Abstract
The heterodimeric integration host factor (IHF) is a site-specific DNA-binding and DNA-bending protein from Escherichia coli. It plays essential roles in a variety of DNA transactions including recombination, transcription and DNA replication. IHF’s ability for concerted binding and bending of DNA is key to its biological function. Here we report the design, characterization and application of a single polypeptide chain IHF, termed scIHF2. In a novel approach for protein engineering, we inserted almost the entire α-subunit of IHF into the β-subunit. DNA binding and DNA bending assays revealed that purified wild-type IHF and scIHF2 behave very similarly. Further, scIHF2 is required for site-specific integrative recombination by phage λ integrase and for pSC101 replication in a ΔIHF E.coli host. It also triggers site-specific integrative and excisive recombination in vitro to the same extent as the wild-type protein. We also demonstrate that scIHF2 is stably expressed in HeLa cells, that it is localized primarily in the cell nucleus and that it triggers integrative recombination in mammalian cells by wild-type integrase. Hence, scIHF2 may be used as a novel regulatory cofactor for recombination or other DNA transactions in mammalian cells that require or benefit from sequence-specific high precision DNA bending.
INTRODUCTION
Integration host factor (IHF) is a heterodimeric Escherichia coli protein that plays important roles in a variety of cellular processes including site-specific recombination, transcription, replication and DNA compaction [reviewed in Nash (1) and Goosen and van de Putte (2)]. IHF exerts its biological functions through binding at specific DNA sites characterized by a limited consensus sequence (3). The crystal structure of IHF complexed to a sequence from the phage λ H′ site revealed strong (>160°) protein-induced DNA bending (4). IHF thus is the strongest sequence-specific DNA-bending protein identified to date [reviewed in Travers (5) and Ellenberger and Landy (6)].
IHF’s two homologous α- and β-subunits of ∼10 kDa each are 30% identical in sequence. Synchrotron X-ray footprinting studies revealed that IHF recognizes its specific site through a multistep mechanism that involves concerted binding and bending of DNA (7). DNA bending is mainly achieved by the intercalation of conserved prolines from each arm of the heterodimer into the minor groove. Both IHF subunits stabilize the bend around the protein by electrostatic interactions with DNA. Further, the protein exhibits a rather high affinity for some specific binding sites. For example, the Kd for the IHF–H′ complex is ∼1 nM (8,9).
The ability of IHF to introduce DNA bending appears key to its biological function. This became clear from early studies of IHF’s role as an essential cofactor in phage λ site-specific recombination where the protein serves as an important architectural element in the assembly of recombinogenic nucleoprotein complexes [reviewed in Azaro and Landy (10)]. It is, as such, an essential and sufficient protein cofactor for the λ integrative recombination pathway, which employs DNA sequences attP and attB.
IHF is also involved in the excisive pathway which employs hybrid DNA sites attL and attR. Excisive recombination, however, requires a second DNA-bending cofactor, the Xis protein. Hence, IHF and Xis tightly regulate the directionality of λ site-specific recombination in the natural host E.coli. In this context, the main function of IHF is to facilitate the formation of high-precision DNA loops that allow integrase (Int) monomers to contact two separate DNA sites simultaneously (10).
We recently transferred the λ Int system to mammalian cells and demonstrated that mutant Ints perform both integrative and excisive recombination reactions on episomal and genomic substrates at a significant level in the absence of protein cofactors (11–13). The directionality of recombination was therefore lost when we employed mutant Ints in eukaryotic cells. However, we also demonstrated that purified IHF pre-bound to a DNA substrate for integrative recombination and transfected into mammalian cells stimulates wild-type Int-mediated recombination. This result revealed an interesting possibility of regaining control over the directionality of recombination in mammalian cells through the employment of protein cofactors and wild-type Int (13). However, it is known that separate over-expression of IHF α- and β-subunits in E.coli results in unstable polypeptides and insoluble aggregates (14,15). Hence, in order to overcome the technical difficulties associated with the necessity of synthesizing two IHF subunits at about the same level in mammalian cells, we decided to construct a single polypeptide chain IHF named scIHF2.
Here we describe the design of scIHF2, which is based on molecular modeling of the existing co-crystal structure with wild-type IHF (4). Our biochemical characterization of the purified, His-tagged protein revealed that scIHF2 exhibits properties very similar to wild-type IHF with respect to DNA binding and bending. Furthermore, scIHF2 promotes both site-specific integrative recombination and pSC101 replication in E.coli. Long-term and high-level scIHF2 expression in HeLa cells revealed that the recombinant protein is located primarily inside the nucleus and that it is apparently not cytotoxic for mammalian cells kept in culture. We demonstrate further that scIHF2-expressing HeLa cells support site-specific integrative recombination catalyzed by wild-type Int.
MATERIALS AND METHODS
Molecular modeling
Based on the atomic coordinates of IHF (PDB accession number 1IHF), the β-chain was first opened between residues Q39 and G40. Five residues (GGSGG) were positioned manually between the N-terminus of the α-chain at L3 and β-Q39 (α-A2 was deleted). Similarly, two glycine residues were manually modeled in order to connect β-G40 and α-A94, omitting the terminal three residues of the IHF crystal structure. The modifications only involved protein regions that appear to be flexible in the crystal structure, judged by comparatively high temperature factors. Further, the modified regions are not part of secondary structure elements. The manually modeled scIHF2 coordinates were then regularized using a simulated annealing regimen performed with the program CNS (16). An ensemble of 10 energetically minimized structures obtained from this procedure superimpose very well. No significant structural changes were detectable in the part of IHF that was not modified.
DNA constructs
ScIHF2 expression vectors for E.coli were constructed as follows: pTrcscIHF2 was cloned via assembly PCR. Three separate PCRs were performed using primer pairs IHF5-1/IHF3-3*, IHF5-4/IHF3-4 and IHF5-5/IHF3-3. Primers were designed in such a way that PCR fragments contain the coding region for one overlapping linker region. The himA and the himD coding sequences served as templates. In a final PCR using primers IHF5-1 and IHF3-3, the scIHF2 coding region was assembled and subsequently cloned into the NcoI and XbaI site of pTrc99a (Pharmacia). Primer sequences are as follows: IHF5-1, 5′-AACTGCAGCCACCATGGGCACCA AGTCAGAATTGATAGAAAGACT-3′; IHF3-3*, 5′-GTAA GACCGCCGCTTCCACCCTGCGCAAGAGTCGAGGCC ATATGCT-3′; IHF5-4, 5′-GGGTGGAAGCGGCGGTC TTACAAAAGCTGAAATGTCAGAATAT-3′; IHF3-4, 5′- ACGCTCGCCCCCACCAGCGTTTTCGACCCGGCTTTT TAAC-3′; IHF5-5, 5′-AACGCTGGTGGGGGCGAGCGT ATTGAAATCCGCG-3′; IHF3-3, 5′-CTAGTCTAGATT ATCAACCTGAGATATTGGCGCG-3′.
The His-tagged version of pTrcscIHF2 was generated accordingly by PCR using primers IHFHIS5, 5′-CAT GCCATGGGGGCTAGCACCAAGTCAGAATTGATAGA AAGACT-3′; and IHFHIS3, 5′-GCTCTAGAGAATTCTTA TCAGTGGTGGTGGTGGTGGTGGCCTGATCCACCGTAGATATTGGCGCGATCG-3′. pETscIHF2 was constructed by inserting the scIHF2 coding sequence including the His tag into pET11d (New England Biolabs). All DNA constructs were sequenced.
Mock and Int expression vectors pCMV, pCMVSSInt, pCMVSSInt-h and pCMVSSInt-h/218, in which the recombinase is under the control of the human cytomegalovirus (CMV) promoter, have been described (11). An expression vector for His-tagged scIHF2, termed poIHF2P, was used for the generation of the scIHF2 HeLa cell lines. Expression of scIHF2 is under the control of a hybrid promoter composed of the CMV immediate-early enhancer fused to the chicken β-actin promoter, termed CAGGS, whereas the expression of the puromycin resistance gene is driven by the phosphoglycerate kinase (PGK) promoter. The scIHF2 coding region was amplified by PCR from pTrcscIHF2 with primers IHF-5-1, 5′-aactgcagccaccatgggcaccaagtcaga-3′; and IHF-His-3, 5′-gct ctagagaattcttatcagtggtggtggtggtggtggcctgatccaccgtagatattggcgcgatcg-3′. The product was cut with PstI–XbaI and subcloned into PstI–XbaI-cleaved pCMVSSInt-h/218, thereby replacing the Int-h/218 coding sequence. The resulting vector (pCMVSSoIHF2His) was used as template for the amplification of SS-scIHF2His-PA in a PCR with primers SS-5-NheI, 5′-cccaagcttgctagcaattcgctgtctgcgagg-3′; and bPARP-BamHI, 5′-cgggatccccagctggttctttccg-3′. The CAGGS promoter was amplified with Caggs-5-EcoR I, 5′-ccggaattctcttcttcttcgtattccttctct-3′; and Caggs-3-Nhe I, 5′-ctagctagcaggcagcgtcgcagcgactcc-3′, using pTriEx-1 as template (Novagen). PCR fragments were cut with NheI–BamHI or EcoRI–NheI, respectively, and cloned into the EcoRI–BamHI sites of pGEM7+ (Promega). A PGK-puromycinR-PA fragment was subsequently inserted into the blunted SapI site of the resulting vector. Substrate vectors for integrative and excisive recombination containing attB/attP (pλIR) or attL/attR (pλER) as direct repeats have been described (13). pNC9WT is a derivate of pNC9 (17) and was generated via PCR. The complete Int coding region, including the pTrc99a promoter, was amplified from pTrcInt (17) and inserted between the directly repeated attB and attP sequences of pNC9. All plasmids were purified using affinity chromatography (Qiagen)
Purification of scIHF2
BL21-DE3 cells bearing pETscIHF2 were cultured in 1 l LB medium until the OD600 reached 0.4–0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM) was added to induce expression of scIHF2 for 4 h. Cells were harvested and resuspended in lysis buffer (100 mM NaH2PO4, 10 mM Tris, 8 M urea pH 8.0). After sonication, the extract was cleared by centrifugation at 16 100 g for 15 min at room temperature. The supernatant was mixed with ∼5 ml of Ni-NTA–agarose (Qiagen) and incubated overnight at room temperature. The resin was packed into a small column and scIHF2 was eluted with a buffer containing 100 mM NaH2PO4, 10 mM Tris, 8 M urea pH 4.5. After excessive dialysis against 50 mM Tris–HCl pH 8.0, 1 mM EDTA, 1 mM dithiothreitol (DTT), 50 mM NaCl, scIHF2 was stored at –20°C in the same buffer containing 50% glycerol. An additional round of purification under native conditions was initiated by dialyzing the stored protein against 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole pH 8.0. After incubation with Ni-NTA–agarose overnight at 4°C, scIHF2 was eluted in a buffer containing 50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole pH 8.0. The protein was stored again at –20°C as described above. Although the second round of purification appeared not to improve the purity of the protein to a significant extent, all experiments reported here were performed with this batch. Purified wild-type IHF was a kind gift of D. Esposito. Protein concentrations were determined using the Bio-Rad protein assay kit with IgG as a standard.
DNA binding and bending assays
The Kd of IHF and scIHF2 was determined using a 32P-labeled 34mer oligonucleotide comprising H′: 5′-GCCAAAAAAG CATTGCTTATCAATTTGTTGCACC-3′. Binding reactions were performed in 20 µl of buffer containing 0.5× TBE, 17% glycerol, 5 mM EDTA, 200 µg/ml bovine serum albumin (BSA), 0.1 nM labeled DNA and 10 nM or less protein. Reactions were incubated at room temperature for 40 min and loaded onto a 15% polyacrylamide gel which had been pre-run in 0.5× TBE at 4°C and 250 V for 1 h. Gel images were quantified using a Bio-Rad Molecular Imager System.
Specific competition was performed with unlabeled H′ as competitor DNA. The stoichiometry of unlabeled to labeled H′ was 480-fold or less. Salmon sperm DNA (Sigma) with an average fragment length of ∼400 bp was used as unspecific competitor DNA. The stoichiometry of competitor DNA to labeled H′ DNA was 2700-fold or less.
DNA bending was analyzed using a 32P-labeled 217 bp attL fragment containing a single H′ site positioned close to its center. Reactions were performed in 20 µl with 70 fmol attL in binding buffer without BSA, but with 365 fmol salmon sperm DNA present. Incubation was for 1 h at room temperature, after which samples were loaded on a 7.5% polyacrylamide gel. Gels were run in 0.5× TBE at room temperature and 150 V for 3.5 h.
Cell culture
HeLa and scIHF2 cell lines were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, streptomycin (0.1 mg/ml) and penicillin (100 U/ml). Cells were passaged twice before transfection.
Stable scIHF2 cell lines were generated by electroporation of approximately 8 × 106 cells with 40 µg of poIHF2P at 250 V and 960 µF using a Gene Pulser (Bio-Rad). Stable cell lines were selected with puromycin (20 µg/ml; Sigma).
Western blot analysis
Long-term scIHF2 expression in transgenic HeLa cell lines was monitored after 4 and 8 months continuous culturing. Cell lysates from 8 × 105 cells were prepared by boiling in SDS sample buffer (New England Biolabs) for 5 min. Equal amounts of proteins were separated in a denaturating 12.5% (w/v) polyacrylamide gel and transferred onto a PVDF membrane (Immobilon P, Millipore). The membrane was blocked with 1% blocking solution (Boehringer-Mannheim) and incubated with rabbit polyclonal antibodies raised against wild-type IHF. Bound antibody was detected with anti-rabbit peroxidase-conjugated secondary antibody (Amersham Biosciences). Anti-actin antibodies (Sigma) were used to control for differences in the amount of protein loaded per well.
Immunostaining
Cells were grown on glass coverslips to 50–70% confluence, washed with phosphate-buffered saline (PBS), fixed in 4% formaldehyde/PBS for 15 min, washed again, and incubated for 10 min in 50 mM ammonium chloride/PBS. Coverslips were then treated for 5 min with 0.4% Triton X-100/PBS. Blocking occurred in 1% milk powder/0.5% saponin (Sigma)/PBS for 30 min. Rabbit-α-IHF polyclonal antibodies were incubated with glass coverslips for 1.5 h. The second antibody, α-rabbit-IgG–fluorescein isothiocyanate (FITC) (Sigma), was subsequently added for 1 h. Both antibodies were diluted in 1% milk powder/0.5% saponin/PBS. Between the two antibody incubations, cells were washed four times with 0.5% saponin/PBS. In order to stain the nuclei, 4′,6-diamidino-2-phenylindole (DAPI; Sigma) was added to the second antibody solution at a concentration of 2 µg/ml. The coverslips were then transferred onto glass slides (cell side downward) and embedded in antifade reagent component A (SlowFade Light Antifade Kit, Molecular Probes). Staining was analyzed with a Laser Scan Microscope (LSM, Zeiss).
Functional assays in E.coli
Plasmid SG86 contains a functional pSC101 origin of replication (18). Accordingly, replication and maintenance of SG86 requires IHF. SG86 and pTrcscIHF2 were co-introduced into CSH26-ΔIHF cells which were plated on LB-agar containing 40 µg/ml tetracycline, 60 µg/ml chloramphenicol, 50 µg/ml ampicillin and 0.5 mM IPTG at 37°C. Transformants were expanded in liquid culture overnight under the same conditions, and plasmids were isolated using affinity chromatography (Qiagen). In vivo recombination assays were performed in CSH26-ΔIHF essentially as described previously (17) employing an Int-expression substrate vector pNC9WT and either pTrcscIHF2 or pTrc99a.
Functional assays in HeLa cells
Parental and transgenic HeLa cells were harvested and washed with PBS, and resuspended in RPMI 1640 without l-glutamine or phenol red (Life Technologies). Co-transfection was performed with approximately 7 × 106 cells at 960 µF and 250 mV using a Gene pulser (Bio-Rad). A total of 60 µg of plasmid DNA was used per transfection, with equal amounts of expression and substrate vector. Green fluorescent protein (GFP) expression was analyzed on a FACScalibur (Becton Dickinson) at 48 h after electroporation. A single-cell suspension was prepared, dead cells were stained with 7-amino-actinomycin D (Sigma, Germany), and excluded when FACS data were analyzed with CellQuest™ software (Becton Dickinson). The transfection efficiency was >90% and determined for each experiment by co-transfecting 30 µg of pCMV (mock) with 30 µg of pEGFP-C1.
In vitro recombination assays
Reactions were performed essentially as described (19) in 25 µl of buffer containing 42 fmol of att sites and a 60- or 180-fold molar excess of IHF or scIHF2, respectively. Reactions were kept at room temperature for 40 min, followed by DNA precipitation and digestion with NcoI. Restriction fragments were analyzed in a 0.8% agarose gel run in TBE buffer and visualized through ethidium bromide staining.
RESULTS
Design of a single chain IHF
An inspection of the published co-crystal structure of IHF bound to the H′ site of λ attL revealed that the N- and C-terminal ends of the α-subunit are located on one side of the complex, while the corresponding termini of the β-subunit are positioned on the opposite side (Fig. 1A). This essentially precluded any possibility of connecting the two subunits via a traditional C- to N-terminal peptide linker. However, upon closer inspection, we also noticed that the two termini of the α-subunit are in rather close proximity to a connecting region (β-Q39 to β-E44) between the second α-helix and the first β-sheet of the β-subunit (Fig. 1A). We therefore decided to insert almost the entire α-subunit into this region of the β-subunit.
Figure 1.
Structures of IHF and scIHF2. (A) Co-crystal structure of IHF and the H′ site of attL, as published by Rice et al. (4), with the α- and β-subunit drawn as ribbons in cyan and green, respectively. The N- and C-terminal ends of the α-subunit are marked, as well as the short linker region in the β-subunit that was chosen as an insertion point for the α-subunit to generate scIHF2. (B) scIHF2–H′ model. The two linkers used to connect the two subunits are shown in yellow. The protein is colored in a gradient from blue over white to red following the N- to C-terminus. (C) Superimposition of the IHF structure and the scIHF2 model. (D) The same as (B), but zoomed and tilted around the horizontal axis to highlight the two linkers (yellow) and the respective residues that were chosen to connect the subunits. The figure was prepared with the program Dino (http://www.dino3d.org).
First, we modeled a five amino acid linker that connects residue β-Q39 with the N-terminus of the α-subunit at position α-L3 (linker 1), thus bridging the 11 Å distance between the two residues in the co-crystal structure (Figs 1B and D, and 2). The freed β-G40 was then connected with α-A94 at the C-terminus of the α-subunit via a second linker, which bridges ∼5 Å (Figs 1B and D, and 2). Importantly, a superimposition of the IHF structure and the energy-minimized scIHF2 model revealed no significant perturbations that were introduced by the two linkers (Fig. 1C).
Figure 2.
Amino acid sequence of scIHF2. The two peptide linkers are highlighted in italic letters and are underlined. Note also that a glycine residue is present at the second position of the N-terminus in the protein expressed in eukaryotic cells. The protein expressed in E.coli contains instead an alanine and a serine residue at the second and third position, respectively (pETscIHF2), or a glycine, alanine and serine at the second to fourth position, respectively (pTrcscIHF2). These additions should stabilize the protein in both eukaryotic and prokaryotic cells (20).
Purification and biochemical properties of scIHF2
In order to simplify the purification procedure for scIHF2, we generated an E.coli expression vector (pETscIHF2) that contains six histidine codons added to the C-terminus of the coding sequence (Fig. 2). We reasoned that the short tag would not significantly interfere with either DNA binding or DNA bending because the C-terminus appears to be at a location rather remote from DNA (Fig. 1B and D). Due to our cloning strategy, we also inserted an alanine and serine residue at the second and third position, respectively, between methionine and threonine originally present in the β-subunit. A eukaryotic expression vector contains instead a glycine residue at the second position (Fig. 2) in order to stabilize the protein in mammalian cells (20). The calculated molecular weight of the recombinant, His-tagged protein expressed in eukaryotes is therefore 22 536 Da.
The tag allowed us to purify between 30 and 50 mg of >95% pure scIHF2 from a 1 l culture (Fig. 3A). DNA band-shift assays employing a radiolabeled oligonucleotide comprising the λ H′ site revealed a Kd of 0.24 nM for IHF, which is in excellent agreement with previous measurements (8,9), while scIHF2 exhibits a 3-fold higher Kd (Fig. 3B–D). However, a Kd of 0.79 nM for scIHF2 indicates that the recombinant protein still exhibits a high affinity for H′. This was confirmed by a series of competition experiments using specific and unspecific competitor DNA (data not shown).
Figure 3.

Specific DNA-binding by scIHF2. (A) Purified IHF and scIHF2 (5 and 10 µg of each) were analyzed by (15%) SDS–PAGE together with molecular weight markers (M) run at the left side of the Coomassie-stained gel. (B) DNA binding assay employing 0.1 nM radiolabeled H′ DNA and ≤10 nM IHF. (C) The same as (B), but employing scIHF2. (D) Quantitation of DNA binding assays shown in (B) and (C). The mean Kd values for IHF and scIHF2, determined from two independent experiments performed with either protein, were 0.24 and 0.79 nM, respectively.
We next analyzed scIHF2’s DNA-bending capacity and compared it with that of the parental protein. We again employed DNA band-shift assays using either a 217 bp radiolabeled fragment comprising attL or the H′ oligonucleotide. Within the limits of resolution of these assays, we could not detect any significant differences in DNA bending between wild-type IHF and scIHF2 (Fig. 4A and B).
Figure 4.
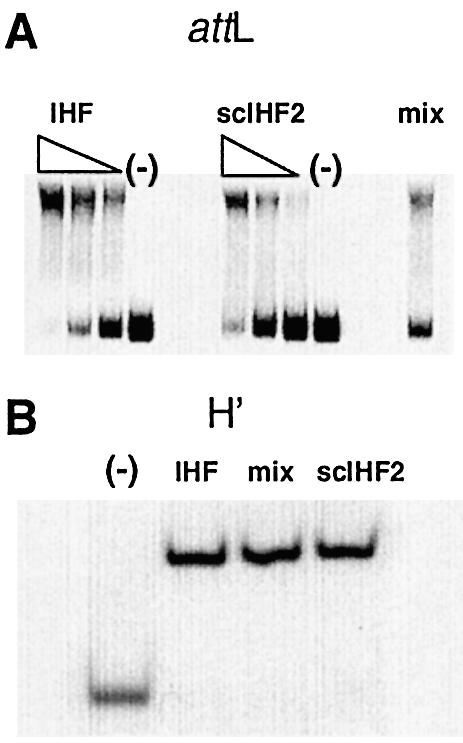
Analysis of DNA bending induced by scIHF2. (A) DNA bending analyzed with a radiolabeled 217 bp attL fragment (70 fmol) and either a 20-fold (or less) excess of IHF or a 40-fold (or less) excess of scIHF2, as indicated. Pre-assembled IHF–attL and scIHF2–attL complexes were also analyzed together (mix). (B) DNA bending analyzed with a radiolabeled oligonucleotide comprising the H′ site with a 20-fold molar excess of IHF or a 50-fold excess of scIHF2. Pre-assembled DNA–protein complexes were also analyzed together (mix).
ScIHF2 promotes λ recombination and pSC101 replication
It appeared from our biochemical analysis that scIHF2 behaves very similarly to wild-type IHF. However, it remained possible that small differences in the degree of protein-induced DNA bending could not be detected by our assays. Since high-precision DNA bending by IHF is required for site-specific recombination (21), a first sensitive test to investigate this possibility further was to employ integrative recombination in E.coli strain CSH26-ΔIHF, which lacks functional IHF protein subunits.
Cells were co-transformed with (i) pNC9WT, a substrate vector carrying attP and attB recombination sites as direct repeats flanking the gene for wild-type Int under the control of an inducible promoter; together with (ii) either an inducible expression vector for scIHF2 (pTrcscIHF2) or a mock vector (pTrc99a). Liquid cultures were induced with IPTG for 5–6 h in order to allow expression of both Int and scIHF2, and plasmid DNA was isolated. Recombination between attP and attB deletes a DNA segment containing the Int expression cassette from the substrate vector, which can be monitored easily by restriction analysis. In the example shown in Figure 5A, it is evident that expression of scIHF2 triggers efficient integrative recombination by wild-type Int, while the mock vector showed no effect. We frequently observed a rather high level of recombination even in the uninduced state, which is most probably due to leaky expression of Int and scIHF2 in co-transfected cells.
Figure 5.
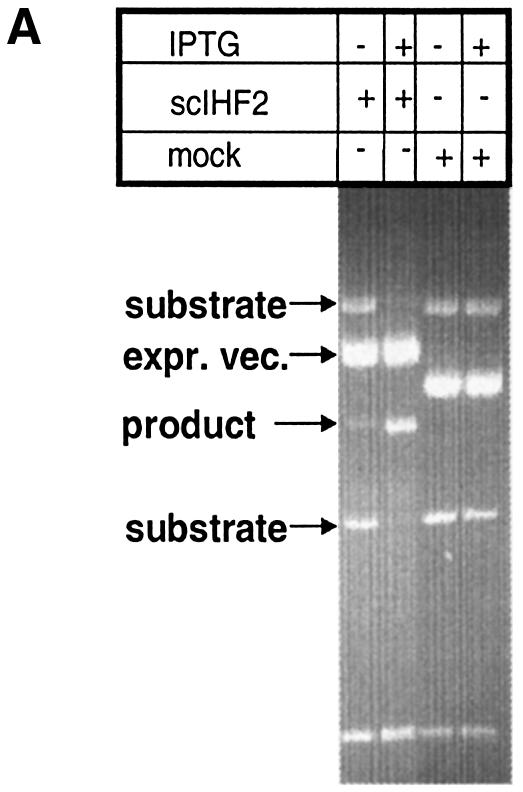
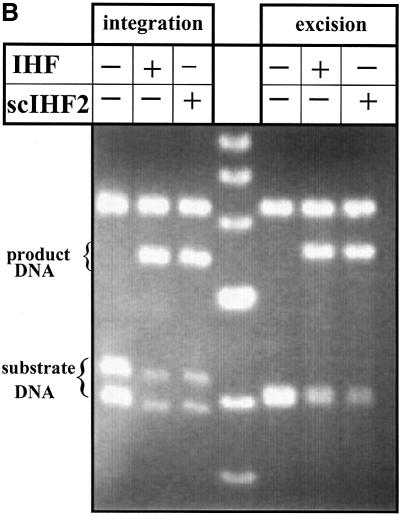
(A) scIHF2 promotes integrative recombination in a ΔIHF E.coli host. pNC9WT, bearing a recombination cassette of directly repeated attB and attP sites flanking the gene for wild-type Int, was co-transformed into CSH26-ΔIHF with either an expression vector for scIHF2 or a mock vector. Stable transformants were induced for 5–6 h with IPTG, and recombination was monitored by agarose gel electrophoresis of AvaI-digested plasmid DNA. (B) In vitro recombination assays. Plasmid substrates for integrative recombination (pλIR) or for excisive recombination (pλER) were incubated with Int (plus Xis in the case of pλER) and either wild-type IHF or scIHF2. Reactions without IHF were used as controls. Recombination can be detected by an altered restriction pattern obtained with NcoI. The top DNA band represents both substrate and product DNA. A DNA marker is run in the middle lane.
In a second test, we employed in vitro assays in order to directly compare the efficiencies with which wild-type IHF and scIHF2 promote integrative and excisive recombination. We adopted reaction conditions described by Cassell and Segall (19) and used plasmid substrates that contain either directly repeated attB and attP sites to test for integrative recombination, or attL and attR sites to analyze excisive recombination. Recombination on either plasmid substrate thus leads to the splicing-out of a DNA segment from the substrate vector, which can be monitored easily by restriction digest (see Fig. 8, top panels). As shown in Figure 5B, wild-type and scIHF2 trigger both types of recombination reactions to about the same extent during 40 min incubation.
Figure 8.
Stimulation of integrative recombination by wild-type Int in scIHF2 transgenic cells. The two top panels depict the substrates for integrative (pλIR) and excisive (pλER) recombination as well as the expected products. These substrates were co-transfected together with either an expression vector for wild-type Int, mutant Int-h, or mutant Int-h/218 or a mock control vector into either parental HeLa cells or transgenic scIHF2 (H/IHF) cells. At 48 h after transfection, fractions of GFP-expressing cells were determined by FACS. Note that the scale for the y-axis [GFP-expressing cells (%)] is different for the two top and bottom panels. We show mean values with standard deviations from 5–6 independent transfections. CMV, cytomegalovirus promoter; GFP, green fluorescent protein; Tstop, transcriptional stop signal.
IHF as an architectural accessory factor also plays an essential role in the initiation of DNA replication at the pSC101 origin (22). Escherichia coli cells lacking functional IHF subunits cannot be used for the propagation of plasmids bearing this origin. Hence, in order to provide further evidence that scIHF2 is biologically active through DNA binding and bending, we tested whether the recombinant protein supports replication of plasmid SG86 that contains a minimal origin derived from pSC101 (18). Experiments employing again E.coli strain CSH26-ΔIHF revealed that only co-transformation of SG86 with pTrcscIHF2 leads to stably transformed cells, and that both vectors could be recovered from liquid cultures. Importantly, all our controls were unable to grow under the same conditions (data not shown).
ScIHF2 triggers λ Int-mediated recombination in human cells
We established thus far that scIHF2 is functional as an essential cofactor in two different DNA transactions in E.coli and in in vitro recombination assays, and decided to generate stable scIHF2–HeLa transgenic cell lines in preparation for functional tests in mammalian cells. These cell lines were characterized first by Southern blotting, PCR and DNA sequencing (data not shown). They contain different copy numbers of the scIHF2 transgene under the control of a strong, constitutively active promoter.
Western analysis performed with crude cell extracts prepared from transgenic cells revealed that, over a period of at least 8 months, high-level scIHF2 expression remains unchanged and that no major proteolytic degradation products are detectable (Fig. 6). These results imply that the recombinant protein appears to be tolerated by mammalian cells kept in culture.
Figure 6.

Long-term expression of scIHF2 in transgenic HeLa cells. Crude cell extracts were prepared from one scIHF transgenic HeLa cell line (H/IHF6) at 1, 4 and 8 months after its generation and analyzed by western blotting. We used an extract prepared from the parental HeLa cell line as negative control. Antibodies raised against actin were used to determine differences in gel loading. Purified scIHF2 (∼10 ng) was used as a positive control.
In order to localize scIHF2, we performed immunostaining with polyclonal antibodies raised against wild-type IHF. At 20 h after cell plating, we find scIHF2 primarily inside the nucleus, while parental HeLa cells show only background staining (Fig. 7). Such a result may be expected for a functional DNA-binding protein of ∼23 kDa, which most probably enters the nucleus by intracellular diffusion.
Figure 7.
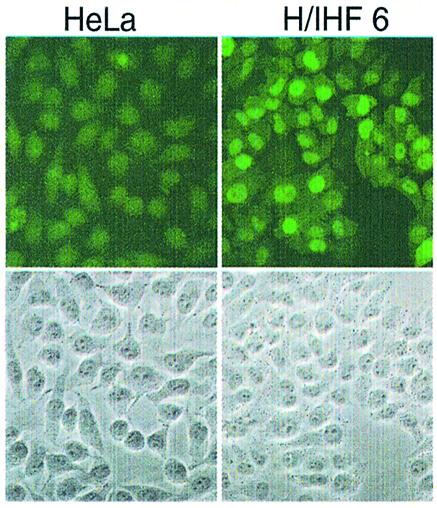
Immunostaining of parental and scIHF2 transgenic HeLa cells. HeLa cells and H/IHF6 cells were plated onto glass coverslips and 20 h later immunostained using polyclonal antibodies raised against IHF. Phase-contrast images of the sections are shown below as reference.
We next performed functional tests in scIHF2–HeLa cells. As shown before, wild-type Int is only marginally active in integrative and excisive recombination on episomal substrates in HeLa cells (13). By applying the same test system, i.e. co-transfection of Int expression and substrate vectors (see Fig. 8, top panels), and then comparing transgenic scIHF2–HeLa (H/IHF) with parental HeLa cells, we show here that the presence of scIHF2 stimulates integrative recombination catalyzed by wild-type Int 4- to 8-fold (Fig. 8, lower left panel). This stimulatory effect is confined to the integrative recombination pathway since scIHF2 does not exhibit a similar effect on excisive recombination (Fig. 8, lower right panel). Further, the activities of mutant integrases, Int-h and Int-h/218, which already catalyze both reactions in the absence of IHF with high efficiency (13), are not significantly affected by the presence of scIHF2 (Fig. 8, middle panels). However, it is also clear from this analysis that the 4- to 8-fold stimulatory effect on integrative recombination by wild-type Int is by far not sufficient to raise the overall efficiency of recombination to the level observed with mutant Ints.
DISCUSSION
We reported here on our approach to generate a functional single chain IHF that can be used in eukaryotic cells. To our knowledge, this is the first example of the design of a recombinant protein in which one entire protein subunit has been inserted into another. We think that it should be possible to apply this strategy also for other heteromeric protein complexes whose three-dimensional structures are known.
The fact that scIHF2 supports site-specific recombination and pSC101 replication in an E.coli host lacking parental IHF has important implications for the structure of scIHF2. First, integrative as well as excisive recombination requires high precision DNA bending by IHF in order to generate functional recombinogenic nucleoprotein complexes (21). Our scIHF2 must therefore be able to facilitate a very similar, if not identical, type of DNA looping upon binding to IHF sites present in attP. Secondly, the functional IHF site as part of the pSC101 minimal origin differs from the λ sites at permissively variable bases within the consensus element as well as in the composition of the upstream located poly(dAT) element and its connecting spacer (23). Hence, scIHF2 recognizes different biologically significant consensus sequences and performs IHF’s function(s) at these sites. Together, this implies that most, if not all, side chains which interact directly or indirectly with DNA are appropriately positioned in scIHF2.
The equivalents of IHF in eukaryotes are the HMG-domain proteins. Some of them show limited sequence specificity, such as the LEF-1 and SRY proteins, while other, more abundant members of this family are sequence independent (5,24). Like IHF, these proteins are involved in DNA transactions such as transcription and recombination. However, the degree of protein-induced DNA bending is significantly smaller (<120°) than that observed with IHF (>160°) (24). We showed here that scIHF2 is stably expressed in transgenic HeLa cells, and that the protein is found primarily inside the nucleus. A conservative estimate of the number of scIHF2 molecules, which is based on several western blot analyses, indicates that between 20 000 and 200 000 copies must be present per cell. A preliminary bioinformatics approach then identified over 90 000 consensus IHF-binding sites scattered more or less randomly throughout the human genome. About 15 000 of those sites have a suitable poly(dAT) element positioned at an appropriate distance upstream. Hence, those latter DNA sites represent segments which could potentially be bent strongly upon scIHF2 binding. We think that at least some of those sites must be accessible for scIHF2, thereby contributing to the rather strong nuclear immunostaining that we observed in some cells. Importantly, the presence of scIHF2 seems not to affect the viability of these cells.
The λ recombination system is more complex than, for example, the Cre/loxP, the Flp/FRT or the phiC31 integrase system (10,25). This higher level of complexity results in a very stringent control over the directionality of recombination. It also increases the fidelity of the system with respect to the choice of recombination partner sequences and the generation of recombinant products (10,26). For example, Int-mediated integration of the phage attachment site attP into genomic sequences other than the 21 bp comprising natural partner attB is a rare event in E.coli cells that express IHF (27). We have demonstrated here that scIHF2 stimulates integrative recombination by wild-type Int on episomal substrates 4- to 8-fold. The very low level of excisive recombination catalyzed by wild-type Int remained nearly unaffected by the presence of scIHF2. This is most likely to be due to the lack of the Xis protein in HeLa cells. A close inspection of the bottom two panels in Figure 8 also reveals that the level of excisive recombination by wild-type Int in HeLa cells is 2- to 3-fold higher than that observed for integrative recombination in HeLa cells. It is possible that the HMG1 or HMG2 proteins, which were shown to stimulate excisive recombination in vitro (28), trigger these reactions even in the absence of IHF and Xis in eukaryotic cells. However, the higher background value obtained with the mock control for excisive recombination complicates this interpretation.
Functional intasomes are therefore apparently formed only on attP and, perhaps, also on attL, but not on hybrid site attR which would require Xis. One potentially important application of the wild-type λ Int system in mammalian cells could thus include scIHF2 for the safe and controlled integration of foreign DNA into one of about 20 suitable yet distinct attB-like sequences that we have identified in the human genome (J.Li and P.Dröge, unpublished results). The high fidelity of the λ system could then limit the frequency of unwanted integration events into other genomic locations as well as illegitimate intragenomic recombination observed with other, more promiscuous site-specific recombination systems (29,30). Eventually such a system could become important in safe genome modifications of, for example, human stem cells for gene therapy purposes. The high fidelity of the λ system may outweigh the rather low efficiency of the integrative recombination reaction that we observe even in the presence of scIHF2.
Potential applications for scIHF2 in mammalian cells are, however, not confined to a role in recombination. We speculate that the recombinant protein may also be useful in studies investigating the structure and function of eukaryotic nucleoprotein complexes inside a living cell. In addition, scIHF2 may be employed in biopharmaceutical production techniques where, through its DNA-bending capacity, it may keep a promoter free of nucleosomes and, thus, more accessible for the transcriptional machinery. This could boost and/or maintain a desired high level of gene expression. Finally, there may exist a possibility to use scIHF2 transgenic cell lines to direct retroviral integration to specific sites in a genome since it was shown that wild-type IHF targets human immunodeficiency virus type 1 cDNA at protein-induced bends in vitro (31).
Acknowledgments
ACKNOWLEDGEMENTS
Special thanks go to S. D. Goodman who provided IHF antibodies and plasmid SG86. He also suggested the use of the minimal pSC101 origin of replication. Thanks also go to A. Segall who provided purified Int and Xis. This work was financed by a Boehringer Ingelheim Fonds predoctoral fellowship to N.C., a predoctoral scholarship from NTU to Q.B., and by research grants from the German Science Foundation (DFG), the Center for Molecular Medicine Cologne and the URC at NTU to P.D.
REFERENCES
- 1.Nash H.A. (1996) The HU and IHF proteins: accessory factors for complex protein–DNA assemblies. In Lin,E.C.C. and Lynch,A.S. (eds), Regulation of Gene Expression in E.coli. R. G. Landes Company, Austin, TX. pp. 149–179. [Google Scholar]
- 2.Goosen N. and van de Putte,P. (1995) The regulation of transcription initiation by integration host factor. Mol. Microbiol., 16, 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Goodrich J.A., Schwartz,M.L. and McClure,W.R. (1990) Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF). Nucleic Acids Res., 18, 4993–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice P.A., Yang,S-w., Mizuuchi,K. and Nash,H. (1996) Crystal structure of an IHF–DNA complex: a protein-induced DNA U-turn. Cell, 87, 1295–1306. [DOI] [PubMed] [Google Scholar]
- 5.Travers A. (1997) DNA–protein interactions: IHF—the master bender. Curr. Biol., 7, R252–R254. [DOI] [PubMed] [Google Scholar]
- 6.Ellenberger T. and Landy,A. (1997) A good turn for DNA: the structure of integration host factor bound to DNA. Structure, 5, 153–157. [DOI] [PubMed] [Google Scholar]
- 7.Dhavan G.M., Crothers,D.M., Chance,M.R. and Brenowitz,M. (2002) Concerted binding and bending of DNA by E.coli integration host factor. J. Mol. Biol., 315, 1027–1037. [DOI] [PubMed] [Google Scholar]
- 8.Yang S-w. and Nash,H.A. (1995) Comparison of protein binding to DNA in vivo and in vitro: defining an effective intracellular target. EMBO J., 14, 6292–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman S.D., Velten,N.J., Gao,Q., Robinson,S. and Segall,A.M. (1999) In vivo selection of integration host factor sites. J. Bacteriol., 181, 3246–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azaro M.A. and Landy,A. (2002) λ Int and the λ Int family. In Craig,N.L., Craigie,R., Gellert,M. and Lambowitz,A. (eds), Mobile DNA II. ASM Press, Washington, DC, pp. 118–148. [Google Scholar]
- 11.Lorbach E., Christ,N., Schwikardi,M. and Dröge,P. (2000) Site-specific recombination in human cells catalyzed by phage λ integrase mutants. J. Mol. Biol., 296, 1175–1181. [DOI] [PubMed] [Google Scholar]
- 12.Christ N. and Dröge,P. (2002) Genetic manipulation of mouse embryonic stem cells by mutant lambda integrases. Genesis, 32, 203–208. [DOI] [PubMed] [Google Scholar]
- 13.Christ N., Corona,T. and Dröge,P. (2002) Site-specific recombination in eukaryotic cells mediated by mutant λ integrases: implications for synaptic complex formation and the reactivity of episomal DNA segments. J. Mol. Biol., 319, 305–314. [DOI] [PubMed] [Google Scholar]
- 14.Nash H.A., Robertson,C.A., Flamm,E., Weisberg,R.A. and Miller,H.I. (1987) Overproduction of Escherichia coli integration host factor, a protein with nonidentical subunits. J. Bacteriol., 169, 4124–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H. and Chong,S. (2003) Visualization of coupled protein folding and binding in bacteria and purification of the heterodimeric complex. Proc. Natl Acad. Sci. USA, 100, 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brünger A.T., Adams,P.D., Clore,G.M., DeLano,W.L., Gros,P., Grosse-Kunstleve,R.W., Jiang,J.S., Kuszewski,J., Nilges,M. and Pannu,N.S. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- 17.Christ N. and Dröge,P. (1999) Alterations in the directionality of lambda site-specific recombination catalyzed by mutant integrases in vivo. J. Mol. Biol., 288, 825–836. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto-Gotoh T., Franklin,F.C., Nordheim,A. and Timmis,K.N. (1981) Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene, 16, 227–235. [DOI] [PubMed] [Google Scholar]
- 19.Cassell G.D. and Segall,A.M. (2003) Mechanism of inhibition of site-specific recombination by Holliday junction-trapping peptide WKHYNY: insights into phage λ integrase-mediated strand exchange. J. Mol. Biol., 327, 413–429. [DOI] [PubMed] [Google Scholar]
- 20.Bachmair A., Finley,D. and Varshavsky,A. (1986) In vivo half-life of a protein is a function of its amino-terminal residue. Science, 234, 179–186. [DOI] [PubMed] [Google Scholar]
- 21.Nunes-Düby S.E., Smith-Mungo,L.I. and Landy,A. (1995) Single base-pair precision and structural rigidity in a small IHF-induced DNA loop. J. Mol. Biol., 253, 228–242. [DOI] [PubMed] [Google Scholar]
- 22.Biek D.P. and Cohen,S.N. (1989) Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 in Escherichia coli: mutations in the topA gene allow pSC101 replication in the absence of IHF. J. Bacteriol., 171, 2066–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenzel T.T., Patel,P. and Bastia,D. (1987) The integration host factor of E.coli binds to bent DNA at the origin of replication of the plasmid pSC101. Cell, 49, 709–717. [DOI] [PubMed] [Google Scholar]
- 24.Giese K., Cox,J. and Grosschedl,R. (1992) The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell, 69, 185–195. [DOI] [PubMed] [Google Scholar]
- 25.Lewis J.A. and Hatfull,G.F. (2001) Control of directionality in integrase-mediated recombination: examination of recombination directionality factors (RDFs) including Xis and Cox proteins. Nucleic Acids Res., 29, 2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radman-Livaja M., Shaw,C., Azaro,M., Biswas,T., Ellenberger,T. and Landy,A. (2003) Arm sequences contribute to the architecture and catalytic function of a lambda integrase–Holliday junction complex. Mol. Cell, 11, 783–794. [DOI] [PubMed] [Google Scholar]
- 27.Miller H.I. and Friedman,D.I. (1981) An E.coli gene product required for λ site-specific recombination. Cell, 20, 711–719. [DOI] [PubMed] [Google Scholar]
- 28.Segall A.M., Goodman,S.D. and Nash,H.A. (1994) Architectural elements in nucleoprotein complexes: interchangeability of specific and non-specific DNA binding proteins. EMBO J., 13, 4536–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt E.E., Taylor,D.S., Prigge,J.R., Barnett,S. and Capecchi,M.R. (2000) Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc. Natl Acad. Sci. USA, 97, 13702–13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thyagarajan B., Olivares,E.C., Hollis,R.P., Ginsburg,D.S. and Calos,M.P. (2001) Site-specific genomic integration in mammalian cells mediated by phage phiC31 integrase. Mol. Cell. Biol., 21, 3926–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bor Y.-C., Bushman,F.D. and Orgel,L. (1995) In vitro integration of human immunodeficiency virus type 1 cDNA into targets containing protein-induced bends. Proc. Natl Acad. Sci. USA, 92, 10334–10338. [DOI] [PMC free article] [PubMed] [Google Scholar]