Abstract
The prolactin secretory response to subcutaneous injection of orphanin FQ/nociceptin (OFQ/N) was measured in wild-type and OFQ/N knockout mice. These injections were given with and without isoflurane anesthesia, to determine if isoflurane would affect the prolactin secretory response. OFQ/N injection significantly increased prolactin levels in males and females, regardless of genotype, with a more robust response in females. Isoflurane pretreatment did not affect prolactin levels in controls or in animals injected with OFQ/N. This is the first report that exogenously administered OFQ/N stimulates prolactin secretion in mice and that brief isoflurane exposure does not significantly affect this response.
Keywords: opiates, opioid, anesthesia, gender, stress
1. Introduction
Orphanin FQ/Nociceptin (OFQ/N) shares high sequence homology with the classic opiate peptides, especially dynorphin [11,17]. However, it does not bind to the traditional opiate receptors but binds with high affinity to its own receptor, ORL-1 [18]. ORL-1 is found in high concentrations in the hypothalamus [11], including the median eminence [13], indicating that OFQ/N is likely involved in regulating neuroendocrine processes.
Like other endogenous opiates, OFQ/N administration increased prolactin (PRL) secretion in the rat [2]. However, OFQ/N does not always produce an opiate-like effect, and there is conflicting evidence regarding its function. OFQ/N knockout mice display anxiety related behaviors and elevated corticosterone (CORT) levels [10]. Also, OFQ/N has been shown to attenuate the anxiety and fear response when given to rodents via intracerebroventricular (ICV) injection [8]. However, this was challenged by Fernandez et al. [5], who demonstrated that OFQ/N given by ICV injection to rodents at similar doses acted as an anxiogenic, not an anxiolytic. Furthermore, ICV OFQ/N increased corticosterone (CORT) levels in unstressed rats [4], indicating activation of the hypothalamic-pituitary-adrenal axis.
The stress caused by injections and handling may further complicate interpretation of the physiological functions of OFQ/N [12]. To minimize these possible complications, administration of a mild inhalant anesthesia, such as isoflurane, which is quickly eliminated from an animal via the lungs [1], may decrease the stress response associated with handling [15]. The purpose of the current study was to examine the effects of OFQ/N on circulating PRL levels in male and female OFQ/N knockout mice and to determine if pretreatment with isoflurane anesthesia affects the PRL secretory response.
2. Methods
2.1 Animals
OFQ/N knockout mice were generated as described previously [10]. Briefly, nonlitter F1 and F2 mice were intercrossed to obtain F2 and F3 mice, respectively, on a 129/Ola × C57BL/6 hybrid background. Experiments were performed using mice obtained from a breeding colony that was established at Miami University with initial breeding pairs provided by R. Reinscheid. Male and female knockout and wild-type mice (2-3 months old, 17-32g) were provided food and water ad libitum. Animals were housed 2-3 per cage under controlled temperature (21°C) and light (12h light: 12h dark). All experiments were conducted following the guidelines of the Animal Welfare Act and were approved by Miami University's Institutional Animal Care and Use Committee (IACUC).
2.2 Anesthesia and injections
Animals were placed in an anesthetic chamber and exposed to 15 sec of isoflurane anesthesia. Immediately after removing mice from the chamber, they were injected subcutaneously with 30 μg OFQ/N (Sigma, St. Louis, MO) or an equal volume of saline (0.1 ml) or they were not injected. Animals were sacrificed 10 minutes after receiving an injection and/or isoflurane exposure. Control animals remained in their home cages until sacrificed at times corresponding to those of treated animals. Basal PRL levels were determined in control animals that did not receive any treatment before sacrifice. At the time of sacrifice, trunk blood was collected and centrifuged (3000Xg) to obtain plasma. Plasma was stored at −20°C until assayed for hormone concentrations.
2.3 Radioimmunoassay
Plasma PRL concentrations were measured in duplicate samples by double antibody radioimmunoassay (RIA). Reagents for the RIA were obtained from NIDDK's National Hormone and Pituitary Program (NHPP) and Dr. A. F. Parlow. PRL was iodinated using Na125I (Perkin-Elmer, MA) as described by Greenwood and Hunter [7]. PRL levels are expressed in ng/ml and were determined using a standard curve of mouse PRL reference prep-3. The upper and lower limits for the PRL assay were 400 and 0.8 ng/ml, respectively. The intraassay coefficient of variation was 10% and the interassay coefficient of variation was 16.5% across three assays.
2.4 Statistics
A three factor ANOVA model was used to analyze the PRL levels. In order to make the variability within each sex-treatment-genotype subgroup comparable (as is required in ANOVA), the PRL levels were log transformed. Means of the transformed data were compared using t statistics. For both sexes, the Bonferroni multiple comparison technique was used to ensure that the probability of a type I error occurring anywhere in the set of comparisons for that sex was no more than 0.05.
3. Results
Subcutaneous injection of OFQ/N alone significantly increased circulating PRL levels in both female (Figure 1A) and male (Figure 1B) knockout and wild-type mice (p<0.0001), with a more robust response in females. Isoflurane pretreatment did not affect the PRL secretory response to OFQ/N. Regardless of treatment, there was no significant difference in the PRL response to OFQ/N between knockout and wild-type mice of the same sex. In addition, animals given isoflurane before saline injections did not have significantly different levels of circulating PRL than either control animals (basal) or animals injected with saline only.
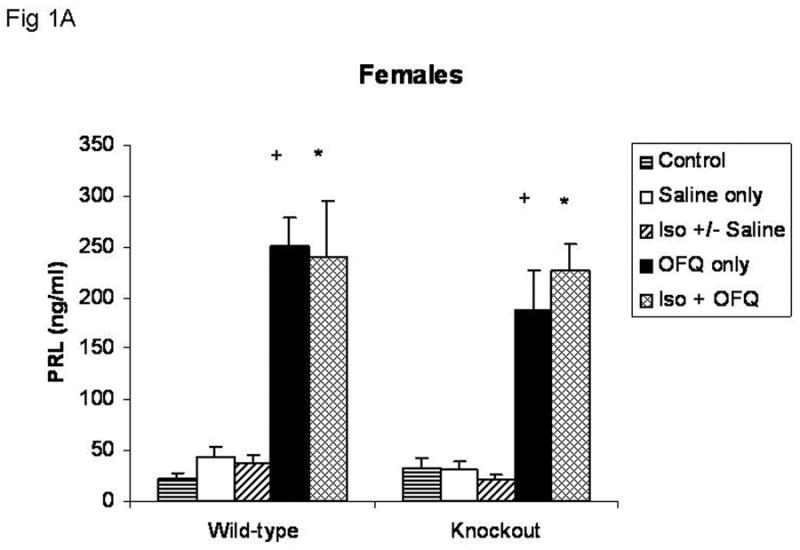
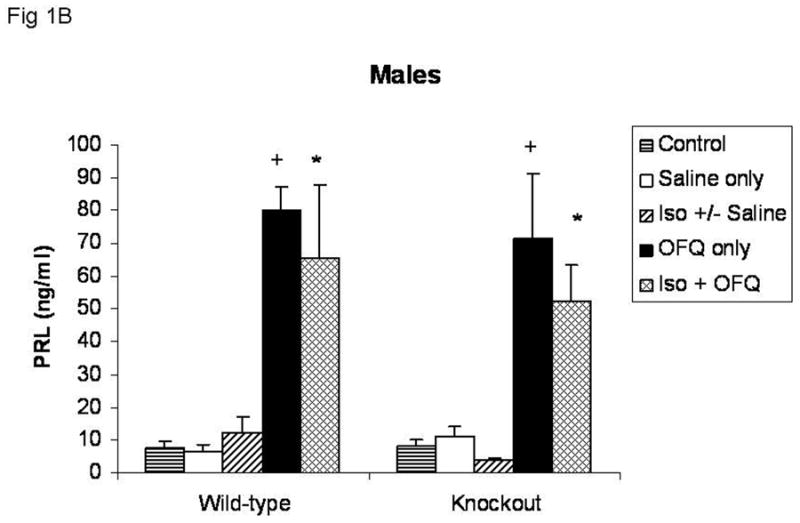
Fig. 1A – Effects of OFQ/N with and without isoflurane anesthesia pretreatment on the prolactin secretory response in female wild-type and knockout mice. Groups of animals that were not exposed to isoflurane were injected with OFQ/N (30 μg, sc in 0.1 ml saline) (OFQ only) or an equal volume of saline (0.1ml) (Saline only). Groups of animals briefly exposed to isoflurane were given the same dose of OFQ/N (Iso + OFQ), an equal volume of saline or no injection. There was no difference in the prolactin levels between animals pretreated with isoflurane that received saline or no injection, so these values were pooled (Iso +/− Saline). Control animals did not receive any treatment. Values are means ± SEM.
Control (WT=8; KO=8); Saline only (WT=8; KO=7); Iso +/− Saline (WT=15; KO=16); OFQ only (WT=7; KO=6); Iso + OFQ (WT=4; KO=6).
+ Significantly different from Saline only, within the same genotype (p<0.0001).
* Significantly different from Iso +/− Saline, within the same genotype (p < 0.0001).
Fig. 1B Effects of OFQ/N with and without isoflurane anesthesia pretreatment on the prolactin secretory response in male wild-type and knockout mice. Groups of animals that were not exposed to isoflurane were injected with OFQ/N (30 μg, scin 0.1 ml saline) (OFQ only) or an equal volume of saline (0.1ml) (Saline only). Groups of animals briefly exposed to isoflurane were given the same dose of OFQ/N (Iso + OFQ), an equal volume of saline or no injection. There was no difference in the prolactin levels between animals pretreated with isoflurane that received saline or no injection, so these values were pooled (Iso +/− Saline). Control animals did not receive any treatment. Values are means ± SEM.
Control (WT=8; KO=8); Saline only (WT=9; KO=8); Iso +/− Saline (WT=17; KO=23); OFQ only (WT=6; KO=8); Iso + OFQ (WT=6; KO=7).
+ Significantly different from Saline only, within the same genotype (p<0.0001).
* Significantly different from Iso +/− Saline (p < 0.0001), within the same genotype.
4. Discussion
This is the first report demonstrating that subcutaneous OFQ/N administration increases circulating levels of PRL in mice. In fact, to our knowledge, this is the first report that OFQ/N stimulates PRL release in mice, regardless of route of administration. Opioid peptides have previously been shown to be transported across the blood-brain barrier by specific transporters [6]. Although less than 1% of a peptide injected into the circulation crosses the blood-brain barrier, it is enough to cause physiological changes [14]. Indeed, Kastin et al. [9], demonstrated that morphine and a potent analog of Metenkephalin (injected intraperitoneally) crossed the blood-brain barrier in male rats as indicated by changes in cortical electroencephalographic readings. In our experiments, the injection itself was not sufficient to stimulate PRL release because neither wildtype nor knockout controls had elevated PRL levels following the saline injection.
Regardless of sex or genotype, no difference in resting PRL levels was detected between groups exposed to isoflurane anesthesia prior to injection and those that were not pretreated with isoflurane. Furthermore, this method of anesthesia does not appear to affect the PRL secretory response to OFQ/N. These data are in agreement with Reburn and Wynne-Edwards [15], who reported that isoflurane did not affect basal PRL secretion in dwarf hamsters. Administration of OFQ/N to OFQ/N knockout mice produced a significant increase in plasma PRL levels within 10 minutes. Because OFQ/N acts at a specific ORL1 receptor and it does not bind to other opiate receptor subtypes [16], our results indicate that the OFQ/N knockout mice have functional ORL-1 receptors, even in the absence of the peptide. In fact, OFQ/N knockout mice have been shown to upregulate ORL-1 receptors in areas of the brain such as the hypothalamus, suggesting that OFQ/N receptors are responding to the loss of OFQ/N [3].
In summary, OFQ/N injected subcutaneously produces a significant increase in circulating PRL levels in OFQ/N male and female wild-type and knockout mice, indicating that the knockout mice do not lose their sensitivity to OFQ/N stimulation. Furthermore, pretreatment with isoflurane anesthesia did not affect the PRL secretory response to OFQ/N administration, nor did it affect basal levels of PRL. Because basal levels of PRL were not affected by isoflurane, this rapidly metabolized anesthetic may be useful in minimizing any stress associated with handling when investigating PRL regulation by OFQ/N.
Acknowledgments
This work was supported by NIH grant DK061956 to PC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bienert A, Bartmann CP, von Oppen T, Poppe C, Schiemann V, Deegen E. Standing behavior in horses after inhalation anesthesia with isoflurane (Isoflo) and postanesthetic sedation with romifidine (Sedivet) or xylazine (Rompun) Dtsch Tierarztl Wochenschr. 2003;110(6):244–248. [PubMed] [Google Scholar]
- 2.Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone release in male and female rats. Brain Res. 1998;807:228–233. doi: 10.1016/s0006-8993(98)00802-6. [DOI] [PubMed] [Google Scholar]
- 3.Clarke S, Chen Z, Hsu MS, Hill RG, Pintar JE, Kitchen I. Nociceptin/Orphanin FQ Knockout Mice Display Up-Regulation of the Opioid Receptor Expression in the Brain. Neuroscience. 2003;117:157–168. doi: 10.1016/s0306-4522(02)00750-9. [DOI] [PubMed] [Google Scholar]
- 4.Devine DP, Watson SJ, Akil H. Orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic-pituitary-adrenal axis. Neuroscience. 2001;102:541–553. doi: 10.1016/s0306-4522(00)00517-0. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/Orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacol. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- 6.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- 7.Greenwood FC, Hunter WM. The preparation of 131I - labelled human growth hormone of high specific radioactivity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Jr, Nothacker HP, Civelli O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. P Natl Acad Sci USA. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastin AJ, Pearson MA, Banks WA. EEG evidence that morphine and an enkephalin analog cross the blood-brain barrier. Pharmacol Biochem Behav. 1991;40:771–774. doi: 10.1016/0091-3057(91)90084-f. [DOI] [PubMed] [Google Scholar]
- 10.Köster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. P Natl Acad Sci USA. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier JC, Mollereau LT, Suaudeau C, Moisand C, Alvinerie P, Butor J, Guillemot J, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 12.Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75(2):333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- 13.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with 125I-[14Tyr]-orphanin FQ binding. J Comp Neurol. 1999;412:563–605. [PubMed] [Google Scholar]
- 14.Pan W, Kastin AJ. Polypeptide delivery across the blood-brain barrier. Curr Drug Targets CNS Neurol Disord. 2004;3:131–136. doi: 10.2174/1568007043482525. [DOI] [PubMed] [Google Scholar]
- 15.Reburn C, Wynn-Edwards KE. Cortisol and prolactin concentrations during repeated blood sample collection from freely moving, mouse-sized mammals (Phodopus spp.) Comp Med. 2000;50(2):184–198. [PubMed] [Google Scholar]
- 16.Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273(3):1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- 17.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 18.Reinscheid RK, Nothacker HP, Civelli O. The orphanin FQ/nociceptin gene: structure, tissue distribution of expression and functional implications obtained from knockout mice. Peptides. 2000;21:901–906. doi: 10.1016/s0196-9781(00)00226-6. [DOI] [PubMed] [Google Scholar]


