Abstract
During development of the central nervous system, neurons and glia are generated from immature neural progenitor cells (NPCs). Basic fibroblast growth factor (FGF-2) is a mitogen for these cells both in vitro and in vivo. However, it is not known whether other members of the FGF family have similar mitogenic effects on NPCs. We have found that FGF-4, in addition to FGF-2, is a mitogen for NPCs isolated from fetal and adult central nervous systems. Other family members have no proliferative effects on these cells. FGFs transduce signals to the cytoplasm through a family of transmembrane tyrosine kinase receptors (FGFR-1–4) or their isoforms. The high-affinity receptor binding sites are found in two regions of the FGF-2 molecule. We have examined the involvement of these sites in mitogenic signaling. Synthetic peptides corresponding to sequences in FGF-2 receptor binding sites were examined in [3H]thymidine incorporation assays for their agonist or antagonist activity. A 10-aa sequence present in the first receptor binding domain has been found to act as an antagonist, blocking the mitogenic effects of FGF-2. Chemical crosslinking studies using 125I-labeled FGF-2 showed specific reduction in binding of radiolabeled FGF-2 to its receptors present on the membranes of NPCs. The identification of this sequence will assist in the study of pathways involved in signal transduction for mitogenesis in these cells and elucidate the role of FGF-2 and FGF-4 during normal development and in the pathogenesis of disease.
During development, the vertebrate central nervous system is regionalized to establish the fundamental units for later development and function, and the cells that make up these regions are generated. The neuroectoderm, from which the neural tube forms, generates all cell types present in the adult brain on a precise schedule. Lineage analysis of cells in the developing brain has revealed that neurons and glia are derived from multipotent precursor or stem cells (1–4). Although the temporal sequence leading to pattern formation and neurogenesis is well described, the signals regulating these events and the molecular mechanisms involved are poorly understood. Emerging evidence indicates that mitogens and trophic factors, including fibroblast growth factors (FGFs), function together to bring about these events. Many of the FGFs are expressed in early mammalian embryos, suggestive of their roles in development (4, 5).
The FGF family is comprised of 10 related, but genetically distinct, polypeptides, FGF-1 through 10, with a highly conserved core region and an overall 30–55% sequence identity (5–8). Although considerable homology exists between FGFs, their functions differ significantly. Examination of effects of FGFs in vitro showed that FGF-1 and FGF-2 are both survival factors for neuronal cells isolated from different regions of the fetal brain (9, 10). At high concentrations, FGF-2 is also a mitogen for neural progenitor cells (NPCs) isolated from fetal (11–15) and adult rat (16–18) and mouse (19) brain. FGF-5 is a survival factor for cultured embryonic spinal motoneurons (20) and promotes differentiation of cultured septal cholinergic and raphe serotonergic neurons (21). FGF-6 and FGF-7 are expressed in restricted cell types; FGF-6 is expressed in cells of myogenic lineage (22) and FGF-7 in cells of epithelial origin (23). FGF-9 is a mitogen for glial cells, BALB/c3T3 fibroblasts, and oligodendrocyte-type 2 astrocyte progenitor cells (24). In vivo effects of FGFs have also been described. FGF-2 influences the anteroposterior neural patterning, and FGF-3 functions in the posterior primitive streak (25, 26). FGF-1, FGF-2, FGF-4, and FGF-8 are essential parts of a signaling network required for growth and patterning of the developing limb (4, 5, 7, 27, 28). Finally, FGF-5 regulates the growth cycle of hair (29). Thus, members of the FGF family play an essential role in cellular proliferation, differentiation, survival, and tissue patterning during vertebrate embryogenesis and in adult central nervous system neurogenesis.
FGFs interact with cell surface low-affinity heparan sulfate proteoglycan and high-affinity FGF receptors (FGFR-1–4) or their isoforms that confer different ligand-binding specificities and affinities (7, 30–32). For example, the isoform of FGFR-1 containing immunoglobulin-like domains II and IIIc binds FGF-2 with higher affinity than FGF-4, but both FGFs stimulate DNA synthesis, phosphorylation of the receptor, and proliferation of cells expressing this receptor (33). Conversely, FGFR-3-mediated mitogenicity of FGF-2 is lower than that of FGF-4 (34). Engineered, mitogenetically responsive cell lines expressing all the major splice variants of FGFRs have been used to explore relevant ligand–receptor variant pairs involved in mitogenic activity of FGFs (35). Only FGF-1 could activate all receptor variants, whereas other FGFs showed preference toward specific splice forms. These results suggest that the type of receptors or receptor variants on the target cells, and differential interactions of FGFs with these receptors, determine how FGFs may separately or synergistically participate in development of the central nervous system.
Different approaches have been used to identify the functional domains of FGFs that participate in heparin and receptor binding and activation. Using synthetic peptides spanning the entire sequence of FGF-2, Baird and colleagues (36–38) identified two regions of FGF-2 corresponding to amino acids 33–77 and 115–129 involved in receptor activation in mesenchymal and PC12 cells. A peptide containing amino acids 25–39 supported hippocampal neuronal survival whereas a peptide containing residues 112–155 inhibited the survival and growth of these cells (39). The second putative receptor binding domain (115–129) has also been implicated in inhibiting the binding of FGF-2 to its receptor on primary hippocampal neurons (38, 39). Sites of FGF-2 molecule involved in high-affinity receptor binding and proliferation of endothelial cells have been mapped by using synthetic peptides (40) or by exchanging a loop structure (amino acids 118–122) with the corresponding loop sequence of FGF-1 (41). Similar approaches have been used to map the functional domains of FGF-1 and FGF-7 (42–44). Thus, small peptides are useful in elucidating the sequences involved in specific functions of a molecule.
While recent studies reported that FGF-2 is a mitogen for NPCs (11–19), it is not known whether other members of the FGF family have similar effects on these cells. In addition, little is known about the mechanisms and the involvement of putative receptor binding domains of FGF-2 on mitogenic signaling in NPCs. Here we report that, among the FGFs tested, only FGF-2 and FGF-4 are mitogens for NPCs. A comparison of amino acid sequences revealed a striking similarity between 10 amino acids of FGF-2 (68–77) and FGF-4 (122–131). Synthetic peptides corresponding to these sequences behave as antagonists and inhibit the proliferation of NPCs by FGF-2 and FGF-4 and block the binding of 125I-labeled FGF-2 to receptors present on NPCs. Peptides derived from similar regions of FGF-1 and FGF-5 were inactive. These results show that this 10-aa sequence, Glu-Arg-Gly-Val-Val-Ser-Ile(Phe)-Lys-Gly-Val, is sufficient to elicit the mitogenic effects of FGF-2 and FGF-4 on NPCs.
MATERIALS AND METHODS
Peptide Synthesis.
Synthetic peptides were obtained from two different sources: Scripps Research Institute Peptide Synthesis Facility, La Jolla, CA, and SynPep, Dublin, CA, to ensure that the observed effects of the peptides were not artifactual. Peptides were synthesized by solid-phase methodology and purified by HPLC.
Cell Cultures.
Hippocampi (E17) or spinal cords (E14) from Fischer rat embryos were dissected out, and NPCs were isolated as described previously (11, 12). Briefly, the tissues were washed in PBS and repeatedly triturated with a medium- to small-bore Pasteur pipette to dissociate the cells. After washing in DMEM/Ham’s F-12 (DMEM/F12) medium (GIBCO), the cells were resuspended in the same medium containing N2 supplements (GIBCO; N2 medium), counted, and used in thymidine incorporation or crosslinking assays without culturing. NPCs from adult (3 months old) Fischer rat hippocampus have been maintained in culture in the presence of 20 ng/ml FGF-2 (a gift from Andrew Baird, The Whittier Institute, La Jolla, CA) through multiple passages (17). These cells were grown in N2 medium for 2–3 days before the experiments to decrease the effects of FGF-2, which can last for 24–48 hr in neurons and astrocytes (45). Adult NPCs continue to divide in the absence of FGF-2, albeit at a slower rate. Cells were trypsinized, washed in DMEM/F12 medium, resuspended in N2 medium and counted. Baby hamster kidney (BHK) cells were grown in Earl’s MEM containing 5% fetal bovine serum. For affinity labeling experiments, cells were incubated in 0.5% serum containing medium overnight.
[3H]Thymidine Incorporation Assay.
To assess the mitogenic effects of different FGFs, embryonic progenitor cells isolated from E16 hippocampi were plated in 96-well plates precoated with polyornithine/laminin (11) and incubated with 20 ng/ml of the growth factors and [3H]thymidine (specific activity = 25 Ci/mmol; Amersham) for 48 hr in a 37°C incubator under 5% CO2. For FGF-4 (R & D Systems) dose–response curve, embryonic progenitors were incubated with different concentrations of FGF-4 and [3H]thymidine. Additive effects of FGFs were measured by plating cells with 20 ng/ml FGF-2, FGF-4, or FGF-5 (R & D Systems) alone or in combinations. The effects of the peptide on thymidine uptake by embryonic or adult NPCs were examined by mixing cells (10,000–15,000) with peptides (80 μM) in Eppendorf tubes and incubating at room temperature for 30 min. FGF-2 or FGF-4 (20 ng/ml) was added to appropriate tubes before plating in 96-well plates. Control cultures did not receive any FGF. To test for the toxicity of the peptides, FGF-2 was either added first, incubated, and then treated with peptides or they were added simultaneously. No difference between the treatments was observed. [3H]Thymidine (1 μCi per well) was added 24 hr after the cells were seeded and then incubated for an additional 24 hr. Cells were washed twice with PBS and lysed in 0.4 M NaOH. Cell lysates were neutralized with HCl and counted in a β-counter (Beckman). All experiments were done in triplicate.
Crosslinking to the Receptors.
Crosslinking of 125I-FGF-2 to cells and the inhibition of the binding by peptides were done as described previously (38). Briefly, cells (106) were taken up in 200 μl of binding buffer (DMEM/F12, 20 mM Hepes, pH 7.4/20 mM KCl/0.2% gelatin) and incubated with peptides (80 μM) for 30 min. Eighteen-kilodaltons form of 125I-FGF-2 (10 ng/ml; specific activity = 1,200 Ci/mmol) was added, and the cells were incubated with gentle agitation for 3 hr on ice. After washing with cold PBS twice, cells were taken up in PBS containing enzyme inhibitors (PBS/E; 1 mM phenylmethylsulfonyl fluoride/0.1 mg/ml aprotinin/0.1 mg/ml leupeptin) and 0.05 mM disuccinimidyl suberate (DSS; Sigma) freshly dissolved in Me2SO. Samples were incubated at room temperature for 15–20 min, and the reactions were stopped by addition of 0.2 M glycine in PBS. After washing with PBS/E, the cells were pelleted and then resuspended in 15 μl of 1% Triton X-100 in PBS/E. Samples were mixed with 15 μl of 2× Laemmli’s sample buffer, boiled for 5 min, and electophoresed on an SDS/7.5% polyacrylamide gel followed by autoradiography.
RESULTS
FGF-4, Like FGF-2, Is a Mitogen for NPCs.
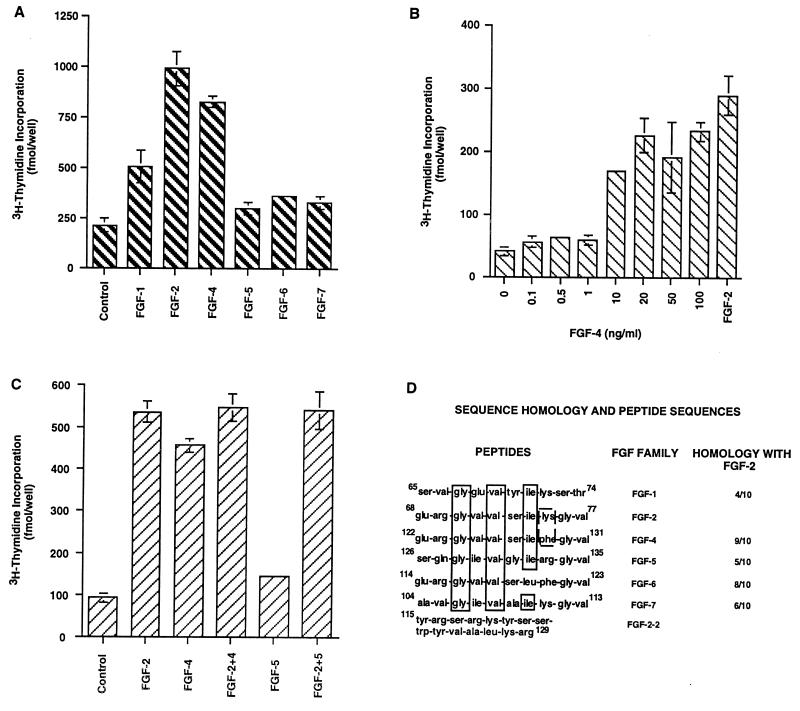
Using thymidine incorporation assay, we found that FGF-2 and FGF-4 had the highest proliferative effects on embryonic hippocampal NPCs, FGF-1 showed lower activity, and FGF-5, FGF-6, and FGF-7 lacked any mitogenic activity (Fig. 1A). Examination of dose-dependent effects of FGF-4 on embryonic hippocampal NPCs showed that FGF-4 has similar dose-dependent effects (Fig. 1B) as FGF-2 (11). FGF-4 is a survival factor up to 1 ng/ml, whereas at or >10 ng/ml, it is a mitogen for these cells. The maximum proliferative effect was observed at 20 ng/ml. Similar dose-dependent survival and proliferative effects of FGF-2 and FGF-4 on embryonic spinal cord NPCs were observed (data not shown). The combination of FGF-2 and FGF-4 at 20 ng/ml was not additive (Fig. 1C), and a combination of FGF-2 and FGF-5, a non-mitogen for these cells, showed the same effect as FGF-2 alone. FGF-2 and FGF-4 thus may be acting through common receptor(s); therefore we hypothesized that their primary sequence contains a common receptor binding site(s) that differentiates them from FGF-5.
Figure 1.
Effects of FGFs on [3H]thymidine incorporation by embryonic hippocampal NPCs. (A) FGF-1, FGF-2, FGF-4 to FGF-7 (20 ng/ml each) were added to cells. Although FGF-1 showed some incorporation, FGF-2 and FGF-4 had the highest effects. The values for FGF-5 to -7 were not different from that of control (no FGF). (B) Dose-dependent effects of FGF-4 on NPCs showed a proliferative effect at or above 10 ng/ml. FGF-2 (20 ng/ml) was included as an internal control. (C) Additive effects of FGF-2, FGF-4, and FGF-5. Combination of FGF-2 and FGF-4 did not increase the thymidine incorporation by the cells. Combination of FGF-2 and FGF-5 showed the proliferative effects of FGF-2. (D) Sequence identity between FGFs in the 10-aa region. The common amino acids between all the FGFs are boxed (solid lines). The amino acids not common between FGF-2 and FGF-4 are boxed in broken lines. FGF-2–2 represents the sequence of second receptor binding site of FGF-2.
Biological Activities of FGF-Derived Peptides on NPCs.
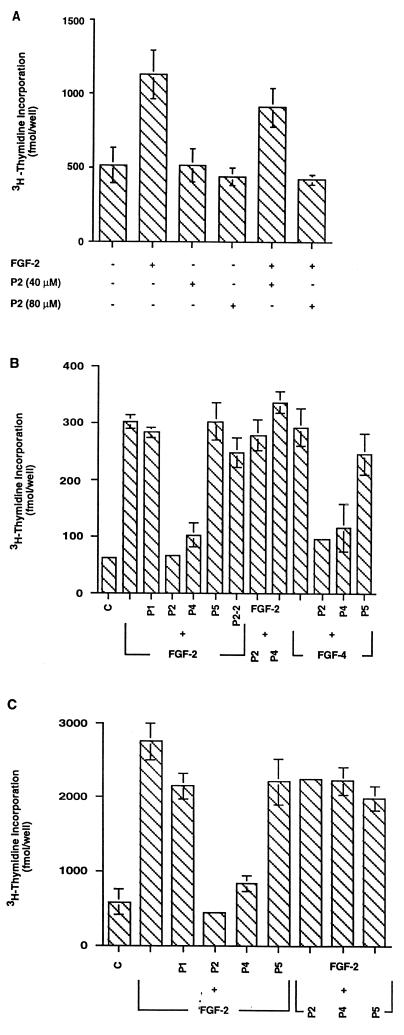
A comparison of the primary sequences of human FGF-1 to FGF-7 showed that a 10-aa sequence (residues 68–77) in FGF-2 has the highest sequence identity (90%) with a similar fragment of FGF-4 (Fig. 1D). This FGF-2 peptide sequence lies within one putative receptor binding site (amino acids 33–77) (12, 13). We subsequently synthesized 10-aa peptides comprising the homologous sequences of FGF-1 (65–74; peptide P1), FGF-2 (68–77; peptide P2), FGF-4 (122–131; peptide P4), FGF-5 (126–135; peptide P5), and a putative second receptor binding site of FGF-2 (115–129; peptide P2–2) and tested their ability to promote or inhibit the proliferation of embryonic and adult hippocampal NPCs. The peptide P2 showed no agonistic effects on embryonic hippocampal NPCs in concentrations up to 80 μM. However, when these cells were treated with 40 μM P2 before the addition of FGF-2 (20 ng/ml), the mitogenic effect of FGF-2 was inhibited by ≈30–40%, and a peptide concentration of 80 μM completely blocked the proliferative effect of FGF-2 (Fig. 2A). Similar results were obtained with the peptide from FGF-4 (P4; data not shown).
Figure 2.
Effects of peptides on thymidine incorporation by embryonic (A and B) or adult (C) hippocampal NPCs. (A) Although peptide P2 had no effects by itself, it inhibited thymidine uptake by embryonic NPCs in a dose-dependent manner. (B) Both peptides P2 and P4 inhibited the mitogenic effects of FGF-2 and FGF-4, but peptides P1, P2–2 or P5 had no effect (P + FGF-2 or P + FGF-4). Addition of FGF-2 before the addition of peptides P2 and P4 (FGF-2 + P2 or P4) abolished their inhibitory effects, indicating that the peptides are not toxic. (C) Peptides showed similar effects on cultured adult hippocampal NPCs. Addition of FGF-2 before peptides P2 or P4 (FGF-2 + P2 or P4) abolished their inhibitory effects.
To examine whether peptides derived from FGF-2 and FGF-4 act through the same receptor on embryonic and adult NPCs, we performed [3H]thymidine incorporation assay on each cell type. NPCs from embryonic brain are primary noncultured cells and adult brain-derived NPCs have been cultured for a long period of time in high concentration of FGF-2. A competitive assessment of the five peptide sequences on the proliferative activity of FGF-2 and FGF-4 on embryonic NPCs was performed (Fig. 2B). Cells incubated in 80 μM P1 or P5 before the addition of FGF-2 showed no inhibition of [3H]thymidine incorporation, whereas cells preincubated with P2 or P4 followed by FGF-2 displayed a significant inhibition. The specificity of the 10-aa sequence on the mitogenic activity of FGF-2 was supported by the P2–2 peptide, which indicated that this second receptor binding site of FGF-2 did not block cellular proliferation. That the mitogenic activity of FGF-4 also is contained within this 10-aa sequence is indicated by findings that only P2 and P4 inhibited the proliferative activity of FGF-4 (Fig. 2B).
Mitogenic effects of FGF-2 on adult hippocampus-derived NPCs could be blocked by peptides P2 and P4 but not by P1 or P5 (Fig. 2C). The peptides did not function as agonists (not shown). In addition, removal of peptides by washing before the addition of FGF-2 reverses their blocking effects (data not shown). These results suggest that the active peptides act similarly on primary embryonic and propagated adult hippocampal NPCs and that prolonged FGF-2 treatment does not change the response of the FGF receptor(s) on the adult NPCs.
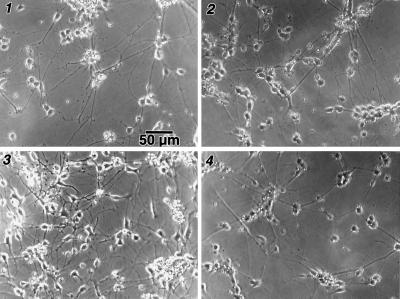
The inhibition of cell proliferation in the presence of P2 and P4 was not due to toxicity. When cells (embryonic or adult) were incubated with FGF-2 either before or concurrently with P2 or P4, thymidine incorporation did not differ from control cultures containing FGF-2 alone (Fig. 2 B and C; FGF-2 + P2 or P4 or P5). Consistent with this observation, microscopic examination of the cells cultured under various experimental conditions showed similar morphologies (Fig. 3).
Figure 3.
The morphology of embryonic cells in cultures grown in N2 medium (1), treated with FGF-2 (2), or FGF-4 (3), or pretreated with peptide P2 followed by FGF-2 (4) was similar, indicating that the peptides were not toxic.
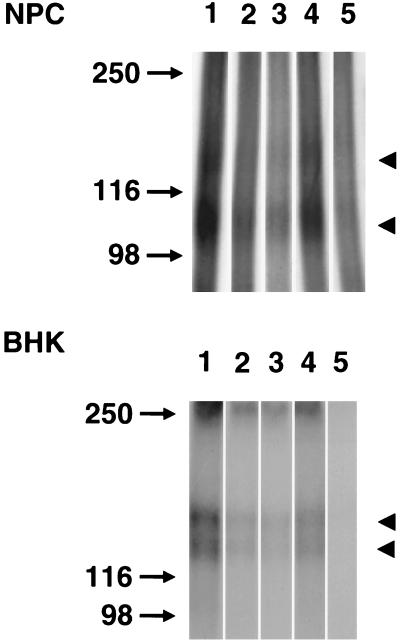
To identify the receptors expressed on NPCs, crosslinking studies using adult hippocampal NPCs were performed (Fig. 4, Upper). A previous study had characterized two membrane proteins of 135 and 85 kDa as receptors for FGF-2 in cultures of fetal hippocampal neurons (38). In the absence of any peptide (Fig. 4, lane 1), two labeled polypeptides of apparent molecular masses of 150 and 100 kDa (arrowhead) were detected. Allowing for the molecular mass of FGF-2, the sizes of the two proteins appear to be ≈135 and 85 kDa, respectively. The peptides P2 and P4 inhibited the labeling of receptors 135 and 85 kDa (lanes 2, 3), whereas P5 had little effect (lane 4). Cold FGF-2 (lane 5) abolished the binding of FGF-2 to both the receptors. Similar results were obtained with embryonic hippocampal NPCs (data not shown). Peptides P2 and P4 thus inhibited affinity labeling of receptors on NPCs, a ligand/receptor binding required for signal transduction to elicit a mitogenic effect.
Figure 4.
Inhibition of 125I-FGF-2 affinity labeling by peptides. (Upper) Adult hippocampal NPCs were treated with peptides P2, P4, or P5 before the addition of radiolabeled FGF-2. The molecular mass markers are indicated on the left. Arrowheads on the right indicate the two receptor polypeptides present in NPCs. The bands appear to be 150 and 100 kDa. Allowing for the molecular mass of FGF-2, the molecular masses of these proteins appear to be about 135 and 85 kDa, respectively. (Lower) The panel of peptides was examined for their ability to inhibit receptor affinity labeling on BHK cells. The bands appear to be 150- and 130-kDa doublet (arrowheads). Peptides P2 and P4 were more effective in blocking of the binding of FGF-2 on both of the receptors, whereas peptide P5 showed lower inhibitory effects.
Inhibitory Effects of the Peptides Are Not Restricted to NPCs.
The specificity of these peptides in blocking interactions of FGF-2 with its receptor(s) in other cell types was examined by using BHK cells. Consistent with a previous report (36), BHK cells have a labeled doublet at ≈150 and 130 kDa (Fig. 4, Lower, lane 1). Peptides P2 and P4 strongly inhibited binding to both the receptor molecules (lanes 2 and 3), whereas peptide P5 (lane 4) was only slightly effective. Unlabeled FGF-2 abolished binding of 125I-FGF-2 to both the receptors (lane 5). The second putative receptor binding domain of FGF-2 (amino acids 115–129) is reported to be involved in receptor activation in BHK cells (36, 38). Thymidine incorporation assay with the peptides P2, P2–2, and P4 showed that they block the proliferative effects of FGF-2 on BHK cells by approximately 22%, 17%, and 18% (P values: 0.0093, 0.0372, and 0.0295), respectively, whereas the effect of peptide P5 was statistically insignificant (P > 0.2). Thus, although the inhibitory effects of the peptides on BHK cells were statistically significant, they were not as effective as on NPCs.
DISCUSSION
Our study demonstrates that both FGF-2 and FGF-4 are mitogens for cultured NPCs, and they act through a common receptor to activate the proliferative signals. Although functions of FGF-2 and FGF-4 are different, they share some common properties (5, 37). Human FGF-4 gene encodes a 206-aa protein that contains a signal sequence (5). While the N-terminal 80 amino acids of FGF-4 are unique, the remaining 126 amino acids have only 40% sequence homology with FGF-2. Remarkably, FGF-2 and FGF-4 showed a 90% homology in a 10-aa region present within the first putative receptor binding domain of FGF-2, with only Lys being substituted by Phe in FGF-4. This 10-aa sequence is rich in hydrophobic, polar, and charged residues. Crystal structure analysis showed that residues 71–77 of FGF-2 form the β strand 5, part of a six-stranded antiparallel β barrel (46). Our results indicate this homologous 10-aa region present within the first putative receptor binding domain of FGF-2 is involved in ligand binding to the FGF receptor(s) and subsequent inhibition of its mitogenic activity. FGF-6, a nonmitogen for NPCs, has 8 of 10 amino acids in common with FGF-2 and 9 with FGF-4 (Fig. 1D). However, peptide containing the homologous 10-aa sequence of FGF-6 did not block the activity of FGF-2 (unpublished data). The lack of mitogenicity of FGF-6 may be due to the double substitutions of Leu in FGF-2 and FGF-4 for Ile and Phe for Lys in FGF-2.
The extracellular region of FGFR-1 contains three immunoglobulin-like domains (I, II, and III) (30). An alternative mRNA splicing pattern in FGFR-1 to -3 creates variants lacking domain I but containing domain II and three alternate forms of domain III (a, b, c), which are important in determining ligand specificity (30). The full-length form of FGFR-1 (I, II, and IIIc) binds both FGF-1 and FGF-2 with equal affinity (34, 47). Isoform containing domains II and IIIc binds FGF-1 with 15- to 20-fold higher affinity than FGF-4 (33) whereas splice variants with domain II and IIIb have 20-fold higher affinity for FGF-1 than FGF-2 (48, 49). Both FGF-2 and FGF-4 activate the IIIc splice form of FGFR-1, -2, and -3 (35). Although it is not known which receptor isoforms are expressed in NPCs, it is likely that common 10-aa residues in FGF-2 and FGF-4 are binding to the same receptor splice form(s). A second putative receptor binding domain of FGF-2 (amino acids 115–129) involved in receptor binding and biological activity in BHK, 3T3, PC12, and endothelial cells (36–39) did not inhibit ligand-receptor binding in our assay but this domain may still be involved in receptor activation by the intact FGF-2 molecule.
Our study shows that the 10-aa peptides functioned only as antagonists. Previous studies using small peptides showed that they have both antagonistic and agonistic functions (34–39, 50, 51). Many extracellular matrix proteins like fibronectin, vitronectin, and fibrinogen contain a tripeptide Arg-Gly-Asp as their cell recognition site (50, 51). In immobilized form, the Arg-Gly-Asp-containing peptide functions as an agonist by promoting cell attachment similar to that of fibronectin, whereas in solution the same peptide inhibited the attachment of cells to a surface coated with fibronectin (50). Peptides derived from FGF-2 sequence also showed agonistic and antagonistic properties (36–39). Whereas some peptides blocked FGF-2-induced neurite outgrowth from PC12 cells and survival of ciliary neurons, others stimulated cell–substratum adhesion and survival of embryonic hippocampal neurons (37, 39). In contrast to these studies, peptides derived from nerve growth factor sequence functioned only as antagonists and inhibited neurite outgrowth from embryonic neurons in culture (52). The lack of agonistic activity of the 10-aa peptide in our study may be due to the fact that the peptide is occupying the binding site for FGF-2 or FGF-4 on the receptor(s) and blocking their binding, but by itself has no capacity to activate signaling pathway leading to mitogenesis.
While the concentration of peptides required to block the activity in our assay is relatively high (80 μM), this concentration was not toxic to cells (see Results). Previous studies also have used high concentrations of peptides, as smaller peptides are less potent than the larger peptides or the parent molecule (39). For example, the Arg-Gly-Asp peptide has been used at the 10–200 μM range to inhibit the binding of fibrinogen to thrombin-stimulated platelets (51). Nerve growth factor-derived peptides inhibited neurite outgrowth from embryonic neurons at a concentration of 2 mM (52), a concentration not toxic to cells.
Affinity crosslinking of 125I-FGF-2 to NPCs showed the presence of two membrane proteins of 135 and 85 kDa, similar to those previously characterized for neurons cultured from fetal hippocampus (38). The FGF-2- and FGF-4 derived peptides (P2 or P4) inhibited the binding of 125I-FGF-2 to both the proteins, although the inhibition of binding to 135-kDa polypeptide appeared to be relatively higher. These results indicate that the binding of FGF-2 to both the receptors may be important for signal transduction needed for proliferation of NPCs.
A thymidine incorporation assay with BHK cells showed that peptides P2, P2–2, P4, or P5 blocked the proliferative effects of FGF-2 by only 20% or less. However, affinity crosslinking studies showed that the peptides P2 and P4 strongly inhibited the binding of 125I-FGF-2 to BHK cells. Thus, although the inhibitory effects of the peptides on BHK cells are significant, they are not as effective in blocking their proliferation. This is likely due to the fact that BHK cells have more FGF receptors and a lower dose requirement of FGF to induce proliferation. To this point we have found that BHK cells require very low concentrations of FGF-2 (500 pg/ml) for their proliferation (data not shown). Alternatively, different FGFR-1 or receptor variants may be involved in binding of FGFs and subsequent mitogenic signaling in BHK cells.
To our knowledge, the study presented here is the first report of involvement of 10-aa domain of FGF-2 in its mitogenic activity. The identification of a sequence in FGF-2 and FGF-4 that functions as an antagonist would likely help in elucidating pathways involved in signal transduction for mitogenesis by FGF-2 and FGF-4, and clarify the role of FGF during normal development and in pathological conditions.
Acknowledgments
We thank M. L. Gage for help in preparation of the manuscript and Drs. L. Fisher and E. Engvall for helpful criticism. This work was supported by separate contracts and grants from the National Institute of Neurological Disorders and Stroke, National Institute on Aging, International Spinal Research Trust, the American Paralysis Association, and the Hollfelder Foundation.
ABBREVIATIONS
- FGF
fibroblast growth factor
- FGF-2
basic fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- NPCs
neural progenitor cells
- BHK
baby hamster kidney
References
- 1.McConnell S K. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 2.Hatten M E, Heintz N. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- 3.Temple S, Qian X. Curr Opin Neurobiol. 1996;6:11–17. doi: 10.1016/s0959-4388(96)80003-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T P, Rossant J. Curr Opin Genet Dev. 1995;5:485–491. doi: 10.1016/0959-437x(95)90053-j. [DOI] [PubMed] [Google Scholar]
- 5.Basilico C, Moscatelli D. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 6.Baird A. Curr Opin Neurobiol. 1994;4:78–86. doi: 10.1016/0959-4388(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 7.Mason I J. Cell. 1994;78:547–552. doi: 10.1016/0092-8674(94)90520-7. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki M, Miyake A, Tagashira S, Itoh N. J Biol Chem. 1996;271:15918–15921. doi: 10.1074/jbc.271.27.15918. [DOI] [PubMed] [Google Scholar]
- 9.Walicke P, Cowen W M, Ueno N, Baird A, Guillemin R. Proc Natl Acad Sci USA. 1986;83:3012–3016. doi: 10.1073/pnas.83.9.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walicke P. J Neurosci. 1988;8:2618–2627. doi: 10.1523/JNEUROSCI.08-07-02618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray J, Schinstein M, Peterson D, Gage F H. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray J, Gage F H. J Neurosci. 1994;14:3548–3564. doi: 10.1523/JNEUROSCI.14-06-03548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick T J, Richards L J, Bartlett P F. Mol Cell Neurosci. 1995;6:2–15. doi: 10.1006/mcne.1995.1002. [DOI] [PubMed] [Google Scholar]
- 15.Ray J, Palmer T D, Suhonen J, Takahasi J, Gage F H. In: Isolation, Characterization, and Utilization of CNS Stem Cells. Gage F H, Christen Y, editors. Heidelberg: Springer; 1997. pp. 129–149. [Google Scholar]
- 16.Richards L J, Kilpatrick T J, Bartlett P F. Proc Natl Acad Sci USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gage F H, Coates P W, Palmer T D, Kuhn H G, Fisher L J, Suhonen J O, Peterson D A, Suhr S T, Ray J. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer T D, Ray J, Gage F H. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 19.Gritti A, Parati E A, Cova L, Frolichsthal P, Wanke E, Faravelli L, Morassutti D J, Roisen F, Nickel D D, Vescovi A L. J Neurosci. 1995;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes R A, Sendtner M, Goldfarb M, Lindholm D, Thoenen H. Neuron. 1993;10:369–377. doi: 10.1016/0896-6273(93)90327-n. [DOI] [PubMed] [Google Scholar]
- 21.Lindholm D, Hartikka J, Berzaghi M P, Castren E, Tzimagiorgis G, Hughes R A, Thoenen H. Eur J Neurosci. 1994;6:244–252. doi: 10.1111/j.1460-9568.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Martin G R. Dev Biol. 1993;158:549–554. doi: 10.1006/dbio.1993.1212. [DOI] [PubMed] [Google Scholar]
- 23.Aaronson S A, Bottaro D P, Miki T, Ron D, Finch P W, Fleming T P, Ahn J, Taylor W G, Rubin J S. Ann NY Acad Sci. 1991;638:62–77. doi: 10.1111/j.1749-6632.1991.tb49018.x. [DOI] [PubMed] [Google Scholar]
- 24.Naruo K, Seko C, Kuroshima K, Matsutani E, Sasada R, Kondo T, Kurokawa T. J Biol Chem. 1993;268:2857–2864. [PubMed] [Google Scholar]
- 25.Doniach T. Cell. 1995;83:1067–1070. doi: 10.1016/0092-8674(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 26.Mansour S L, Goddard J M, Capecchi M R. Development (Cambridge, UK) 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- 27.Crossley P H, Minowada G, MacArthur C A, Martin G R. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 28.Vogel A, Rodriguez C, Izpisúa-Belmonte J C. Development (Cambridge, UK) 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- 29.Hebert J M, Rosenquist T, Gotz J, Martin G R. Cell. 1994;78:1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 30.Givol D, Yayon A. FASEB J. 1992;6:3362–3369. [PubMed] [Google Scholar]
- 31.Johnson D E, Williams L T. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 32.Gray T E, Eisenstein M, Shimon T, Givol D, Yayon A. Biochemistry. 1995;34:10325–10333. doi: 10.1021/bi00033a002. [DOI] [PubMed] [Google Scholar]
- 33.Mansukhani A, Moscatelli D, Talarico D, Levytska V, Basilico C. Proc Natl Acad Sci USA. 1990;87:4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ornitz D M, Leder P. J Biol Chem. 1992;267:16305–16311. [PubMed] [Google Scholar]
- 35.Ornitz D M, Xu J, Colvin J S, McEwen D G, MacArthur C A, Coulier F, Gao G, Goldfarb M. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 36.Baird A, Schubert D, Ling N, Guillemin R. Proc Natl Acad Sci USA. 1988;85:2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schubert D, Ling N, Baird A. J Cell Biol. 1987;104:635–643. doi: 10.1083/jcb.104.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walicke P A, Feige J-J, Baird A. J Biol Chem. 1989;264:4120–4126. [PubMed] [Google Scholar]
- 39.Walicke P A, Harrison C. In: Growth Factors and Alzheimer’s Disease. Hefti F, Brachet P, Will B, Christen Y, editors. Heidelberg: Springer; 1991. pp. 175–192. [Google Scholar]
- 40.Yayon A, Aviezer D, Safran M, Gross J L, Heldman Y, Cabilly S, Givol D, Katchalski-Katzir E. Proc Natl Acad Sci USA. 1993;90:10643–10647. doi: 10.1073/pnas.90.22.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seddon A P, Aviezer D, Li L-Y, Bohlen P, Yayon A. Biochem. 1995;34:731–736. doi: 10.1021/bi00003a004. [DOI] [PubMed] [Google Scholar]
- 42.Seno M, Sasada R, Kurokawa T, Igarashi K. Eur J Biochem. 1990;188:239–245. doi: 10.1111/j.1432-1033.1990.tb15395.x. [DOI] [PubMed] [Google Scholar]
- 43.Wong P, Hampton B, Szylobryt E, Gallagher A M, Jaye M, Burgress W H. J Biol Chem. 1995;270:25805–25811. doi: 10.1074/jbc.270.43.25805. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki K, Oomura Y, Figurov A, Morita N, Yanaihara N. Brain Res Bull. 1995;38:185–191. doi: 10.1016/0361-9230(95)00092-s. [DOI] [PubMed] [Google Scholar]
- 45.Bottaro D P, Fortney E, Rubin J S, Aaronson S A. J Biol Chem. 1993;268:9180–9193. [PubMed] [Google Scholar]
- 46.Walicke P A, Baird A. J Neurosci. 1991;11:2249–2258. doi: 10.1523/JNEUROSCI.11-07-02249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu X, Komiya H, Chirino A, Faham S, Fox G M, Arakawa T, Hsu B T, Rees D C. Science. 1990;251:90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]
- 48.Dionne C, A, Crumley G, Bellor F, Kaplow J M, Searfoss G, Ruta M, Burgess W H, Jaye M, Schlessinger J. EMBO J. 1990;9:2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werner S, Duan D R, de Vries C, Peters K, Johnson D J, William L T. Mol Cell Biol. 1992;12:82–88. doi: 10.1128/mcb.12.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruoslahti E, Pierschbacher M D. Science. 1987;238:490–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 51.Plow E F, Pierschbacher M D, Ruoslahti E, Marguerie G A, Ginsberg M H. Proc Natl Acad Sci USA. 1985;82:8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longo F M, Mobley W C. In: Growth Factors and Alzheimer’s Disease. Hefti F, Brachet P, Will B, Christen Y, editors. Heidelberg: Springer; 1991. pp. 39–60. [Google Scholar]