Abstract
Context
In some individuals, chronic tension-type headache fails to respond to tricyclic antidepressant medications that often serve as first-line therapy.
Objective
To evaluate the clinical efficacy of paroxetine hydrochloride for chronic tension-type headache not responding to amitriptyline hydrochloride.
Design and Setting
Open-label trial of paroxetine conducted at 2 outpatient sites in Ohio.
Participants and Intervention
Thirty-one adults (mean age, 37 years; 20 women) with chronic tension-type headache (mean, 25 headache days per month) who had failed to respond (less than 30% improvement) to treatment with either amitriptyline (n = 13) or matched placebo (n = 18). All participants were treated with paroxetine (up to 40 mg per day) in a 9-month protocol.
Outcome Measures
Monthly headache index calculated as the mean of pain ratings (0 to 10 scale) recorded by participants in a diary 4 times per day, number of days per month with at least moderate pain (pain rating of 5 or greater), and analgesic medication use.
Results
In patients who had not responded to amitriptyline, paroxetine failed to reduce chronic tension-type headaches or analgesic medication use. In patients who had not responded to placebo, paroxetine produced modest reductions in chronic tension-type headaches and analgesic use.
Conclusions
We found no evidence that chronic tension-type headaches that failed to respond to tricyclic antidepressant therapy with amitriptyline improved when subsequently treated with paroxetine. More support was found for the efficacy of paroxetine in patients with chronic tension-type headaches who had failed to respond to placebo.
Keywords: headache, paroxetine, amitriptyline, tension-type
Tricyclic antidepressant medications remain the primary drug therapy for chronic tension-type headache, with amitriptyline the first-line treatment.1-6 Even so, preliminary support for the effectiveness of certain selective serotonin reuptake inhibitors is provided by open-label studies reporting positive results with fluoxetine and paroxetine,7,8 although negative results also have been reported.9
We report pilot data on the effectiveness of paroxetine with chronic tension-type headache in 2 groups of patients: those unresponsive to tricyclic antidepressant medication (amitriptyline) or those unresponsive to matched placebo. Treatment of the first group with paroxetine allows an assessment of the effectiveness of paroxetine in nonresponders to amitriptyline; treatment of the second group allows an assessment of the effectiveness of paroxetine in patients who could be assumed unresponsive to placebo.
METHODS
Patients who had been treated with tricyclic antidepressant medication (amitriptyline to 100 mg per day and if not tolerated or effective, nortriptyline to 75 mg per day) or matched placebos in an earlier double-blind trial and who were classified as nonresponders by both daily diary recordings (less than 30% reduction in headache index) and neurologist judgment (rated unimproved or only slightly improved) were enrolled in this study.6 Patients treated with tricyclic antidepressant medication had been treated for a mean of 9.1 months (SD, 2.3), and patients treated with placebo were treated for a mean of 7.3 months (SD, 2.3) before being transferred to paroxetine. Patients who were nonresponders to tricyclic antidepressant medication (TANR group) and patients who were nonresponders to placebo (PLNR group) were examined separately.
After previous study medication (placebo or tri-cyclic antidepressant) had been tapered, paroxetine therapy was initiated at 10 mg per day and adjusted over the course of 2 months to 30 mg per day as tolerated, with an increase to 40 mg per day allowed during the subsequent evaluation period. Clinic visits were scheduled during months 0, 1, 2, 3, 5, and 9 of paroxetine therapy. During paroxetine therapy, treating neurologists remained unaware as to whether patients had failed to respond to antidepressant medication or to placebo in the previous trial.
Response to tricyclic antidepressant medication or matched placebo in the previous trial was assessed by 1 month of daily diary recordings collected before and during the final month of tricyclic antidepressant or placebo treatment. Response to paroxetine in the current study was assessed by a month of diary recordings during months 3 and 9 of therapy.
MATERIALS
Headaches and Analgesic Medication Use
Patients recorded headache activity in a diary 4 times a day using an 11-point rating scale with 5 anchors that ranged from 0 = “no pain” to 10 = “extremely painful—I can't do anything when I have a headache.”10-13 The diary also provided spaces to record analgesic and study medication use each day.
A headache index, the mean of all diary ratings, was calculated over 1-month periods to obtain a measure of overall headache activity.10,11 The number of days per month with a headache of at least moderate severity (pain rating of 5 or greater) and weighted analgesic medication consumption (number of pills weighted by analgesic potency) also served as outcome measures.11,14,15 For example, simple or combined nonprescription analgesics are assigned a weight of 1; prescription-strength nonsteroidal anti-inflammatory agents, a weight of 2; and compound (eg, butalbital- or codeine-containing) analgesics, a weight of 3.
Beck Depression Inventory
The Beck Depression Inventory (BDI) is a 21-item self-report measure that assesses symptoms of depression.16 The BDI is widely used in the assessment of individuals with recurrent headache disorders.17
RESULTS
Patients
Inclusion criteria for the previous trial were an age of 18 to 65 years and a diagnosis, on 2 separate evaluations, of chronic tension-type headache according to criteria of the International Headache Society (IHS).18 Relevant exclusion criteria were: an IHS diagnosis of analgesic abuse headaches,18 migraine headache more than 1 day per month, pain disorder other than headache (eg, arthritis) as primary pain problem, and contraindication to tricyclic antidepressant medication. Additional details can be found in Holroyd et al.6
Demographic and clinical characteristics for the PLNR group (n = 18) and the TANR group (n = 13) are presented in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics*
| Nonresponders |
||
|---|---|---|
| Feature | Placebo Group | Tricyclic Antidepressant Group |
| Age at entry, y | 37.1 (3.2) | 35.4 (2.8) |
| Female, % | 72.2 | 46.2 |
| White, % | 100 | 92.3 |
| Headache characteristics | ||
| Headache index | 2.7 (.29) | 2.5 (.35) |
| Headache days/month | 25.7 (1.22) | 25.2 (1.59) |
| At least moderate severity days/month† | 13.9 (1.55) | 12.2 (2.39) |
| Comorbid migraine diagnosis, % | 22.2 | 7.7 |
| Disease duration, y | ||
| Problem headaches | 10.3 (2.26) | 16.1 (2.52) |
| At current frequency | 5.7 (1.25) | 11.0 (2.61) |
Values expressed as mean (SE) unless otherwise specified.
Pain rating of 5 or greater (on a scale of 0 to 10).
Adherence and Dropouts
Nonresponders to Placebo
Neurologists rated all patients as over 90% compliant at the dose-adjustment visits. Eleven of 18 patients, however, discontinued paroxetine therapy before the 9-month visit: 7 because of dissatisfaction with their treatment response, 2 because of side effects (1, diarrhea and constipation and 1, skin rash), and 2 gave no reason.
Nonresponders to Tricyclic Antidepressant Medication
Neurologist ratings of adherence were high with all patients over 90% adherent at the dose-adjustment visits. Ten of 13 patients, however, discontinued paroxetine therapy by the 9-month visit: 5 because of dissatisfaction with their treatment response and 5 due to side effects (1, difficulty concentrating, anxiety, nervousness, and worsening headaches; 2, sexual dysfunction; 1, gastric upset and dizziness; and 1, depression).
Dosing
Nonresponders to Placebo
The highest tolerated dose varied for the 18 patients: 10 mg for 3 patients, 20 mg for 7, 30 mg for 7, and 40 mg for 1. Participants who tolerated higher doses of paroxetine remained in the trial for longer periods (r = .85; P < .001).
Nonresponders to Tricyclic Antidepressant Medication
The highest tolerated dose varied for the 13 patients: 10 mg for 2 patients, 20 mg for 4, 30 mg for 3, and 40 mg for 4. Participants who tolerated higher doses of paroxetine remained in the trial for longer periods (r = .78; P < .01).
Headache Activity and Analgesic Medication Use
Nonresponders to Placebo
Before treatment with paroxetine, placebo had failed to yield improvement on any headache measure: a 19% increase in mean headache index and less than 1% reduction in days of at least moderate pain and weighted analgesic use. As can be seen in Figure 1, paroxetine therapy yielded moderate improvements in measures of headache activity and analgesic medication consumption at both the 3- and 9-month evaluations. The results of t tests examining mean changes from baseline to the 3- and 9-month evaluations with last point carried forward for dropouts are reported in Table 2, where it can be seen that improvements on all 3 outcome measures were statistically significant. Moreover, 7 (39%) of 18 patients recorded clinically significant (50% or greater) reductions in their headache index on paroxetine.19 Even so, 3 (43%) of these 7 improved patients discontinued paroxetine therapy either because of adverse events or dissatisfaction with treatment results before the 9-month evaluation. As a result, only 4 (22%) of 18 patients showed clinically significant improvement and were sufficiently satisfied with treatment to continue paroxetine therapy through the 9-month evaluation.
Fig 1.
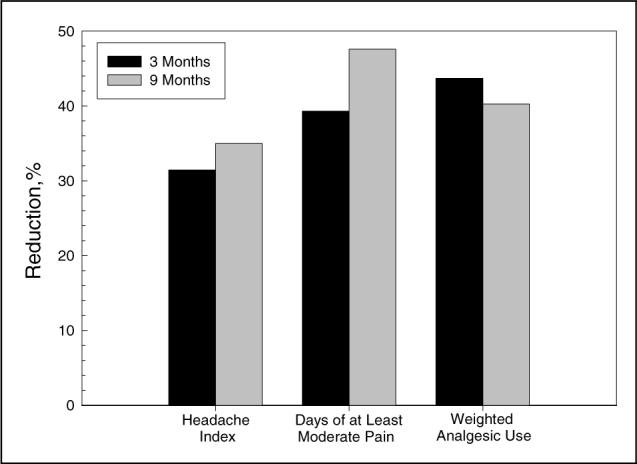
Mean percentage reduction in headache index, days of at least moderate pain, and weighted analgesic use with paroxetine hydrochloride in patients who had failed to respond to treatment with placebo.
Table 2.
Outcome Variables and Depression Inventory*
| Baseline | 3 Months | 9 Months | |
|---|---|---|---|
| Placebo nonresponders | |||
| Headache index | 3.2 | 2.2† | 2.1† |
| At least moderate pain, days/month | 14.5 | 8.8† | 7.6‡ |
| Weighted analgesic use | 34.8 | 19.6§ | 20.8§ |
| Beck Depression Inventory | 7.7 | 6.6 | 6.6 |
| Tricyclic antidepressant nonresponders | |||
| Headache index | 2.6 | 2.4 | 2.4 |
| At least moderate pain, days/month | 11.0 | 9.5 | 9.7 |
| Weighted analgesic use | 39.2 | 37.4 | 41.7 |
| Beck Depression Inventory | 6.4 | 6.4 | 6.4 |
Values are means. t tests were conducted on end-point data to examine mean differences.
P < .001.
P < .01.
P < .05.
Partial correlations (controlling for baseline scores) revealed that treatment outcome and paroxetine dose were unrelated at the 3-month evaluation (P > .05), but positively related at the 9-month evaluation (r = −.61; P < .05 for headache index; r = −.52; P < .05 for days of at least moderate pain). Treatment outcome, however, was unrelated to time in the trial (all P values > .05).
Nonresponders to Tricyclic Antidepressant Medication
Amitriptyline had yielded no improvement in headache index and only modest improvement in the remaining measures before paroxetine treatment: 1% increase in headache index, 28% reduction in days of at least moderate pain, and 31% reduction in weighted analgesic use. It can be seen in Figure 2 and Table 2 that only minimal improvements in the 2 headache measures were observed with paroxetine treatment; in fact, weighted analgesic use showed a slight increase during paroxetine therapy at the 9-month evaluation. Only 2 (15%) of 13 patients showed a clinically significant (50% or greater) reduction in the headache index on paroxetine, and only 1 of these patients was sufficiently satisfied with treatment to continue with paroxetine therapy through the 9-month evaluation.
Fig 2.
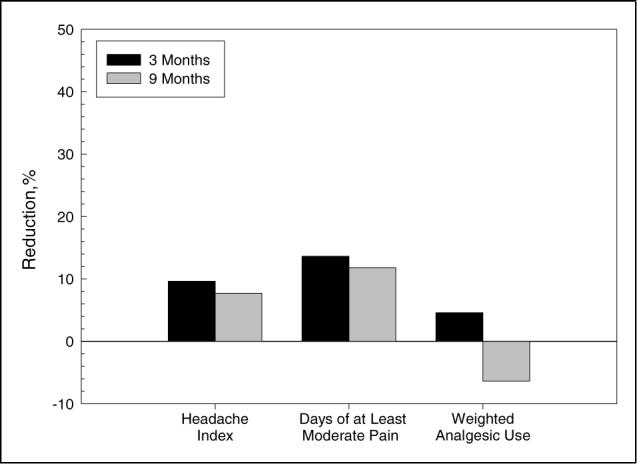
Mean percentage reduction in headache index, days of at least moderate pain, and weighted analgesic use with paroxetine hydrochloride in patients who had failed to respond to treatment with amitriptyline hydrochloride.
Partial correlations (controlling for baseline scores) indicated that treatment outcome was unrelated to paroxetine dose or time in the trial (all P values > .05).
Depression
Mean BDI scores were in the reference range for normal and were unchanged with paroxetine treatment in both treatment groups (Table 2).
COMMENTS
We found no evidence that patients who had failed to respond to tricyclic antidepressant therapy with amitriptyline were likely to benefit if subsequently treated with paroxetine. Only 2 of 13 TANRs recorded clinically significant reductions in their headache index when treated with paroxetine, and only 1 of these patients was sufficiently satisfied to continue paroxetine therapy through the 9-month protocol. In addition, no reduction in analgesic medication consumption was observed when patients were changed from tricyclic antidepressant medication to paroxetine.
Notably more positive results were observed in patients who had been placebo nonresponders in the previous trial. In PLNRs, paroxetine yielded mean improvements of 30% to 40% or greater on headache and analgesic medication consumption measures, with about 40% of patients showing clinically significant (50% or greater) reductions in their headache index. Nevertheless, treatment acceptance remained a problem: only half of the patients who showed clinically significant improvements in their headache index were sufficiently satisfied to continue on paroxetine through the 9-month protocol. At best, these results provide qualified support for the use of paroxetine in the management of chronic tension-type headache.
To our knowledge, no previous study has evaluated the effectiveness of paroxetine specifically for chronic tension-type headache that has failed to respond to tricyclic antidepressant medication. Langemark and Olesen, however, reported a poor response to paroxetine relative to sulpiride in chronic tension-type headache.9 Unfortunately, the global assessment measure used in that study does not allow their results and our results to be compared directly. In contrast, Foster and Bafaloukos reported “mixed migraine/tension-type” headaches that had shown “a poor response to at least two medications” (page 587) responded to paroxetine, with 44 (73%) of 60 paroxetine-treated patients showing clinically significant (50% or greater) improvements on a global measure of headache days.7 These findings raise the possibility that paroxetine is more effective for transformed or chronic migraine than for chronic tension-type headache.20,21 Additional research would be required to assess this possibility.
Our results provide no support for the use of paroxetine to treat chronic tension-type headache that has failed to respond to amitriptyline, the first-line therapy.1-5 We did, however, find that paroxetine was modestly effective in controlling chronic tension-type headache that had failed to respond to treatment with placebo. These latter results, however, come with several caveats. Even when paroxetine was effective, it was poorly accepted with about half of patients failing to continue on paroxetine through the 9-month protocol. In addition, this was an open-label trial, even though in a previous trial patients had failed to respond to placebo and, thus, placebo responders were likely removed from the sample. Finally, only a small number of patients were treated in this study.
Acknowledgments
Support for this research was provided, in part, by a grant from The National Institute of Neurological Disorders and Stroke, National Institutes of Health (NS32374). Appreciation is expressed to M. Katherine Davis-Rosanbalm, Douglas French, Kimberly Hill, Gay L. Lipchik, Pete Malinoski, Angela Nicolosi, Carol Nogrady, Cornelia Pinnell, France Talbot, and Sharon Waller. SmithKline Beecham (now GlaxoSmithKline) donated paroxetine hydrochloride for this project.
REFERENCES
- 1.Schoenen J, Wang W. Tension-type headache. In: Goadsby PJ, Silberstein SD, editors. Headache. Butterworth-Heinemann; Boston: 1997. pp. 177–200. [Google Scholar]
- 2.Couch JR. Medical management of recurrent tension-type headache. In: Tollison CD, Kunkel RS, editors. Headache Diagnosis and Treatment. Williams & Wilkins; Baltimore: 1993. pp. 151–162. [Google Scholar]
- 3.Couch JR, Micieli G. Prophylactic pharmacotherapy. In: Olesen J, Tfelt-Hansen P, Welch KM, editors. The Headaches. Raven Press, Ltd; New York: 1993. [Google Scholar]
- 4.Mathew NT, Bendtsen L. Prophylactic pharmacotherapy of tension-type headache. In: Olesen J, Tfelt-Hansen P, Welch KM, editors. The Headaches. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 667–674. [Google Scholar]
- 5.Kunkel RS. Diagnosis and treatment of muscle contraction (tension-type) headaches. Med Clin North Am. 1991;75:595–603. doi: 10.1016/s0025-7125(16)30435-7. [DOI] [PubMed] [Google Scholar]
- 6.Holroyd KA, O'Donnell FJ, Stensland M, Lipchik GL, Cordingley GE, Carlson B. Management of chronic tension-type headache with tricyclic antidepressant medication, stress-management therapy, and their combination: a randomized controlled trial. JAMA. 2001;285:2208–2215. doi: 10.1001/jama.285.17.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache. 1994;34:587–589. doi: 10.1111/j.1526-4610.1994.hed3410587.x. [DOI] [PubMed] [Google Scholar]
- 8.Walker Z, Walker RW, Robertson MM, Stansfeld S. Antidepressant treatment of chronic tension-type headache: a comparison between fluoxetine and desipramine. Headache. 1998;38:523–528. doi: 10.1046/j.1526-4610.1998.3807523.x. [DOI] [PubMed] [Google Scholar]
- 9.Langemark M, Olesen J. Sulpiride and paroxetine in the treatment of chronic tension-type headache. An explanatory double-blind trial. Headache. 1994;34:20–24. doi: 10.1111/j.1526-4610.1994.hed3401020.x. [DOI] [PubMed] [Google Scholar]
- 10.Holroyd KA, Nash JM, Pingel JD, Cordingley GE, Jerome A. A comparison of pharmacological (amitriptyline HCl) and nonpharmacological (cognitive-behavioral) therapies for chronic tension headaches. J Consult Clin Psychol. 1991;59:387–393. doi: 10.1037//0022-006x.59.3.387. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard EB, Andrasik F. Management of Chronic Headaches: A Psychological Approach. Pergamon Press; Elmsford, NY: 1985. [Google Scholar]
- 12.Blanchard EB, Andrasik F, Neff DF, Jurish SE, O'Keefe DM. Social validation of the headache diary. Behav Ther. 1981;12:711–715. [Google Scholar]
- 13.McKee M. Headache diary. In: Tollison CD, Kunkel RS, editors. Headache Diagnosis and Treatment. Williams & Wilkins; Baltimore: 1993. pp. 321–327. [Google Scholar]
- 14.Holroyd KA, Martin PR. Psychological treatments for tension-type headache. In: Olesen J, Tfelt-Hansen P, Welch KM, editors. The Headaches. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 643–649. [Google Scholar]
- 15.Coyne L, Sargent J, Segerson J, Obourn R. Relative potency scale for analgesic drugs: use of psychophysical procedures with clinical judgments. Headache. 1976;16:70–71. doi: 10.1111/j.1526-4610.1976.hed1602070.x. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 17.Penzien DB, Rains JC, Holroyd KA. Psychological assessment of the recurrent headache sufferer. In: Tollison CD, Kunkel RS, editors. Headache: Diagnosis and Treatment. Williams and Wilkins; Baltimore: 1993. pp. 39–50. [Google Scholar]
- 18.Headache Classification Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(suppl 7):1–96. [PubMed] [Google Scholar]
- 19.Blanchard EB, Schwarz SP. Clinically significant changes in behavioral medicine. Behav Assess. 1988;10:171–188. [Google Scholar]
- 20.Silberstein SD, Lipton RB, Solomon S, Mathew NT. Classification of daily and near-daily headaches: proposed revisions to the IHS criteria. Headache. 1994;34:1–7. doi: 10.1111/j.1526-4610.1994.hed3401001.x. [DOI] [PubMed] [Google Scholar]
- 21.Mathew NT. Transformed migraine. Cephalalgia. 1993;13(suppl 12):78–83. doi: 10.1177/0333102493013S1217. [DOI] [PubMed] [Google Scholar]


