Abstract
The yeast REV3 gene encodes the catalytic subunit of DNA polymerase zeta (pol ζ), a B family polymerase that performs mutagenic DNA synthesis in cells. To probe pol ζ mutagenic functions, we generated six mutator alleles of REV3 with amino acid replacements for Leu979, a highly conserved residue inferred to be at the pol ζ active site. Replacing Leu979 with Gly, Val, Asn, Lys, Met or Phe resulted in yeast strains with elevated UV-induced mutant frequencies. While four of these strains had reduced survival following UV irradiation, the rev3-L979F and rev3-L979M strains had normal survival, suggesting retention of pol ζ catalytic activity. UV mutagenesis in the rev3-L979F background was increased when photoproduct bypass by pol η was eliminated by deletion of RAD30. The rev3-L979F mutation had little to no effect on mutagenesis in an ogg1Δ background, which cannot repair 8-oxo-guanine in DNA. UV-induced can1 mutants from rev3-L979F and rad30Δrev3-L979F strains primarily contained base substitutions and complex mutations, suggesting error-prone bypass of UV photoproducts by L979F pol ζ. Spontaneous mutation rates in rev3-L979F and rev3-L979M strains are elevated by about 2-fold overall and by 2- to 8-fold for C to G transversions and complex mutations, both of which are known to be generated by wild-type pol ζ in vitro. These results indicate that Rev3p-Leu979 replacements reduce the fidelity of DNA synthesis by yeast pol ζ in vivo. In conjunction with earlier studies, the data establish that the conserved amino acid at the active site location occupied by Leu979 is critical for the fidelity of all four yeast B family polymerases. Reduced fidelity with retention of robust polymerase activity suggests that the homologous rev3-L979F allele may be useful for analyzing pol ζ functions in mammals, where REV3 deletion is lethal.
Keywords: pol ζ, translesion synthesis, mutagenesis, yeast
1. INTRODUCTION
DNA polymerase ζ(pol ζ) is one of many eukaryotic DNA polymerases [1-3], and it has a particularly important role in mutagenesis [4]. Pol ζ consists of an accessory subunit encoded by the REV7 gene and a polymerase catalytic subunit encoded by the REV3 gene [5]. Cells disrupted for REV3 have reduced levels of DNA damage-induced mutagenesis [4], thereby implicating pol ζ in mutagenic translesion DNA synthesis. REV3 has many functions [4,6,7]. It is needed for the majority of spontaneous mutagenesis [4,8-13], and it contributes to mutagenesis associated with impairment of the major replicative polymerases [11], with defective DNA repair [14-19], with double-strand break repair [20-22], with transcription [23], and with somatic hypermutation of immunoglobulin genes [24,25]. Consistent with these multiple mutagenic functions, pol ζ lacks an intrinsic proofreading exonuclease [5] and it is promiscuous in extending mismatched primer termini [26-29]. Consistent with its role in mutagenic synthesis in vivo, purified yeast pol ζ synthesizes DNA in vitro with lower fidelity than the major replicative polymerases, pol α, pol δ and pol ε[30].
As a member of the B family of DNA polymerases, pol ζ shares homology with pol α, pol δ and pol ε, as well as with enzymes that replicate bacteriophage genomes, such as T4 pol and RB69 pol. Residues in motifs A, B and C that form the polymerase active site are particularly conserved. In the crystal structure of RB69 pol [31], an invariant tyrosine in motif A interacts with the ribose of the incoming dNTP. Adjacent to this tyrosine is a hydrophobic residue, methionine in pol ε or leucine in other Family B members, e.g., Leu979 in pol ζ. In the major replicative polymerases, replacing this residue with certain other amino acids reduces DNA synthesis fidelity in vitro [32-36], due to increased dNTP misinsertion efficiency, increased mismatch extension efficiency and/or decreased proofreading efficiency [see also 37,38]. Consistent with these biochemical properties, strains encoding these mutant polymerases have elevated spontaneous mutation rates that likely reflect reduced DNA replication fidelity in vivo [32-37,39,40]. Based on these studies of other Family B polymerases, we hypothesized that Leu979 in Rev3p may have an important role in determining the fidelity of DNA synthesis by pol ζ. To test this, and ultimately to obtain a more thorough understanding of multiple pol ζ functions in mutagenic DNA transactions in vivo, here we have undertaken a search for amino acid replacements in Rev3p that are likely to retain pol ζ activity yet result in elevated spontaneous and damage-induced mutagenesis in yeast. This approach to study rev3 mutator alleles is intended to add to studies in which pol ζ function has been inferred from rev3 disruption, a strategy has been highly informative in yeast [4] but less informative in mice because rev3 disruption results in embryonic lethality [41-43].
2. MATERIALS AND METHODS
2.1. Media and growth conditions
Yeast strains were grown nonselectively on YPDA media (1% yeast extract, 2% Bacto-peptone, 2% dextrose, 10 mg/L adenine). Synthetic, selective media contained 2% dextrose and appropriate amino acids. Canavanine resistant mutants were identified by growth on synthetic media supplemented with 60 ug/mL canavanine. All growth was at 30°.
2.2. Construction of yeast strains
All strains used in this study were derived from haploid 8C-YUNI101 (MATa his7-2 leu2-3,112 ura3Δbik1::ura3-29RL trp1-1UAG ade 2-1UAA) [44]. The rev3::LEU2 mutant was described previously [44]. All other mutants were constructed by standard one-step (OST) or two-step transplacement (TST) and all strains were verified by PCR and/or sequencing. RAD30 and OGG1 deletions were generated via OST with PCR fragments amplified from pFA6-kanMX4 [45] using primers disRAD30-L and disRAD30-R, or disOGG1-L and disOGG1-R, respectively (for primer sequences see Supplementary Table 1). rev3-L979X alleles were created by site-directed mutagenesis of pREV3Cav2 [44] using the QuickChange Kit (Stratagene). rev3-L979X yeast strains were made by TST with SnaBI-cut mutagenized derivatives of pREV3Cav2. Each of the rev3-L979X yeast strains were verified by sequencing using primers designated REV3seq in Supplementary Table 1.
2.3. Measurements of mutation rates
Spontaneous mutation rates were determined by the method of median [46], using at least 22 independent cultures per experiment. Ultraviolet light (UV)-induced mutation frequencies were measured using 2-4 independent cultures. Cultures were grown to saturation in YPDA liquid, then diluted appropriately and plated onto synthetic media supplemented with canavanine and non-selective synthetic media. Plates were immediately exposed to UVC at the indicated dose and were then incubated at 30°C in the dark. Mutation frequencies were calculated as the average of the number of colonies on canavanine plates divided by the number of colonies on non-selective plates multiplied by the dilution factor.
2.4. Sequence analysis of can1 mutants
UV-induced can1 mutations were isolated following exposure to 20 J/m2 under the conditions described above. At least 24 canavanine resistant isolates were sequenced per strain. A 2.0 kb region covering the 3′ end of CAN1 gene was amplified with primers can1-F and can1-R (Supplementary Table 1). The 5′ end of CAN1 gene was amplified using primers can1-F2 and can1-R (Supplementary Table 1). The PCR fragments were subsequently sequenced on both strands using sequencing primers can1-SF1 to can1-SR3 (Supplementary Table 1).
2.5. Statistical analysis
We first estimated the number of can1 mutants expected to be due to an individual type of mutation in each strain by multiplying the proportion of that mutation observed in the mutation spectrum (Table 1) by the total number of can1 survivors obtained after irradiation with 20 J/m2 UV. We then estimated mutation frequencies of individual mutation types as the estimated number of can1 mutants of each type divided by the total number of can1 survivors. The mutation frequencies of an individual type in each strain were then compared with the wild-type strain using an independent samples t-test for equality of proportions. The variance of this test was adjusted to reflect the number of mutants sequenced. A complete explanation of the two independent samples test for equality of mutation frequencies can be found in the Supplementary Information.
Table 1.
UV-induced mutations in wild-type, rad30 and rev3 yeast strains.
| rad30Δ | ||||||||
|---|---|---|---|---|---|---|---|---|
| wild-type |
rev3-L979F |
rad30Δ |
rev3-L979F |
|||||
| Freq. (×10−6) |
88 [59 - 120] |
200 [50 - 350] |
200 [170 - 230] |
540 [470 - 590] |
||||
| Events | Freq. | Events | Freq. | Events | Freq. | Events | Freq. | |
| A-T to G-C | 4 | 14 | 0 | 0 | 2 | 16 | 0 | 0 |
| G-C to A-T | 8 | 28 | 6 | 48 | 12 | 92c | 10 | 220c |
| A-T to T-A | 6 | 21 | 2 | 16 | 4 | 32 | 2 | 43 |
| A-T to C-G | 0 | 0 | 0 | 0 | 1 | 8 | 0 | 0 |
| G-C to T-A | 1 | 4 | 2 | 16 | 2 | 16 | 4 | 86c |
| Tandem bps | 1 | 4 | 3 | 24 | 0 | 0 | 4 | 86c |
| Indelsa | 3 | 11 | 4 | 32 | 0 | 0 | 2 | 43 |
| Complexb | 2 | 7 | 8 | 64c | 5 | 48 | 3 | 65 |
Cells were exposed to 20 J/m2 of UVC.
Freq. represents mutation frequency. All mutation frequencies are (×10−6). Numbers in brackets represent one standard deviation.
One and two-base insertions and deletions were categorized as indels.
Multiple, closely spaced base substitutions and/or indels were categorized as complex mutations. One of the can1 isolates from the rad30Δ strain contained three single base changes within 30 base-pairs.
Significant difference (p < 0.05) from the REV3 strain. P-values were determined as described in the materials and methods.
3. RESULTS
3.1. Choice of rev3 missense alleles to investigate
Mutator phenotypes had been previously observed for yeast strains containing the following replacements for homologous residues in the three major yeast replicative DNA polymerases: Val, Lys and Met replacements for Leu868 in pol α [32,33], Val, Lys, Met, Phe, Gly and Asn replacements for Leu612 in pol δ [34,39,40] and Phe replacement for Met644 in pol ε [35]. Here we chose to test whether the same replacements for Rev3p-Leu979 might affect the accuracy of DNA synthesis by yeast pol ζ and thereby confer mutator phenotypes characteristic of mutagenic DNA synthesis activity by pol ζ in vivo. Thus, we began this study by constructing yeast strains with Gly, Val, Asn, Lys, Met or Phe replacements for Rev3p-Leu979.
3.2. UV-induced killing and mutagenesis
3.2.1. UV sensitivity and mutagenesis of the six rev3-L979X strains
Because pol ζ has a well-established role in UV-induced survival and mutagenesis, we first examined whether the rev3 missense mutations affected these two parameters. As expected, the rev3Δstrain was more sensitive to UV irradiation than was wild-type yeast (Fig. 1A). The rev3-L979K, rev3-L979G, rev3-L979N and rev3-L979V strains all had intermediate sensitivity, suggesting partial loss of pol ζ activity. In contrast, the rev3-L979F and rev3-L979M strains had wild-type sensitivity to UV irradiation, suggesting greater retention of pol ζ catalytic activity. When UV-induced mutant frequencies at the CAN1 locus were measured, the rev3Δ strain was immutable (Fig. 1B). In contrast, all strains with Rev3p-Leu979 replacements were more UV mutable than wild-type yeast (Fig. 1B), suggesting that these replacements reduce the fidelity of pol ζ as it participates in translesion DNA synthesis (TLS) of UV photoproducts.
Figure 1. UV-induced killing and mutagenesis in yeast strains harboring rev3-L979X alleles.
A. Survival following UV irradiation. B. UV-induced mutagenesis at the CAN1 locus. C. Survival following UV irradiation in rad30 Δstrains. D. UV-induced mutagenesis at the CAN1 locus in rad30 Δ strains. Error bars represent one standard deviation.
3.2.2. UV sensitivity and mutagenesis in the rad30Δrev3-L979F double mutant strain
In wild-type yeast, the majority of UV photoproduct bypass is conducted by pol η, the product of the RAD30 gene. However, in a rad30Δ strain, pol ζ has been implicated in mutagenic bypass [47,48]. Therefore, we next examined UV-induced sensitivity and mutagenesis in a rad30Δrev3-L979F double mutant strain. As expected based on earlier studies [47,49,50], deletion of RAD30 (rad30Δ) slightly reduces survival (Fig. 1C). However, the rad30Δrev3-L979F double mutant strain survives exposure to UV radiation at levels comparable to those for the rad30Δ single mutant (Fig. 1C). This again suggests that L979F pol ζ retains high pol ζ catalytic activity. In addition, the rad30Δrev3-L979F double mutant strain is several-fold more UV mutable than are the single mutant rev3-L979F or rad30Δ strains, and it is much more mutable than the single rev3Δ mutant or the double rad30Δ revΔ mutant strain (Fig. 1B and 1D). These results are consistent with participation of L979F pol ζ in mutagenic TLS of UV photoproducts when pol η is absent.
3.2.3. Specificity of UV-induced mutations in rev3-L979F mutant strains
In order to determine if the spectrum of mutations resulting from in vivo TLS by L979F pol ζ is similar to that of wild-type pol ζ, we determined the types of UV-induced mutations that arose at CAN1 in wild-type and rev3-L979F strains. We also sequenced can1 isolates from isogenic rad30Δ and rev3-L979F rad30Δ strains, which lack TLS by pol η. Yeast cells were irradiated with 20 J/m2 of UVC, a dose that induces mutants in all four strains examined at frequencies that are much higher than the spontaneous frequencies (Fig. 1 and see below).
The UV-induced mutation spectrum in the wild-type strain contained primarily single base substitutions (76%), all of which occurred at dipyrimidine sequences. Of these, eight are consistent with misincorporation opposite the 3′ base of a photodimer and two are consistent with misincorporation opposite the 5′ base of a photodimer. An additional nine single base substitutions arose at tripyrimidine sequences and are thus ambiguous with respect to site of the misincorporation. Among transitions, G-C to A-T substitutions predominated, and among transversions, A-T to T-A changes predominated (Table 1). This specificity is consistent with earlier studies [see also 49 and references therein], and suggests that UV light induces mutations predominantly via C•A and T•T mispairs that preferentially arise at the 3′ base of photodimers. Additionally, the frequencies of UV-induced insertion/deletion (indel) and complex mutations are at least 100-fold higher than the spontaneous mutation rates for these events (compare Tables 1 and 2), suggesting that these mutations also result from mutagenic bypass of UV photoproducts.
Table 2.
Spontaneous mutations in wild-type and rev3 yeast strains.
| wild-type |
rev3-L979F |
rev3-L979M |
rev3Δ |
|||||
|---|---|---|---|---|---|---|---|---|
| Rate (× 10−8) |
26 [23 – 34] |
42 [32 – 60] |
46 [39 – 88] |
20 [11-24] |
||||
| Events | Rate | Events | Rate | Events | Rate | Events | Rate | |
| A-T to G-C | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 2 |
| G-C to A-T | 6 | 6 | 8 | 13 | 5 | 10 | 10 | 8 |
| A-T to T-A | 3 | 3 | 0 | ≤2 | 2 | 4 | 1 | 1 |
| A-T to C-G | 2 | 2 | 0 | ≤2 | 1 | 2 | 1 | 1 |
| G-C to T-A | 5 | 5 | 6 | 10 | 2 | 4 | 1 | 1 |
| G-C to C-G | 1 | 1 | 5 | 8 | 3 | 6 | 3 | 2 |
| Tandem bps | 1 | 1 | 0 | ≤2 | 1 | 2 | 0 | ≤1 |
| Indelsa | 5 | 5 | 0 | ≤2 | 8 | 15 | 5 | 4 |
| Duplications | 1 | 1 | 1 | 2 | 0 | ≤2 | 2 | 2 |
| Complexb | 0 | ≤1 | 4 | 7 | 1 | 2 | 0 | ≤1 |
All rates are (× 10−8). Numbers in brackets are 95% confidence intervals.
One to twelve-base insertions and deletions were categorized as indels.
Multiple, closely spaced base substitutions and/or indels were categorized as complex mutations.
The UV-induced mutation spectrum of the rev3-L979F single mutant strain (Table 1 and Fig. 2) differed from that of wild-type yeast in several ways. Foremost was the presence of eight complex mutations (32% of can1 mutations; for sequences see Supplementary Table 2), which contain multiple, closely spaced base substitutions and/or indel mutations. The frequency of complex mutations was significantly higher in the rev3-L979F mutant compared to the wild-type strain (9-fold, p = 0.0046). This increase is consistent with mutagenic TLS of UV photoproducts by L979F pol ζ that is less accurate than TLS by wild-type pol ζ. The frequencies of tandem double base substitutions and G-C to T-A transversions were also elevated 5-7 fold in the rev3-L979F mutant. Because only 25 clones were sequenced, the number of each type of event is small, such that these increases are not statistically significant. All seven of single base substitutions from the rev3-L979F mutant strain that occurred at dipyrimidines are consistent with misincorporation opposite the 3′ base of the dipyrimidine. This specificity suggests that although L979F pol ζ is less accurate than wild-type pol ζ, it participates in a bypass reaction that retains the preference for misincorporation opposite the 3′ base of a photodimer.
Figure 2. Relative frequencies of UV-induced mutagenesis for various classes of mutations.
White, hatched, grey and solid bars correspond to wild-type, rev3-L979F, rad30 Δ and rev3-L979F rad30 Δ strains, respectively. Mutation frequencies (and one standard deviation) for each class were calculated by multiplying the proportion of the specific type of event in the corresponding mutation spectrum (Table 1) by the total mutation frequency. These data were collected after a dose of 20 J/m2. As the data from the rad30 Δ mutant were more variable than those from the other strains, the error bars for the rad30 Δ mutant are comparatively large.
The rad30Δ single mutant (Table 1 and Fig. 2) had a significantly elevated frequency of UV-induced G-C to A-T transitions compared to the wild-type strain (3-fold, p = 0.019). Also slightly elevated were G-C to T-A transversions (4-fold) and complex mutations containing multiple, closely-spaced nucleotide changes (7-fold). Twenty of 21 single base substitutions from the rad30Δ strain occurred at dipyrimidine or tripyrimidine sequences. Of these, 11 are consistent with misincorporation opposite the 3′ base of a photodimer, two are consistent with misincorporation opposite the 5′ base of a photodimer and seven are ambiguous, as they occurred at tripyrimidine sites. Since pol ζ is present in the rad30Δ single mutant strain, these data are consistent with a contribution of pol ζ to mutagenic TLS of photoproducts that yields single base mispairs preferentially located at the 3′ base of photodimers, as well as complex errors (Table 1, Fig. 2). The complex mutations observed when pol ζ is present and pol η is absent are reminiscent of those generated by purified yeast pol ζ as it copies undamaged DNA in vitro [30].
The UV-induced mutation spectrum of the rad30Δ rev3-L979F double mutant showed combined characteristics of the spectra for the rad30Δ and rev3-L979F single mutant strains (Table 1, Fig. 2). The frequencies of three types of UV-induced mutations were significantly elevated in the rad30Δ rev3-L979F double mutant compared than those of wild-type: G-C to A-T transitions (8-fold, p = 0.009), G-C to T-A transversions (26-fold, p = 0.04), and tandem, double base substitutions (26-fold, p = 0.04). UV-induced complex mutations were also elevated 9-fold, although this elevation was not statistically significant. Of the sixteen base substitution errors in the rad30Δ rev3-L979F double mutant, eleven occurred at dipyrimidines and four at tripyrimidines. All of the mutations that occurred at dipyrimidines are consistent with misincorporation opposite the 3′ base, again implicating misinsertions opposite photodimers as the mutagenic event in rev3-L979F strains. This suggests that the lesions responsible for most of these substitutions in the double mutant strain are photodimers that are normally copied by pol η, such that in its absence (rad30Δ), L979F pol ζ participates in mutagenic TLS. The situation may be slightly different for indels and complex mutations, whose mutant frequencies are similar in the rad30Δ rev3-L979F double mutant and the rev3-L979F single mutant (Table 1, Fig. 2).
3.3. Minimal effect of the rev3-L979F allele on ogg1-dependent mutagenesis
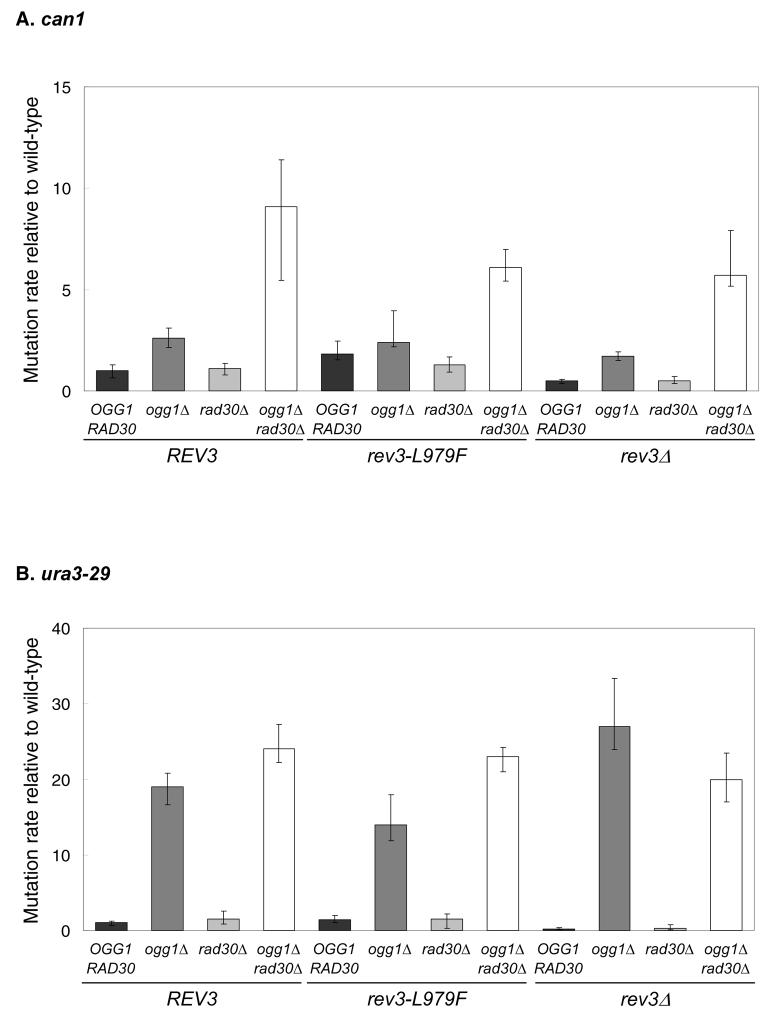
To determine if the rev3-L979F allele has an effect on mutagenesis involving a spontaneously arising oxidative lesion, we examined mutation rates in ogg1Δ mutants, which are deficient for the glycosylase that removes 8-oxo-guanine from DNA. In REV3 strains, ogg1Δ results in an elevated mutation rate at CAN1, and this rate is further elevated in an ogg1Δ rad30Δ double mutant (Fig. 3A). These results are consistent with earlier studies that revealed the roles of Ogg1 and pol η in preventing G-C to T-A substitutions resulting from spontaneous oxidative stress [51,52]. Interestingly, rates are not further elevated in ogg1Δ or ogg1Δ rad30Δ mutant strains that harbor the rev3-L979F allele (Fig. 3A). Similar results were obtained for reversion of the ura3-29 reporter (Fig. 3B), which in an ogg1Δ background is a highly sensitive marker for G-C to T-A transversions [53]. These results imply that, unlike the situation for UV photoproducts, L979F pol ζ has little if any role in mutagenic bypass of spontaneously arising 8-oxo-guanine in DNA in vivo. This is consistent with an earlier study indicating that wild-type pol ζ does not have a major role in mutagenesis in an ogg1Δ strain [51].
Figure 3. Spontaneous mutation rates in ogg1, rad30 and rev3 single, double and triple mutant strains.
Mutation rates were measured for (A) the can1 forward and (b) ura3-29 reversion reporters. Mutation rates shown were normalized relative to the values observed in the wild-type strain, which were 1.72 × 10−7 (95% confidence limits: 1.10 – 2.19 × 10−7) for can1 and 6.57 × 10−9(95% confidence limits: 4.34 – 8.04 × 10−9) for ura3-29. Error bars represent 95% confidence limits.
3.4. Spontaneous mutation rates in rev3-L979F and rev3-L979M strains
In the above experiments, the spontaneous mutation rate at CAN1 for the rev3-L979F strain (3.1 × 10−7, 95% CI = 2.6 – 4.2) was 1.8-fold higher than for the wild-type strain (1.7 × 10−7, 95% CI = 1.1 – 2.2). In a second experiment, the rev3-L979F and rev3-L979M strains had spontaneous mutation rates that were 1.6-fold and 1.8-fold higher, respectively, than in a wild-type strain (Table 2), and 2.1-fold and 2.3-fold higher, respectively, than in the rev3Δ strain. Thus, the rev3-L979F and rev3-L979M alleles confer a weak spontaneous mutator phenotype to yeast. To determine if this effect was specific to any particular types of mutations, independent can1 mutants were sequenced. The results (Table 2) indicate that the rates of G-C to C-G transversions and of complex mutations involving multiple base changes in the rev3-L979F and rev3-L979M strains were higher than in the wild-type or rev3Δ strains. The rev3-L979M strain also had a higher rate of indels, all but one of which were single base deletions, and all of which occurred in non-iterated sequences or sequences with only two repeat units.
4. DISCUSSION
Our current understanding of the cellular functions of DNA polymerase ζ largely derives from comparing the phenotypes of normal cells to those in which the REV3 gene is disrupted or its expression is reduced. These approaches typically reduce both spontaneous and DNA damage-induced mutagenesis, indicating that DNA polymerase ζ contributes to mutagenic DNA synthesis in cells. Here we have taken a different and complementary approach by demonstrating that yeast cells expressing REV3 derivatives with amino acid replacements in the pol ζ active site have mutator phenotypes. The results are informative when compared to studies of other Family B polymerases, and also regarding the role of pol ζ in mutagenic bypass of UV photoproducts and in spontaneous mutagenesis.
4.1. Inferred activity of pol ζ L979X mutants
The reduced survival of rev3-L979V, rev3-L979N, rev3-L979G and rev3-L979K mutant strains following UV irradiation (Fig. 1A) suggests that pol ζ harboring any of these four amino acid replacements is less active than wild-type pol ζ. The inferred reduction in polymerase activity resulting from these replacements is consistent with the observation that yeast strains containing homologous L612N, L612G and L612K replacements in pol δ [40] progress more slowly through S phase and have increased sensitivity to DNA damaging agents. Nonetheless, the rev3-L979V, rev3-L979N, rev3-L979G and rev3-L979K mutant strains apparently retain some pol ζ activity, because their survival (Fig. 1A) and mutant frequencies (Fig. 1B) following UV irradiation are greater than those of a rev3Δstrain. An even higher level of pol ζ activity is apparently retained by the rev3-L979F and rev3-L979M strains, since their survival remains relatively normal following UV radiation (Fig. 1A), even when pol η-dependant bypass of UV-induced cyclobutane pyrimidine dimers is eliminated by deletion of RAD30 (Fig. 1C). Retention of robust polymerase activity by pol ζL979F and L979Mmutants is consistent with previous studies showing nearly wild-type polymerase activity in vitro for pol α L868F and L868M mutants [32,33], a pol δ L612M mutant [34] and a pol ε M644F mutant [35].
4.2. Mutator effect of pol ζL979X mutants compared to other Family B polymerases
All six amino acid replacements for Leu979 in yeast pol ζ result in increased UV-induced mutagenesis (Fig 1B) and the L979F and L979M replacements increase spontaneous mutagenesis (Table 2). Spontaneous mutator phenotypes have also been reported in yeast strains with similar replacements for the homologous amino acids in active site motif A of yeast pol α Leu868 [32,33], pol δ Leu612 [34,37,40]), and pol ε Met644 [35,36]. Those studies further revealed reduced DNA synthesis fidelity by the mutant polymerases, leading us to infer that this is likely to be the case for the pol ζ mutants studied here. If so, the identity of the side chain at this location is a determinant of the fidelity of DNA synthesis in vivo by each of the four eukaryotic Family B DNA polymerases. In the crystal structure of prokaryotic Family B member RB69 pol [31], Leu415 at this position interacts with an adjacent, invariant tyrosine (Tyr416), which in turn interacts with the ribose of the incoming dNTP. Given this location, amino acid replacements for Leu979 in pol ζ, or for homologous residues in pols α, δ and ε, may alter the geometry of the nascent base pair binding pocket and/or the chemistry of the reaction. This in turn may reduce selectivity against incorrect dNTP insertion and/or increase the probability of extending mismatched termini. Indeed, both parameters are affected by replacements for Leu868 in yeast pol α [32,33], L614 in yeast pol δ [34] and Met644 in yeast pol ε [35,36]. Attempts are currently underway to express and purify yeast pol ζ with the L979F replacement, in order to examine its properties in comparison to the wild-type pol ζ.
4.3. Mutagenic bypass of UV photoproducts by pol ζ L979X mutants
The spontaneous mutator phenotypes of the pol α, δ and ε mutant strains reported earlier revealed the importance of this conserved residue in the fidelity of replication of undamaged DNA. The UV-induced mutator phenotype of the pol ζ L979X mutants (Fig. 1) extends this by indicating that Leu979 is a determinant of the fidelity of pol ζ dependent translesion DNA synthesis in vivo. The mutational specificity data imply that the majority of the lesions responsible for UV-induced mutagenesis are dipyrimidine photoproducts. In the wild-type and rev3-L979F strains, where cyclobutane pyrimidine dimers (CPDs) can be efficiently bypassed by pol η, the mutagenic lesions may primarily be 6-4 photoproducts. In the rad30 Δ and rad30 Δ rev3-L979F strains, which lack pol η, a greater contribution of CPDs to pol ζ-dependent mutagenesis seems likely. In all four strains, there is a bias for UV-induced base substitutions at the 3′ base of dipyrimidines. Given the increased frequency of UV-induced base substitutions in rev3-L979F strains (Table 1, Fig. 2) compared to REV3 strains, synthesis by L979F pol ζ is inferred to contribute to mutagensis opposite the 3′ base during photodimer bypass in vivo. Whether this reflects L979F pol ζ misinsertions opposite the damaged bases, extension of mismatched primers, or both, awaits biochemical analysis. However, this in vivo specificity is consistent with kinetic data showing that wild-type yeast pol ζ preferentially misinserts nucleotides opposite the 3′ T as compared to the 5′ T of a cis-syn cyclobutane TT dimer [28,54]. This same bias is shared by human pol η [55] and may reflect differences in base-base hydrogen bonding potential for the 3′ versus 5′ base of a photodimer bound in the polymerase active site, as has been observed for Sulfolobus sulfataricus Dpo4 [56].
Also of note are the higher frequencies of UV-induced tandem double base substitutions in the rev3-L979F and rad30 Δ rev3-L979F mutant strains in comparison to their REV3 counterparts (Fig. 2). None of these were a CC to TT change that could have resulted from “correct” insertion of two dAMPs opposite uracils created by deamination of cytosine in photodimers. Thus, the L979F replacement renders pol ζ more capable of contributing to mutagenesis that requires an initial misinsertion, a second misinsertion from a mismatched primer terminus, and extension of the resulting doubly mismatched terminus. This remarkable promiscuity extends to an even more diverse array of complex UV-induced mutations containing two or more substitutions, deletions and/or insertions (Table 1, Fig 2). These mutations, which are more frequent in the rev3-L979F strains (Table 1, Fig. 2), require formation and extension of aberrant primer-templates having multiple mismatches of different types. The results presented in Table 1 are consistent with the possibility that pol ζ may contribute to the UV-induced triplet mutation formation described in a recent report [57].
4.4. Contribution of pol ζ L979F/M mutants to spontaneous mutagenesis
The remarkable promiscuity of L979F pol ζ revealed in the UV-induced mutation spectra is also apparent during spontaneous mutagenesis, in the form of an increased rate of complex mutations (Table 2). These complex events could result from mutagenic bypass of endogenously arising lesions in DNA. Supporting this possibility is a previous study showing that wild-type pol ζ contributes to formation of spontaneous, complex mutations in a yeast strain defective in nucleotide excision repair [9]. That study provided evidence that the responsible lesion was a modified guanine resulting from spontaneous oxidative stress. This is particularly interesting in relation to our observation that the rev3-L979F allele does not contribute strongly to mutagenesis in ogg1 Δ strains (Fig. 3). This implies that, unlike the situation for UV photoproducts, L979F pol ζ may have little if any role in mutagenic bypass of spontaneously arising 8-oxo-guanine in DNA in vivo. This does not exclude a role for L979F pol ζ in bypass of other oxidative lesions, e.g., those that may be subject to nucleotide excision repair [9] and/or toxic oxidation products of 8-oxo-guanuine [58]. Indeed, some secondary oxidation products of 8-oxo-guanuine result in GC to C-G transversions [59], which is interesting because G-C to C-G transversions are elevated in rev3-L979F and rev3-L979M strains (Table 2).
An additional, nonexclusive possibility is that some of the spontaneous complex mutations and G-C to C-G substitutions seen in the rev3-L979F and rev3-L979M strains result from L979F/M pol ζ-dependent copying of undamaged DNA. This possibility is consistent with the error specificity of wild-type yeast pol ζ as it copies an undamaged DNA template in vitro [30]. Compared to the major replicative DNA polymerases, wild type pol ζ has lower fidelity, yet it can perform processive DNA synthesis [60]. Interestingly, wild type pol ζ generates lacZ mutants in vitro [30] and L979F pol ζ generates can1 mutants in vivo that contain multiple sequence changes separated by several correct base pairs (Table 2; including a mutant with three substitutions in a stretch of 18 base pairs). This combination of inaccurate but processive synthesis is shared only by DNA polymerase κ [61], making these two TLS polymerases prime candidates for generating mutational “showers” in genomes [62]. Such showers of multiple, dispersed sequence changes within a single mutant clone have important implications for the evolutionary fitness of organisms and for the origins of diseases [63].
4.5. Utility of pol ζ mutator alleles
As mentioned in the Introduction, inactivation of the REV3 gene in mice is lethal [41-43,64]. Thus, the present search for mutator alleles of yeast pol ζ that retain robust pol ζ catalytic activity was partly motivated by the desire to study the function of mammalian pol ζ using homologous mouse rev3 missense mutants that are predicted to be viable. The genetic results presented here suggest that mutations equivalent to yeast rev3-L979F and rev3-L979M may be ideal, in that polymerases with these changes appear to retain high polymerase activity but they appear to have lower fidelity. If the equivalent mouse pol ζ mutants are viable and confer mutator effects, then given the multiple functions of pol ζ mentioned in the Introduction, it will be interesting to determine if mice harboring these rev3 alleles have altered sensitivity to environmental stress, altered rates of spontaneous and/or DNA damage induced mutagenesis, altered susceptibility to environmental diseases (e.g., cancer), or altered somatic hypermutation of immunoglobulin genes.
Supplementary Material
Acknowledgements
We thank Marilyn Diaz and Stephanie Nick McElhinny for thoughtful suggestions on this manuscript. This work was supported in part by the Intramural Research Program of the NIH, NIEHS (to TAK) and in part by the Japan Atomic Energy Agency (to ANS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bebenek K, Kunkel TA. Functions of DNA polymerases. Adv Protein Chem. 2004;69:137–165. doi: 10.1016/S0065-3233(04)69005-X. [DOI] [PubMed] [Google Scholar]
- 2.Hubscher U, Maga G, Spadari S. Eukaryotic DNA polymerases. Annu Rev Biochem. 2002;71:133–163. doi: 10.1146/annurev.biochem.71.090501.150041. [DOI] [PubMed] [Google Scholar]
- 3.Shcherbakova PV, Bebenek K, Kunkel TA. Functions of eukaryotic DNA polymerases. Sci Aging Knowledge Environ 2003. 2003:RE3. doi: 10.1126/sageke.2003.8.re3. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence CW. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv Protein Chem. 2004;69:167–203. doi: 10.1016/S0065-3233(04)69006-1. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JR, Lawrence CW, Hinkle DC. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann AR. Translesion synthesis in mammalian cells. Exp Cell Res. 2006;312:2673–2676. doi: 10.1016/j.yexcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Okada T, Sonoda E, Yoshimura M, Kawano Y, Saya H, Kohzaki M, Takeda S. Multiple roles of vertebrate REV genes in DNA repair and recombination. Mol Cell Biol. 2005;25:6103–6111. doi: 10.1128/MCB.25.14.6103-6111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassier C, Chanet R, Henriques JA, Moustacchi E. The effects of three PSO genes on induced mutagenesis : a novel class of mutationally defective yeast. Genetics. 1980;96:841–857. doi: 10.1093/genetics/96.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minesinger BK, Abdulovic AL, Ou TM, Jinks-Robertson S. The effect of oxidative metabolism on spontaneous Pol zeta-dependent translesion synthesis in Saccharomyces cerevisiae. DNA Repair (Amst) 2006;5:226–234. doi: 10.1016/j.dnarep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Minesinger BK, Jinks-Robertson S. Roles of RAD6 epistasis group members in spontaneous polzeta-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics. 2005;169:1939–1955. doi: 10.1534/genetics.104.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northam MR, Garg P, Baitin DM, Burgers PM, Shcherbakova PV. A novel function of DNA polymerase zeta regulated by PCNA. Embo J. 2006;25:4316–4325. doi: 10.1038/sj.emboj.7601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quah SK, von Borstel RC, Hastings PJ. The origin of spontaneous mutation in Saccharomyces cerevisiae. Genetics. 1980;96:819–839. doi: 10.1093/genetics/96.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabbioneda S, Minesinger BK, Giannattasio M, Plevani P, Muzi-Falconi M, Jinks-Robertson S. The 9-1-1 checkpoint clamp physically interacts with polzeta and is partially required for spontaneous polzeta-dependent mutagenesis in Saccharomyces cerevisiae. J Biol Chem. 2005;280:38657–38665. doi: 10.1074/jbc.M507638200. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich E, Eisler H, Steinboeck F. Epistatic participation of REV1 and REV3 in the formation of UV-induced frameshift mutations in cell cycle-arrested yeast cells. Mutat Res. 2006;593:187–195. doi: 10.1016/j.mrfmmm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich E, Holzmann V, Eisler H. Polymerase zeta dependency of increased adaptive mutation frequencies in nucleotide excision repair-deficient yeast strains. DNA Repair (Amst) 2004;3:395–402. doi: 10.1016/j.dnarep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Roche H, Gietz RD, Kunz BA. Specificity of the yeast rev3 delta antimutator and REV3 dependency of the mutator resulting from a defect (rad1 delta) in nucleotide excision repair. Genetics. 1994;137:637–646. doi: 10.1093/genetics/137.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roche H, Gietz RD, Kunz BA. Specificities of the Saccharomyces cerevisiae rad6, rad18, and rad52 mutators exhibit different degrees of dependence on the REV3 gene product, a putative nonessential DNA polymerase. Genetics. 1995;140:443–456. doi: 10.1093/genetics/140.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar S, Davies AA, Ulrich HD, McHugh PJ. DNA interstrand crosslink repair during G1 involves nucleotide excision repair and DNA polymerase zeta. Embo J. 2006 doi: 10.1038/sj.emboj.7600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen X, Jun S, O'Neal LE, Sonoda E, Bemark M, Sale JE, Li L. REV3 and REV1 Play Major Roles in Recombination-independent Repair of DNA Interstrand Cross-links Mediated by Monoubiquitinated Proliferating Cell Nuclear Antigen (PCNA) 2006:13869–13872. doi: 10.1074/jbc.C600071200. [DOI] [PubMed] [Google Scholar]
- 20.Holbeck SL, Strathern JN. A role for REV3 in mutagenesis during double-strand break repair in Saccharomyces cerevisiae. Genetics. 1997;147:1017–1024. doi: 10.1093/genetics/147.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rattray AJ, Shafer BK, McGill CB, Strathern JN. The roles of REV3 and RAD57 in double-strand-break-repair-induced mutagenesis of Saccharomyces cerevisiae. Genetics. 2002;162:1063–1077. doi: 10.1093/genetics/162.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano Y, Sugimoto K. ATR homolog Mec1 controls association of DNA polymerase zeta-Rev1 complex with regions near a double-strand break. Curr Biol. 2006;16:586–590. doi: 10.1016/j.cub.2006.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta A, Jinks-Robertson S. Association of increased spontaneous mutation rates with high levels of transcription in yeast. Science. 1995;268:1616–1619. doi: 10.1126/science.7777859. [DOI] [PubMed] [Google Scholar]
- 24.Diaz M, Verkoczy LK, Flajnik MF, Klinman NR. Decreased frequency of somatic hypermutation and impaired affinity maturation but intact germinal center formation in mice expressing antisense RNA to DNA polymerase zeta. J Immunol. 2001;167:327–335. doi: 10.4049/jimmunol.167.1.327. [DOI] [PubMed] [Google Scholar]
- 25.Diaz M, Lawrence C. An update on the role of translesion synthesis DNA polymerases in Ig hypermutation. Trends Immunol. 2005;26:215–220. doi: 10.1016/j.it.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence CW, Hinkle DC. DNA polymerase zeta and the control of DNA damage induced mutagenesis in eukaryotes. In: Lindahl T, editor. Cancer Surveys: Genetic Instability in Cancer. Vol. 20. 1996. pp. 21–31. [PubMed] [Google Scholar]
- 27.Lawrence CW, Gibbs PE, Murante RS, Wang XD, Li Z, McManus TP, McGregor WG, Nelson JR, Hinkle DC, Maher VM. Roles of DNA polymerase zeta and Rev1 protein in eukaryotic mutagenesis and translesion replication. Cold Spring Harb Symp Quant Biol. 2000;65:61–69. doi: 10.1101/sqb.2000.65.61. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L. Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature. 2000;406:1015–1019. doi: 10.1038/35023030. [DOI] [PubMed] [Google Scholar]
- 29.Acharya N, Johnson RE, Prakash S, Prakash L. Complex formation with Rev1 enhances the proficiency of Saccharomyces cerevisiae DNA polymerase zeta for mismatch extension and for extension opposite from DNA lesions. Mol Cell Biol. 2006;26:9555–9563. doi: 10.1128/MCB.01671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong X, Garg P, Stith CM, Nick McElhinny SA, Kissling GE, Burgers PM, Kunkel TA. The fidelity of DNA synthesis by yeast DNA polymerase zeta alone and with accessory proteins. Nucleic Acids Res. 2006;34:4731–4742. doi: 10.1093/nar/gkl465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin MC, Wang J, Steitz TA. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell. 2001;105:657–667. doi: 10.1016/s0092-8674(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 32.Niimi A, Limsirichaikul S, Yoshida S, Iwai S, Masutani C, Hanaoka F, Kool ET, Nishiyama Y, Suzuki M. Palm mutants in DNA polymerases alpha and eta alter DNA replication fidelity and translesion activity. Mol Cell Biol. 2004;24:2734–2746. doi: 10.1128/MCB.24.7.2734-2746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlov YI, Frahm C, Nick McElhinny SA, Niimi A, Suzuki M, Kunkel TA. Evidence that errors made by DNA polymerase alpha are corrected by DNA polymerase delta. Curr Biol. 2006;16:202–207. doi: 10.1016/j.cub.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Nick McElhinny SA, Stith CM, Burgers PM, Kunkel TA. Inefficient proofreading and biased error rates during inaccurate DNA synthesis by a mutant derivative of Saccharomyces cerevisiae DNA polymerase delta. J Biol Chem. 2006 doi: 10.1074/jbc.M609591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pursell ZP. Regulation of B Family DNA Polymerase Fidelity by a Conserved Active Site Residue: Characterization of M644W, M644L and M644F Mutants of Yeast DNA Polymerase ε. 2007 doi: 10.1093/nar/gkm132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pursell ZP. Evidence that Yeast DNA Polymerase ε Participates in Leading Strand DNA Replication. 2007 doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reha-Krantz LJ, Nonay RL. Motif A of bacteriophage T4 DNA polymerase: role in primer extension and DNA replication fidelity. Isolation of new antimutator and mutator DNA polymerases. J Biol Chem. 1994;269:5635–5643. [PubMed] [Google Scholar]
- 38.Fidalgo da Silva E, Mandal SS, Reha-Krantz LJ. Using 2-aminopurine fluorescence to measure incorporation of incorrect nucleotides by wild type and mutant bacteriophage T4 DNA polymerases. J Biol Chem. 2002;277:40640–40649. doi: 10.1074/jbc.M203315200. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Murphy KM, Kanevets U, Reha-Krantz LJ. Sensitivity to phosphonoacetic acid: a new phenotype to probe DNA polymerase delta in Saccharomyces cerevisiae. Genetics. 2005;170:569–580. doi: 10.1534/genetics.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkatesan RN, Hsu JJ, Lawrence NA, Preston BD, Loeb LA. Mutator phenotypes caused by substitution at a conserved motif A residue in eukaryotic DNA polymerase delta. J Biol Chem. 2006;281:4486–4494. doi: 10.1074/jbc.M510245200. [DOI] [PubMed] [Google Scholar]
- 41.Bemark M, Khamlichi AA, Davies SL, Neuberger MS. Disruption of mouse polymerase zeta (Rev3) leads to embryonic lethality and impairs blastocyst development in vitro. Curr Biol. 2000;10:1213–1216. doi: 10.1016/s0960-9822(00)00724-7. [DOI] [PubMed] [Google Scholar]
- 42.Esposito G, Godindagger I, Klein U, Yaspo ML, Cumano A, Rajewsky K. Disruption of the Rev3l-encoded catalytic subunit of polymerase zeta in mice results in early embryonic lethality. Curr Biol. 2000;10:1221–1224. doi: 10.1016/s0960-9822(00)00726-0. [DOI] [PubMed] [Google Scholar]
- 43.Wittschieben J, Shivji MK, Lalani E, Jacobs MA, Marini F, Gearhart PJ, Rosewell I, Stamp G, Wood RD. Disruption of the developmentally regulated Rev3l gene causes embryonic lethality. Curr Biol. 2000;10:1217–1220. doi: 10.1016/s0960-9822(00)00725-9. [DOI] [PubMed] [Google Scholar]
- 44.Pavlov YI, Shcherbakova PV, Kunkel TA. In vivo consequences of putative active site mutations in yeast DNA polymerases alpha, epsilon, delta, and zeta. Genetics. 2001;159:47–64. doi: 10.1093/genetics/159.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 46.Drake JW. A constant rate of spontaneous mutation in DNA-based microbes. Proc Natl Acad Sci U S A. 1991;88:7160–7164. doi: 10.1073/pnas.88.16.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdulovic AL, Jinks-Robertson S. The in vivo characterization of translesion synthesis across UV-induced lesions in Saccharomyces cerevisiae: insights into Pol zetaand Pol eta-dependent frameshift mutagenesis. Genetics. 2006;172:1487–1498. doi: 10.1534/genetics.105.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence CW. Cellular roles of DNA polymerase zeta and Rev1 protein. DNA Repair (Amst) 2002;1:425–435. doi: 10.1016/s1568-7864(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 49.Kozmin SG, Pavlov YI, Kunkel TA, Sage E. Roles of Saccharomyces cerevisiae DNA polymerases Poleta and Polzeta in response to irradiation by simulated sunlight. Nucleic Acids Res. 2003;31:4541–4552. doi: 10.1093/nar/gkg489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDonald JP, Levine AS, Woodgate R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics. 1997;147:1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Padula M, Slezak G, Auffret van Der Kemp P, Boiteux S. The post-replication repair RAD18 and RAD6 genes are involved in the prevention of spontaneous mutations caused by 7,8-dihydro-8-oxoguanine in Saccharomyces cerevisiae. Nucleic Acids Res. 2004;32:5003–5010. doi: 10.1093/nar/gkh831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haracska L, Yu SL, Johnson RE, Prakash L, Prakash S. Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase eta. Nat Genet. 2000;25:458–461. doi: 10.1038/78169. [DOI] [PubMed] [Google Scholar]
- 53.Pavlov YI, Mian IM, Kunkel TA. Evidence for preferential mismatch repair of lagging strand DNA replication errors in yeast. Curr Biol. 2003;13:744–748. doi: 10.1016/s0960-9822(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 54.Johnson RE, Washington MT, Prakash S, Prakash L. Fidelity of human DNA polymerase eta. J Biol Chem. 2000;275:7447–7450. doi: 10.1074/jbc.275.11.7447. [DOI] [PubMed] [Google Scholar]
- 55.McCulloch SD, Kokoska RJ, Masutani C, Iwai S, Hanaoka F, Kunkel TA. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature. 2004;428:97–100. doi: 10.1038/nature02352. [DOI] [PubMed] [Google Scholar]
- 56.Ling H, Boudsocq F, Plosky BS, Woodgate R, Yang W. Replication of a cis-syn thymine dimer at atomic resolution. Nature. 2003;424:1083–1087. doi: 10.1038/nature01919. [DOI] [PubMed] [Google Scholar]
- 57.Ikehata H, Ono T, Tanaka K, Todo T. A model for triplet mutation formation based on error-prone translesional DNA synthesis opposite UV photolesions. DNA Repair (Amst) 2007;6:658–668. doi: 10.1016/j.dnarep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Neeley WL, Delaney S, Alekseyev YO, Jarosz DF, Delaney JC, Walker GC, Essigmann JM. DNA polymerase V allows bypass of toxic guanine oxidation products in vivo. J Biol Chem. 2007;282:12741. doi: 10.1074/jbc.M700575200. [DOI] [PubMed] [Google Scholar]
- 59.Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505. doi: 10.1021/tx0600043. [DOI] [PubMed] [Google Scholar]
- 60.Garg P, Stith CM, Majka J, Burgers PM. Proliferating cell nuclear antigen promotes translesion synthesis by DNA polymerase zeta. J Biol Chem. 2005;280:23446–23450. doi: 10.1074/jbc.C500173200. [DOI] [PubMed] [Google Scholar]
- 61.Ohashi E, Bebenek K, Matsuda T, Feaver WJ, Gerlach VL, Friedberg EC, Ohmori H, Kunkel TA. Fidelity and processivity of DNA synthesis by DNA polymerase kappa, the product of the human DINB1 gene. J Biol Chem. 2000;275:39678–39684. doi: 10.1074/jbc.M005309200. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Gonzalez KD, Scaringe WA, Tsai K, Liu N, Gu D, Li W, Hill KA, Sommer SS. Evidence for Mutation Showers. PNAS. 2007 doi: 10.1073/pnas.0610902104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drake JW. Mutations in clusters and showers. PNAS. 2007 doi: 10.1073/pnas.0703089104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sloan J, Kirkland AI, Hutchison JL, Green ML. Structural characterization of atomically regulated nanocrystals formed within single-walled carbon nanotubes using electron microscopy. Acc Chem Res. 2002;35:1054–1062. doi: 10.1021/ar010169x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.