the anterior hypothalamus (AHA), and the paraventricular nucleus (PVN) were identified with the mouse atlas by Franklin and Paxinos (1997). These brain areas are known to be part of a neural circuit influencing aggressive behavior (Goodson, 2005; Newman, 1999). Images were captured sequentially using a Nikon E800 microscope. Immunopositive cells in each nucleus were counted with the aid of Neurolucida software (Microbrightfield, Burlington, VT). Control sections in which primary antibodies were not added showed no specific staining.
Statistical Analyses
In Experiment I, behavioral data from the partition tests, as well as all cell count data, were analyzed with two-way ANOVA, testing for effects of genotype, housing condition, and their interaction. Independent t tests were used to evaluate data from aggression tests of singly housed nNOS−/− and WT mice. All hormone data were log transformed prior to statistical analysis and analyzed with two-way ANOVA. In Experiment 2, behavioral data from habituation–dishabituation tests, partition tests, and aggression tests were analyzed with two-way ANOVA testing for effects of drug treatment, housing condition, and their interaction. Planned comparisons were used to test for effects of 3BrN on behavior in singly housed and pair-housed animals. Mean differences were considered statistically significant when p < .05. All data are presented as means ± standard error of the mean (SEM).
Results
Experiment 1: Effects of nNOS Deletion on Social Investigation and Aggression
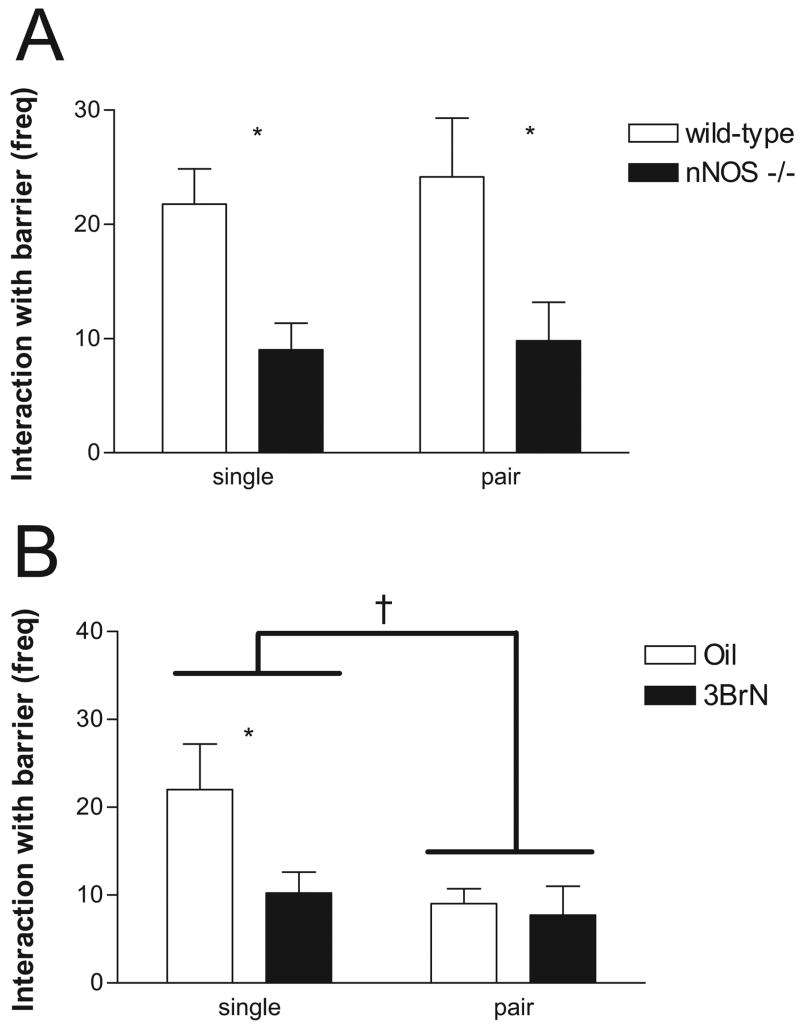
In partition tests, nNOS−/− mice were significantly less likely than WT mice to interact with the barrier by digging underneath or biting the barrier (Figure 1A), F(1, 20) = 13.9, p < .001. There was no effect of pair housing, F(1, 20) = 0.19, p > .05, and no significant interaction. Differences in social investigation were not associated with differences in posttest corticosterone or testosterone concentrations (Table 1).
Figure 1.
Behavioral responses in partition tests in the (A) knockout and (B) 3-bromo-7-nitroindazole (3BrN) studies. In the knockout study (A), wild-type mice (open bars) interacted with the barrier significantly more than did mice with selective deletion of the neuronal nitric oxide synthase gene (nNOS −/−) (black bars, *p < .05 effect of genotype). In the 3BrN study (B), there was a significant difference between 3BrN (black bars) and oil (open bars) treatment groups when mice were singly housed but not when mice were pair housed (*p < .05, effect of 3BrN). Overall, singly housed males interacted with the barrier more than did pair-housed mice (†p = .06, effect of housing). freq = frequency.
Table 1.
Hormone Concentrations (M ± SEM) of Wild-Type Mice and Those With Selective Deletion of the Neuronal Nitric Oxide Synthase Gene (nNOS −/−)
| Wild-type
|
nNOS −/−
|
|||
|---|---|---|---|---|
| Hormone | Single | Pair | Single | Pair |
| Corticosterone (ng/mL) | 141 ± 28.9 | 117 ± 35.1 | 104 ± 30.2 | 140.2 ± 17.6 |
| Testosterone (ng/mL) | 7.5 ± 3.6 | 6.6 ± 4.6 | 5.2 ± 2.7 | 4.3 ± 3.8 |
Note. There was no effect of housing condition or genotype on corticosterone or testosterone concentrations.
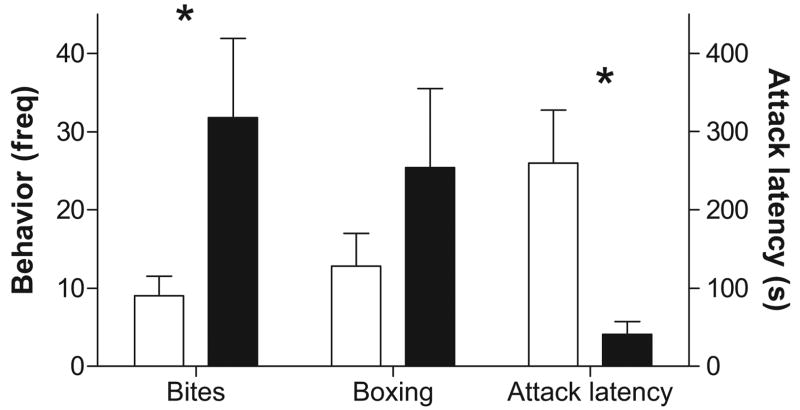
After 2 weeks of single housing, we tested the cagemates of the pair-housed animals in the aggression test. We observed that nNOS−/− mice increased biting (Figure 2), t(9) = 2.38, p < .05, and reduced attack latency (Figure 2), t(9) = 2.89, p < .05, compared with WT mice. The mean frequency of boxing in nNOS−/− was higher than that for WT mice but was not significantly different (Figure 2), t(9) = 1.22, p > .05. In WT mice, there was no effect of housing condition (all ps > .05) on the number of nNOS positive cells in the vLS (M ± SEM: single, 24.0 ± 2.0; pair, 22.6 ± 7.1), pBNST (M ± SEM: single, 45.6.0 ± 7.1; pair, 43.2 ± 3.2), AHA (M ± SEM: single, 96.0 ± 4.4; pair, 90.3 ± 2.3), or PVN (Figure 3; M ± SEM: single, 43.7.0 ± 7.8; pair, 43.1 ± 3.3).
Figure 2.
Aggressive behavior in singly housed wild-type mice (open bars) and mice with selective deletion of the neuronal nitric oxide synthase gene (nNOS −/−; black bars) in Experiment 1. nNOS −/− mice exhibited increased biting and reduced attack latency compared with wild-type males. Freq = frequency. *p < .05, genotype comparison.
Figure 3.
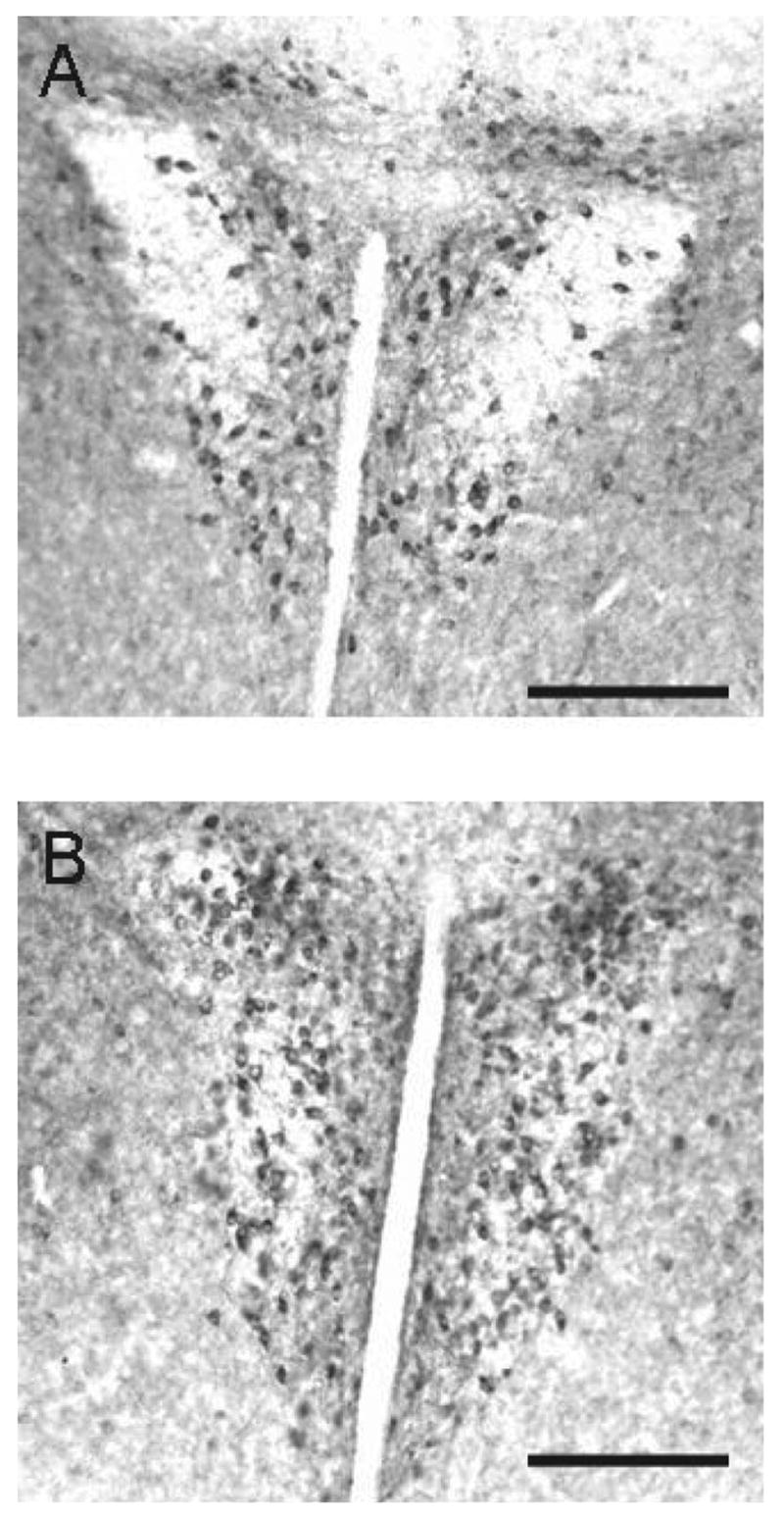
Photomicrographs of neuronal nitric oxide synthase (nNOS) immunoreactive cells in the paraventricular nucleus of singly housed (A) and pair-housed (B) wild-type mice. Scale bar = 180 μm.
Experiment 2: Effects of Neuronal Nitric Oxide Inhibition and Social Housing on Social Investigation and Aggression
In Experiment 2, we observed a significant main effect of 3BrN treatment (Figure 1B), F(1, 32) = 5.17, p < .05, and a nonsignificant trend for housing condition, F(1, 32) = 3.66, p = .06, on the number of times males interacted with the barrier in the partition test. Planned comparisons indicated that 3BrN treatment significantly reduced the number of times singly housed males interacted with the barrier and that this effect was not significant in group-housed mice. There was no significant interaction between drug treatment and housing condition in the partition test.
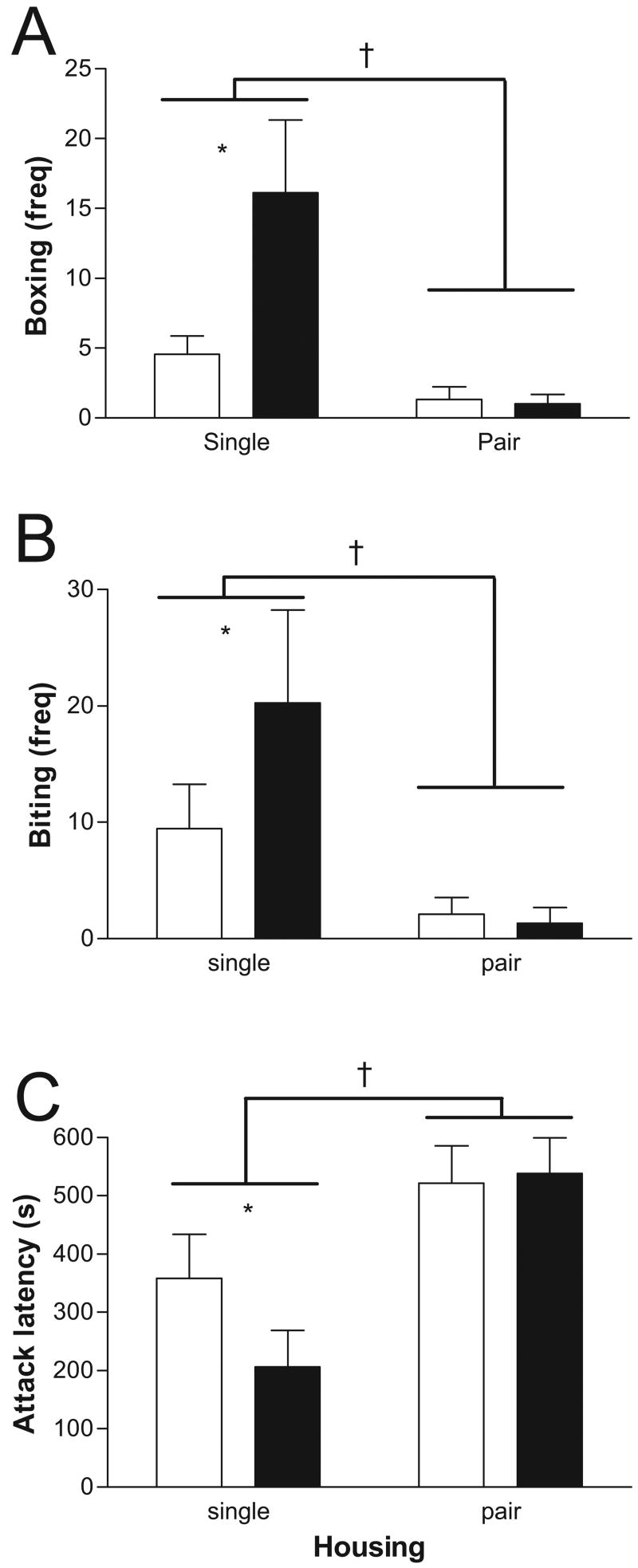
Pair housing had a robust effect on aggression, decreasing biting (Figure 4A), F(1, 31) = 9.5, p < .01, and boxing (Figure 4B), F(1, 31) = 13.1, p = .001, and increasing attack latency (Figure 4C), F(1, 31) = 13.7, p = .001, relative to singly housed males. Although there was no significant main effect of 3BrN treatment for the three aggressive behaviors measured, planned comparisons indicated that 3BrN increased biting and boxing in singly housed males (p < .05) but not in pair-housed males. Similarly, 3BrN reduced attack latency in singly housed males (p < .05) but not in pair-housed males. The interaction between drug treatment and housing condition was significant for boxing behavior, F(1, 31) = 5.5, p < .05, but not for biting or attack latency.
Figure 4.
Aggressive behavior of mice treated with oil (open bars) or 3-bromo-7-nitroindazole (3BrN; black bars). Pair-housed mice reduced biting (A) and boxing (B) and increased attack latency (C) compared with singly housed mice. 3BrN increased aggression in singly housed mice but not pair-housed mice. * p < .05, effect of 3BrN; † p < .05, effect of housing condition.
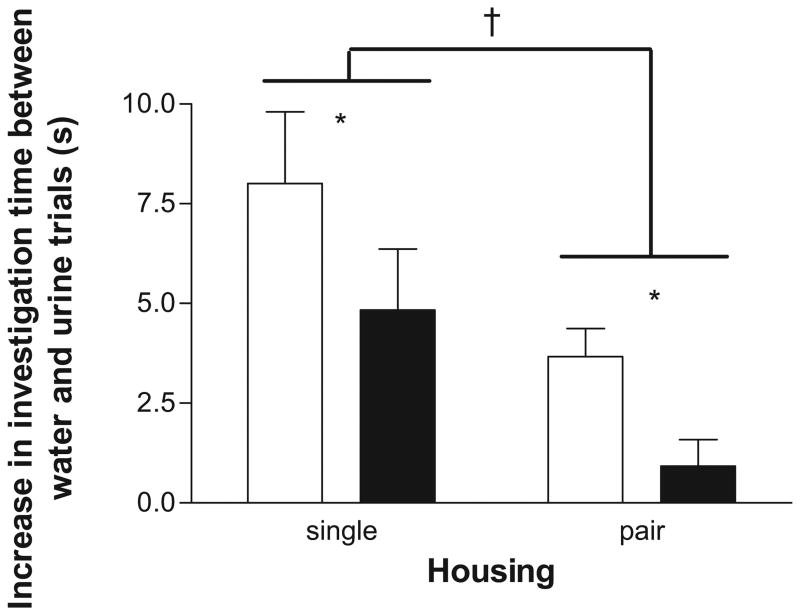
In habituation–dishabituation tests, the first presentation of male urine (Trial 4) resulted in a significant increase in investigation in singly housed oil-treated mice, paired t(9) = 5.0, p < .01, and 3BrN-treated mice, paired t(9) = 4.80, p < .01, relative to the previous water stimulus (Trial 3). In pair-housed animals, initial presentation of urine increased investigation in oil-treated mice, paired t(9) = 5.13, p < .01, but not in 3BrN-treated mice, paired t(9) = 1.36, p > .05. Difference scores (Trial 4 time − Trial 3 time) were computed for each mouse and compared to assess effects of treatment and housing conditions. Treatment with 3BrN significantly inhibited investigation of the initial urine stimulus compared oil treatment (Figure 5), F(1, 38) = 10.7, p < .01. There was also a significant main effect of housing condition (Figure 5), F(1, 38) = 18.6, p < .001, with pair-housed males showing reduced responses to the urine stimulus in comparison with singly-housed males. There was no significant interaction between drug treatment and housing condition on investigation of the initial urine stimulus.
Figure 5.
Behavior of mice treated with oil (open bars) or 3-bromo-7-nitroindazole (3BrN; black bars) in habituation–dishabituation tests. Mice were habituated to presentation of water droplets over three 2-min trials and then presented with a drop of male urine. The difference in investigation time between the third water trial and the initial presentation of male urine was analyzed to assess the motivation to investigate a socially relevant stimulus. 3BrN treatment attenuated investigation responses to initial presentation of male urine samples. Pair housing also reduced investigation responses. * p < .05, effect of 3BrN; † p < .05, effect of housing condition.
Discussion
Previous studies examining responses to social stimuli and aggression in rodents reported that a deficit in social investigation was associated with reduced aggression (Wersinger et al., 2002, 2004). Our data show that social investigation and aggression can be dissociated and that nNOS signaling differentially affects these behaviors. Singly housed mice deficient in nNOS signaling (either knockout or 3BrN-treated) showed a reduction in social investigation in two different tests. Notably, singly housed mice treated with 3BrN showed a significant increase in investigation time in response to male urine, demonstrating that nNOS deficiency does not block the ability to identify social stimuli. Instead, nNOS-deficient mice appear less motivated to explore social stimuli. Despite reduced social investigation, singly housed nNOS-deficient mice increased aggressive behavior when confronted with an intruder. We originally expected that behavior observed in the partition test would reflect increased aggressive motivation in nNOS−/− mice. However, the reduced social investigation associated with nNOS inhibition appears to mask this response. These data suggest that nNOS may play a role in mediating behavioral states in which social withdrawal is associated with heightened aggression. In general, the effects of nNOS on social investigation and aggression were reduced or absent in pair-housed mice, suggesting that the effects of nNOS on behavior is dependent on the social environment.
Recent studies have begun to focus on social investigation in rodents as a model for understanding social withdrawal components of mental disorders. Studies have demonstrated that mice of the C57B6 and FVB/NJ strains spend more time sniffing an unfamiliar mouse behind a barrier compared with mice of the BALB/c, DBA/2J, and A/J strains (Moy et al., 2004; Sankoorikal, Kaercher, Boon, Lee, & Brodkin, 2006). Although aggressive behavior has been characterized in these strains, no study has compared strains for both social investigation and aggression simultaneously. When these behaviors were measured concurrently in AVPr1b−/− mice, social investigation and aggression were positively linked (Wersinger et al., 2004). Our data indicate that this is not a universal observation; murine aggression can be expressed in the context of reduced motivation to investigate social stimuli.
Central serotonergic turnover in the hypothalamus and cerebral cortex mediates the increased aggression caused by the absence of nNOS signaling (Chiavegatto et al., 2001). However, changes in serotonergic signaling alone are not sufficient to explain the decreases in social investigation in nNOS-deficient mice because serotonin-transporter knockout mice show increased aggression but not decreased social investigation (Holmes, Murphy, & Crawley, 2002). It is unlikely that nNOS deficiency blocks the detection of social odors because singly housed mice treated with the nNOS inhibitor responded to presentations of male urine with significant increases in investigation time. We hypothesize that nNOS affects the motivation to engage social stimuli rather than the ability to detect them. Supporting this hypothesis are studies reporting that nNOS signaling increases dopamine release in the medial preoptic area (Lorrain, Matuszewich, Howard, Du, & Hull, 1996), increases sexual motivation (Lagoda, Muschamp, Vigdorchik, & Hull, 2004), and facilitates social memory (Bohme et al., 1993). Thus, deficiencies in nNOS may result in increased aggression through reduced serotonergic function and decreased social investigation through decreases in dopaminergic function.
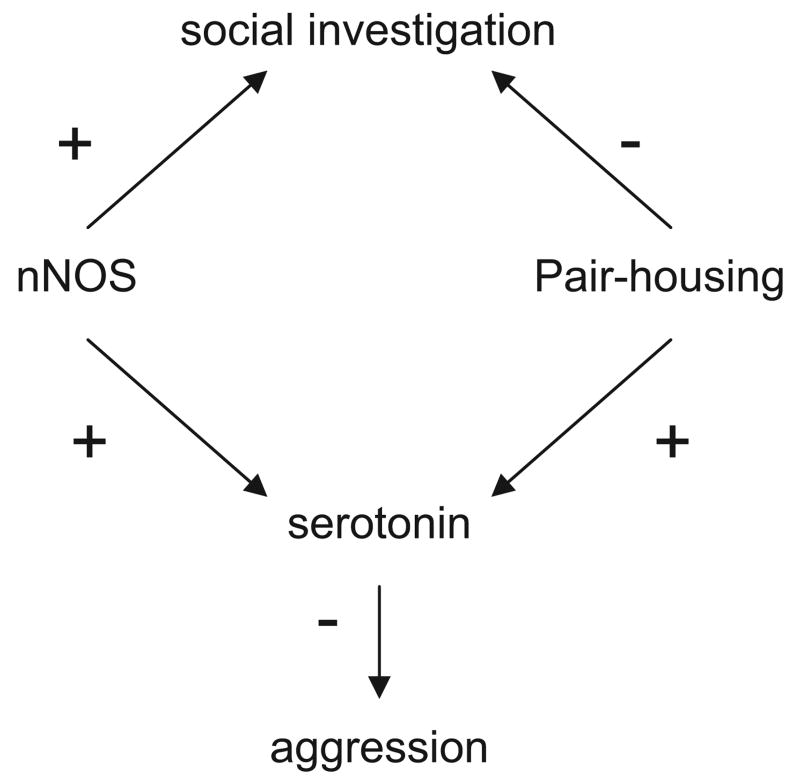
The effects of nNOS signaling on social investigation and aggression were diminished in pair-housed animals. There was no difference in the number of nNOS immunoreactive cells in several brain regions between singly-housed and pair-housed mice, suggesting that the social environment does not affect nNOS protein levels. At present, our data suggest that the effects of housing conditions on nNOS-mediated social behavior are indirect. Social isolation in rodents is associated with decreased expression of 5-hydroxytryptamine (HT)1A receptors (Rilke, Freier, Jahkel, & Oehler, 1998; Schiller, Jahkel, & Oehler, 2006) and increased expression of Vasopressin (V)1A receptors (Albers, Dean, Karom, Smith, & Huhman, 2006). Work in hamsters has demonstrated that 5-HT inhibits vasopressin-mediated aggression (Ferris et al., 1997), so pair housing might decrease aggression by directly and indirectly decreasing vasopressinergic signaling. In addition to increasing aggression, decreased vasopressin signaling would be expected to decrease social investigation, as observed in Experiment 2. We hypothesize that nNOS and social housing affect distinct, but overlapping, physiological pathways to regulate behavior (Figure 6). Serotonin appears to be a common pathway that affects aggression. For social investigation, the effects of nNOS may be mediated primarily via dopamine, whereas the effects of social housing may be mediated mainly via vasopressin.
Figure 6.
Schematic of hypothesized relationships among neuronal nitric oxide synthase (nNOS), aggression, and motivation to explore social cues. We propose that pair housing decreases social investigation by decreasing vasopressin function. A more conclusive link has been defined between pair housing and serotonin function, which we suggest mediates the effect of pair housing on aggression. Prior studies have demonstrated that the effects of nNOS deletion on aggression are mediated by reduced serotonin function. We propose that nNOS increases social investigation by increasing dopamine function.
Developmental studies have identified several cases of increased aggression paired with decreased social investigation. Male rhesus monkeys (Macaca mulatta) that are heterozygous for the short form of the serotonin transporter promoter and raised in peer-only groups (without the mother) exhibit increased social withdrawal and increased aggression compared with individuals homozygous for the long form of the promoter and raised in the same conditions (Barr et al., 2003). These differences are absent in monkeys raised by their mothers. Increased aggression and reduced social investigation was also observed when comparing behavior of domestic dogs (Canis familiaris) and gray wolves (Canis lupis) hand-raised by human caretakers. In interactions with familiar caretakers, C. lupis were more likely to avoid social contact and behave aggressively than C. familiaris (Gacsi et al., 2005). Although this study does not examine intraspecific behavior per se, recent work has demonstrated that C. familiaris are adept at using human social cues in an object choice paradigm (Hare, Brown, Williamson, & Tomasello, 2002), even when reared with minimal human contact. These results suggest that mechanisms of social behavior in Canis familiaris have been co-opted for use in interactions with humans. These studies describe a phenotype that avoids social contact but responds with increased aggression when forced into social situations. This phenotype is similar to certain forms of autism and antisocial personality disorder, in which social withdrawal is associated with increased aggression (Anckarsater, 2006). Decreased vasopressinergic activity is unlikely to explain this phenotype because most mammalian studies indicate that deficits in vasopressin decrease both social investigation (Insel & Fernald, 2004) and aggression (Ferris, 2005). Our data suggest that mice deficient for nNOS signaling will provide a useful model for studying this phenotype. Further investigation of nitric oxide signaling in these populations could provide clues to the mechanistic bases of these behavioral states.
Acknowledgments
We thank L. B. Martin II, K. J. Navara, and Z. M. Weil for helpful discussions, and K. Kassouf, J. E. West, and M. S. Finy for technical assistance. This work was supported by National Institutes of Health (NIH) Grant MH076313 to Brian C. Trainor and NIH Grant MH57535 to Randy J. Nelson.
References
- Albers HE, Dean A, Karom MC, Smith D, Huhman KL. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Research. 2006;1073–1074:425–430. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Anckarsater H. Central nervous changes in social dysfunction: Autism, aggression, and psychopathy. Brain Research Bulletin. 2006;69:259–265. doi: 10.1016/j.brainresbull.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Anderson PK, Hill JL. Mus musculus: Experimental induction of territory formation. Science. 1965;148:1753–1755. doi: 10.1126/science.148.3678.1753. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes, Brain and Behavior. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacology, Biochemistry and Behavior. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proceedings of the National Academy of Sciences USA. 1993;90:9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proceedings of the National Academy of Sciences USA. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Dong H, Murphy A, Valenzuela D, Yancopoulos G, Swanson L, Anderson D. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustaffson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proceedings of the National Academy of Sciences USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: Inhibition by anpirtoline: A 5-HT1b receptor agonist. Neuropsychopharmacology. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Demas GE, Eliasson MJ, Dawson TM, Dawson VL, Kriegsfeld LJ, Nelson LE, Snyder SH. Inhibition of neuronal nitric oxide synthase increases aggressive behavior in mice. Molecular Medicine. 1997;3:610–616. [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. Journal of Neuroscience. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF. Vasopressin/oxytocin and aggression. Novartis Foundation Symposium. 2005;268:190–198. [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: Reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Gacsi M, Gyori B, Miklosi A, Viranyi Z, Kubinyi E, Topal J, Csanyi V. Species-specific differences and similarities in the behavior of hand-raised dog and wolf pups in social situations with humans. Developmental Psychobiology. 2005;47:111–122. doi: 10.1002/dev.20082. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Olaghere-da Silva UB, Nelson RJ. 3-Bromo-7-nitroindazole, a neuronal nitric oxide synthase inhibitor, impairs maternal aggression and citrulline immunoreactivity in prairie voles. Brain Research. 2000;870:80–86. doi: 10.1016/s0006-8993(00)02404-5. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Hormones and Behavior. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. 2002;298:1634–1636. doi: 10.1126/science.1072702. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology. 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN. An experimental approach to the study of learned aggression. Aggressive Behavior. 2000;26:241–256. [Google Scholar]
- Kudryavtseva NN, Bondar NP, Alekseyenko OV. Behavioral correlates of learned aggression in male mice. Aggressive Behavior. 2000;26:386–400. [Google Scholar]
- Kudryavtseva NN, Bondar NP, Avgustinovich DF. Effects of repeated experience of aggression on the aggressive motivation and development of anxiety in male mice. Neuroscience and Behavioral Physiology. 2004;34:721–730. doi: 10.1023/b:neab.0000036013.11705.25. [DOI] [PubMed] [Google Scholar]
- Lagoda G, Muschamp JW, Vigdorchik A, Hull EM. A nitric oxide synthesis inhibitor in the medial preoptic area inhibits copulation and stimulus sensitization in male rats. Behavioral Neuroscience. 2004;118:1317–1323. doi: 10.1037/0735-7044.118.6.1317. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Matuszewich L, Howard RV, Du J, Hull EM. Nitric oxide promotes medial preoptic dopamine release during male rat copulation. NeuroReport. 1996;8:31–34. doi: 10.1097/00001756-199612200-00007. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnusson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes, Brain and Behavior. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86-23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends in Neurosciences. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC, Chiavegatto S, Demas GE. Pleiotropic contributions of nitric oxide to aggressive behavior. Neuroscience and Biobehavioral Reviews. 2006;30:346–355. doi: 10.1016/j.neubiorev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Newman S. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Annals of the New York Academy of Sciences. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. Journal of Neuroscience. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilke O, Freier D, Jahkel M, Oehler J. Dynamic alterations of serotonergic metabolism and receptors during social isolation of low-and high-active mice. Pharmacology, Biochemistry and Behavior. 1998;59:891–896. doi: 10.1016/s0091-3057(97)00509-1. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biological Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Schiller L, Jahkel M, Oehler J. The influence of sex and social isolation housing on pre- and postsynaptic 5-HT1A receptors. Brain Research. 2006;1103:76–87. doi: 10.1016/j.brainres.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nature Genetics. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Soderstrom H, Sjodin AK, Carlstedt A, Forsman A. Adult psychopathic personality with childhood-onset hyperactivity and conduct disorder: A central problem constellation in forensic psychiatry. Psychiatry Research. 2004;121:271–280. doi: 10.1016/s0165-1781(03)00270-1. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WSI. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Molecular Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O’Carroll AM, Young SI. Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Hormones and Behavior. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]