Abstract
Some studies have suggested that asthma may be a risk factor for coronary heart disease and stroke, particularly in women. Child and adult-onset asthma differ according to inflammatory characteristics and gender distribution. We examined whether childhood-onset and adult-onset asthma were associated with carotid artery intima-media thickness (IMT) in men and women in the Atherosclerosis Risk in Communities (ARIC) study. In unadjusted analyses, the weighted mean far wall IMT thickness for women with history of adult-onset asthma was significantly greater than that of women without history of asthma (0.731mm vs. 0.681mm; p<0.0001) while IMT for women with history of childhood-onset asthma (IMT=0.684mm) did not differ substantially from non-asthmatic women. Mean IMT did not differ significantly according to asthma history among men. When the data were fitted to a linear model, the interaction between asthma status and gender was significant (p=0.006). After adjusting for age, race, BMI, smoking status, smoking pack years, diabetes, hypertension, physical activity, education level, and high and low density lipoprotein levels, the mean IMT difference between women with adult-onset asthma and no history of asthma was attenuated but remained significant (0.713mm vs. 0.687mm, p=0.008). In conclusion, adult-onset asthma but not child-onset asthma is associated with increased carotid atherosclerosis among women but not among men.
INTRODUCTION
Previous studies have linked potent inflammatory mediators known as leukotrienes, and the genes that regulate them, to both asthma and atherosclerosis 1–3. Other studies have suggested that asthma itself may be a risk factor for coronary heart disease and stroke, particularly in women 4–6. Despite this evidence, the importance of asthma as a risk factor for atherosclerotic vascular disease is not well-established. In addition, asthma is a heterogeneous disease with many recognized phenotypes7, which may have differential effects on atherosclerosis risk and therefore should be examined separately. One phenotypic distinction involves age of onset with two asthma subtypes, child-onset or adult-onset asthma. These asthma subtypes are distinct entities both for inflammatory pathophysiology 8,9 and patient susceptibility, with adult-onset asthma being more common in women and the child-onset subtype more common in men 10.
In the present study we sought to examine the relationship between asthma and its subtypes (child-onset and adult-onset) with carotid artery intima-media thickness (IMT) in men and women who were participants in the Atherosclerosis Risk in Communities (ARIC) study. We hypothesized that IMT would be greater in subjects with history of either child- or adult-onset asthma, particularly in women.
METHODS
The Atherosclerosis Risk in Communities (ARIC) study is a prospective study of the etiology of atherosclerotic, cardiovascular, and cerebrovascular disease in four communities in North Carolina, Mississippi, Minnesota, and Maryland. The study population of 15,792 men and women ages 45 to 64 years includes both black and white participants. Carotid IMT was measured in subjects during a baseline clinic visit during 1987 to 1989.
For the present study we examined whether far wall IMT measurements of subjects at baseline differed according to self-reported asthma history. The far wall thickness was chosen because it has been shown to be measured more accurately than the near wall11. We excluded 167 subjects because of missing asthma history data, 1154 subjects because of missing carotid IMT data, and 81 subjects due to history of endarectomy because IMT thickness may have been altered in these subjects. Carotid angioplasty and stenting were not yet being performed at the time IMT was measured in these subjects. An additional 763 subjects were excluded because of missing data for smoking, hypertension, diabetes, body mass index (bmi), physical activity, and lipid profile. This left 13,627 subjects available for analysis.
Measurement of Asthma and Covariates
Asthma history of subjects was classified according to whether they answered yes or no to the questions “Ever had asthma?” and “Was it confirmed by a doctor?”. Subjects who reported having asthma but whose diagnosis was not physician confirmed (9.4% of those reporting asthma) were excluded from the study population. We further classified asthmatic subjects as having “adult-onset asthma” if they reported age of onset of 21 years of age or above or as having “childhood-onset asthma” if onset age was before age 21.
Self reported pack-years of smoking and current smoking status were used as measures of smoking behavior. Diabetes was defined as having an 8 or more hour fasting plasma glucose level greater than 126, a non-fasting plasma glucose greater than 200, or self report of diabetes or taking medications for treatment of diabetes12–14. Hypertension was defined as diastolic blood pressure greater than or equal to 90 mm Hg, systolic pressure greater than 140 mm Hg, or self report of taking medication for hypertension in the two weeks prior to interview15. Physical activity was assessed according to self reported frequency, duration, and intensity of physical activity using a questionnaire and scale developed by Baecke et al. This scale features three index variables for physical activity according to work, sports, and leisure (non-sport) 16. Education was measured as a three-level ordinal variable according to the number of years of school completed (<12 years, 12–16 years, or >16 years). Use of beta adrenergic and oral glucocorticoid asthma medications, which have been associated with cardiovascular disease17,18, was assessed for the period of two weeks prior to the baseline clinic visit. Subjects were asked to bring all prescription and non-prescription medications used during this period to the clinic visit and medications were coded according to drug category.
Pulmonary function was assessed by ARIC personnel using a Collins Survey II Volume Displacement spirometer according to established protocol19. Because we hypothesized that increased atherosclerosis among asthmatics would be a result of systemic inflammation and not diminished lung function, which is independently associated with CHD20, we examined whether the asthma-IMT association persisted after controlling for lung function measured as force expiratory volume in one second (FEV1). The latter was categorized according to gender-specific quartiles.
Measurement of Carotid IMT
Carotid IMT was measured using B-mode ultrasound by trained technicians. Measurements were adjusted for reader differences and temporal trends and missing measurements were imputed using maximum likelihood procedures. The overall mean far wall IMT was calculated by averaging the mean far wall measurements for up to six different far wall sites for each subject: the left and right common bifurcation, internal carotid, and common carotid (optimal angle). Further details on IMT measurement procedures in the ARIC study have been published previously21,22.
Analysis
Baseline covariates were compared among subjects reporting no history of asthma, history of childhood-onset asthma, and history of adult-onset asthma separately among men and women using chi-square tests or pooled or unpooled t-tests.
Within each gender, t-tests were used to compare the weighted mean IMT of subjects with adult-onset asthma or childhood-onset asthma to the mean IMT of subjects with no history of asthma. The weight given to the overall mean far wall IMT for specific subjects was determined by dividing the number of far wall sites used to calculate the overall mean for each subject by six (the maximum number of sites). In this way, subjects whose mean IMT was based on more sites were given more weight than those whose mean IMT was determined by fewer measurements. Using a class variable representing asthma status (none, adult-onset, or childhood-onset), we first tested for the asthma-gender interaction and then fit separate multivariable linear regression models for each gender that included age, race, education, body mass index, smoking status, smoking pack years, diabetes, hypertension, physical activity, and high and low density lipoprotein cholesterol levels. In these models, the multivariable adjusted mean IMT for each subtype of asthma was compared to the adjusted mean IMT of subjects with no history of asthma within each gender. We also tested for the interaction of asthma with smoking status in each gender-specific model. We also compared means when this model was refit excluding subjects with diabetes and hypertension. We then compared the means generated from additional multivariable models in which prevalent coronary heart disease, lung function and asthma medication use were also included.
IMT was also evaluated as a dichotomous outcome variable using logistic regression. For this analysis, significant atherosclerosis was defined as having a mean far wall IMT ≥ 1mm. This cutpoint is based upon previous research in this cohort suggesting that it is clinically significant in the prediction of incident coronary heart disease and stroke in both men and women 23,24,. In our study population, this cutoff corresponds to the upper 10.4% and 4.4% of far wall IMT measurements for men and women, respectively. Multivariate analysis was performed using the same control variables as in the regression analysis.
We also performed additional analyses to investigate whether lung function was associated with IMT differently among men and women. Within each gender, we compared crude and multivariable adjusted mean weighted IMT according to gender-specific quartile of FEV1. We performed trend tests within each gender using an ordinal variable for FEV1 quartile to determine if decreasing lung function was associated with increasing mean IMT. Additionally, we also examined IMT according to current or former asthma status as well as asthma duration.
RESULTS
The prevalence of adult-onset asthma was higher in women than in men (3.4% verses 2.2%) while childhood-onset asthma was more common among men than in women (3.1% verses 2.4%) (Chi-Square p<0.0001). The weighted mean far wall carotid IMT was 0.683 mm for women and 0.779 mm for men.
Both men and women with history of adult-onset asthma were older, had less education, lower FEV1, more pack years of smoking, and were more likely to have diabetes and hypertension than their non-asthmatic counterparts (Table 1). Women, but not men, with adult-onset asthma also had elevated BMI, were more likely to be African American, and reported lower leisure physical activity. Women with adult-onset asthma included a higher proportion of current and former smokers compared to non-asthmatic women, while men with adult-onset asthma had a higher proportion of former smokers and less current smokers. In both men and women, childhood-onset asthma was associated with lower FEV1, but to a lesser degree than adult-onset asthma. Childhood-onset asthma was also associated with a greater prevalence of diabetes among women but not among men. Use of beta-adrenergic and glucocorticosteriod drugs was elevated among all asthmatics but was greatest among those with adult-onset asthma. Work and sport physical activity did not differ according to asthma history among either men or women.
Table 1.
Baseline comparison of men and women according to self-reported asthma history.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variable | No History of Asthma | Asthma Onset Age<21 Years | Asthma Onset Age≥21 Years | No History of Asthma | Asthma Onset Age<21 Years | Asthma Onset Age≥21 Years |
| n=5766 | n=189 | n=132 | n=7102 | n=180 | n=258 | |
| Age | 54.6 | 54.1 | 56.0* | 53.8 | 53.0 | 54.6* |
| Education Level | ||||||
| <12 Years | 23.5% | 21.9% | 34.1%** | 22.2% | 20.6% | 30.6%** |
| 12–16 Years | 36.7% | 35.8% | 27.3%** | 45.2% | 50.6% | 38.4%** |
| >16 Years | 39.8% | 42.3% | 38.6%** | 32.6% | 28.9% | 31.0%** |
| Body Mass Index | 27.3 | 27.2 | 27.2 | 27.5 | 27.6 | 28.5** |
| Black Race | 21.4% | 16.4% | 13.6%* | 27.0% | 25.6% | 33.7%* |
| Diabetes | 10.9% | 9.0% | 15.2% | 9.9% | 13.9% | 17.5%** |
| Hypertension | 33.4% | 28.6% | 36.4% | 32.8% | 32.2 % | 43.4%** |
| Prevalent Coronary Heart Disease | 8.0% | 7.7% | 7.6% | 1.9% | 2.3% | 3.2% |
| Smoking Status | ||||||
| Current | 27.6% | 23.8% | 19.7%* | 24.8% | 22.2% | 28.7% |
| Former | 43.8% | 44.4% | 53.8%* | 21.9% | 27.2% | 24.0% |
| Never | 28.6% | 31.8% | 26.5%* | 53.4% | 50.6% | 47.3% |
| Smoking Pack Years | 23.0 | 22.2 | 26.3 | 10.3 | 11.6 | 14.7** |
| Physical Activity | ||||||
| Sports Index | 2.58 | 2.59 | 2.66 | 2.34 | 2.34 | 2.28 |
| Leisure Index | 2.34 | 2.35 | 2.36 | 2.39 | 2.33 | 2.28** |
| Work Index | 2.35 | 2.31 | 2.21 | 2.05 | 2.05 | 2.12 |
| FEV1 (liters) | 3.34 | 3.10** | 2.73** | 2.45 | 2.21** | 2.05** |
| Asthma Medication Use | ||||||
| Beta Adrenergics | 0.6% | 5.3%** | 30.3%** | 0.5% | 13.9%** | 21.3%** |
| Glucocorticoids | 0.5% | 2.1%* | 13.6%** | 1.0% | 3.3%** | 8.9%** |
p<0.05;
p<0.01 comparing subjects within asthma subtype to subjects reporting no history of asthma within each gender
The weighted mean far wall IMT thickness for women with history of adult-onset asthma was significantly greater than that of women without history of asthma (0.731mm vs. 0.681mm; p<0.0001) while IMT for women with history of childhood-onset asthma (IMT=0.684mm) did not differ substantially from non-asthmatic women (table 2). Among women who reported never having smoked, the crude mean IMT was 0.667mm for non-asthmatics, 0.663mm for child-onset asthmatics, and 0.684 for adult-onset asthmatics. Men with either asthma phenotype had lower mean IMT compared to men with no history of asthma but these differences were not statistically significant. Among men who had never smoked, mean IMT was 0.727 for non-asthmatics, 0.724 for child-onset asthmatics, and 0.709 for adult-onset asthmatics. When the data were fitted to a crude linear model, the interaction between asthma status and gender was significant (p=0.006)
Table 2.
Crude and Adjusted Mean Far Wall Carotid Intima Medial Thickness (IMT) Measurement According to Gender and Self-Reported Asthma History
| Men | Women | |||||
|---|---|---|---|---|---|---|
| No History of Asthma | Asthma w/Age of Onset<21 | Asthma w/Age of Onset≥21 | No History of Asthma | Asthma w/Age of Onset<21 | Asthma w/Age of Onset≥21 | |
| n=5766 | n=189 | n=132 | n=7102 | n=180 | n=258 | |
| Crude Weighted* Mean Far Wall IMT (SE) [p-value] ‡ | 0.780 (0.003) | 0.772 (0.018) p=0.62 | 0.757 (0.015) p=0.12 | 0.681 (0.002) | 0.684 (0.013) p=0.81 | 0.731 (0.013) p<0.0001 |
| Model 1** | ||||||
| Multivariate Adjusted Least Square Mean Far Wall IMT (SE) [p-value] ‡ | 0.785 (0.003) | 0.776 (0.014) p=0.51 | 0.760 (0.016) p=0.13 | 0.687 (0.002) | 0.701 (0.011) p=0.21 | 0.713 (0.010) p=0.007 |
| Model 2† | ||||||
| Multivariate Adjusted Least Square Mean Far Wall IMT (SE) [p-value] ‡ | 0.785 (0.003) | 0.775 (0.014) p=0.49 | 0.758 (0.017) p=0.13 | 0.686 (0.002) | 0.705 (0.012) p=0.12 | 0.716 (0.010) p=0.005 |
Weighted according to number of outer wall sites measured (to a maximum of six) to calculate mean outer wall thickness for a given individual. Weight for the ith individual (Wi)=(number of sites)i/6
Adjusted for age, BMI, black race, smoking status and pack years, diabetes, hypertension, education level, low and high density lipoprotein levels, and physical activity
Adjusted for Model 1 covariates plus prevalent coronary heart disease, lung function (FEV1) and use of Glucocorticoid and Beta-Agonist Asthma Medications
p-values for t-tests comparing asthma subtype to non-asthmatics within each gender
We fit separate multivariable models for each gender. After adjusting for age, race, BMI, smoking status, smoking pack years, diabetes, hypertension, physical activity, education level, and high and low density lipoprotein levels, the mean IMT difference between women with adult-onset asthma and no history of asthma was somewhat attenuated but remained significant (0.713mm vs. 0.687mm, p=0.007) (table 2). The adjusted mean IMT for women with childhood-onset asthma (0.703mm) was also greater than that of non-asthmatic women but the difference was non-significant (p=0.21). Mean IMT for men with either type of asthma remained less than that of non-asthmatic men after adjustment. The interaction between smoking status and asthma was not significant within the male model (p=0.055) or female model (p=0.28). Addition of prevalent coronary heart disease, lung function and asthma medication use to the models did not generally change the results (table 2). The results were also similar when we excluded patients with hypertension and diabetes. Among women, adult onset asthmatics had significantly higher mean IMT (IMT=0.688mm; p=0.0096) compared to non-asthmatics (IMT=0.656mm) while child onset asthmatics did not (IMT=0.669mm; p=0.28). Among men, IMT was 0.754mm for nonasthmatics, 0.714 for adult onset asthmatics, and 0.757 for child onset asthmatics.
In analyses of the dichotomized outcome, women with history of adult-onset asthma were approximately twice as likely (odds ratio [OR]: 2.31, 95% CI, 1.34 to 3.96) to have mean far wall IMT thickness of 1mm or greater compared to women without history of asthma (table 3) while no significant association was observed among women with childhood-onset asthma. After multivariate adjustment, the odds ratios for women with adult-onset asthma were somewhat reduced but the association remained significant (Odds Ratio [OR]: 1.79, 95% CI, 1.00 to 3.22). Addition of prevalent coronary heart disease, lung function and asthma medications to the model did not substantially change these estimates (table 3). No significant associations were observed among men with either type of asthma.
Table 3.
Frequencies and Odds Ratios* Comparing Mean Outer Wall Carotid Intima Media Thickness (IMT) ≥1.0mm According to Gender and Self-Reported Asthma History.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| No History of Asthma | Asthma w/Age of Onset<21 | Asthma w/Age of Onset≥21 | No History of Asthma | Asthma w/Age of Onset<21 | Asthma w/Age of Onset≥21 | |
| n=5766 | n=189 | n=132 | n=7102 | n=180 | n=258 | |
| Percentage of subjects with mean outer wall IMT≥1.0mm | 10.4% | 10.1% | 12.9% | 4.3% | 3.3% | 8.5% |
| Crude Odds Ratio (95% CI) | 1.00 (Ref) | 0.83 (0.45, 1.52) | 1.12 (0.59, 2.13) | 1.00 (Ref) | 0.68 (0.23, 1.99) | 2.31 (1.34, 3.96) |
| Model 1** | ||||||
| Multivariate Adjusted Odds Ratio (95% CI) | 1.00 (Ref) | 0.89 (0.46, 1.71) | 0.93 (0.47, 1.84) | 1.00 (Ref) | 0.78 (0.26, 2.35) | 1.79 (1.00, 3.22) |
| Model 2† | ||||||
| Multivariate Adjusted Odds Ratio (95% CI) | 1.00 (Ref) | 0.83 (0.43, 1.63) | 0.79 (0.38, 1.66) | 1.00 (Ref) | 0.84 (0.27, 2.56) | 1.87 (1.01, 3.46) |
Odds ratios weighted according to number of outer wall sites measured (to a maximum of six) to calculate mean outer wall thickness for a given individual. Weight for the ith individual (Wi)=(number of sites)i/6
Adjusted for age, BMI, black race, smoking status and pack years, diabetes, hypertension, education level, low and high density lipoprotein levels, and physical activity
Adjusted for Model 1 covariates plus prevalent coronary heart disease, lung function (FEV1) and use of Glucocorticoid and Beta-Agonist Asthma Medications
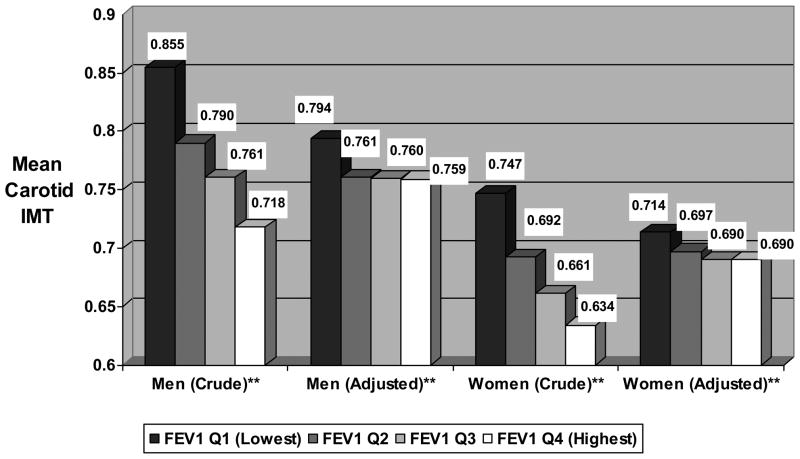
Decreasing lung function was significantly associated with greater carotid intima medial thickness in both men and women (p-values for trend tests<0.0001). Women in the lowest quartile of FEV1 had mean IMT of 0.747mm compared to 0.634mm among women in the highest quartile (figure 1). Among men, those in the lowest quartile of lung function had IMT of 0.855mm compared to 0.718mm for men in the highest quartile. After adjustment for age, smoking, asthma, and other variables, these differences were reduced and IMT remained elevated only among men and women in lowest quartile of FEV1.
Figure 1. Mean Crude and Adjusted* Carotid Intima Medial Thickness According to Gender-Specific Quartile of FEV1.
* Adjusted for age, race, asthma status, BMI, smoking status, smoking pack years, diabetes, hypertension, physical activity, education level, and high and low density lipoprotein levels
**P-Values for all trend tests<0.0001
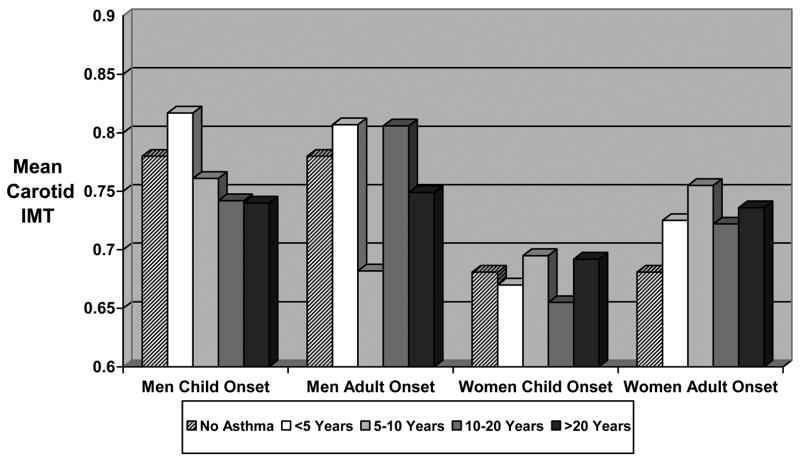
In supplemental analyses, asthma duration was not related to IMT among men or women of any asthma type and did not suggest a dose-response relationship (figure 2). In analyses comparing current and former asthma status with nonasthmatic status (figure 3), crude IMT was significantly greater in women who reported either current (IMT=0.707mm) or former (IMT=0.777mm) adult onset asthma compared with nonasthmatic women. Differences among women with child onset asthma or men with any asthma type were smaller and not significant.
Figure 2. Crude Mean Carotid Intima Medial Thickness According to Asthma Type and Duration.
Figure 3. Crude Mean Carotid Intima Medial Thickness According to Asthma Type and Current Asthma Status.
* p<0.05; ** p<0.0001; Comparing Mean IMT of Former or Current Asthma to No Asthma within Gender
DISCUSSION
We found that adult-onset asthma was associated with carotid atherosclerosis among women. However, asthma was not associated with carotid atherosclerosis among women with childhood-onset asthma or men with either asthma phenotype. Women with adult-onset asthma had crude mean far wall IMT approximately 0.050 mm thicker than their non-asthmatic counterparts, which is comparable in magnitude to the difference between currently smoking women and non-smoking women in this cohort. This difference was reduced by half, but not eliminated, after adjustment for smoking, physical activity, lung function, asthma medication use, and other potential confounders.
The results of our study lend evidence to the notion that asthma is associated with atherosclerosis among women but not among men and further suggest that this relationship is limited to women with adult-onset asthma. Our results are consistent with several previous studies which have suggested that asthma is associated with coronary heart disease and stroke and that these associations are either limited to or more pronounced among women4–6. Toren et al reported stronger associations between asthma and cardiovascular disease among women (All Vascular Disease SMR = 1.9, 95%CI 1.3 to 2.4; Ischemic Heart Disease SMR = 2.5, 95% CI: 1.7 to 3.3) than among men (All Vascular Disease SMR = 1.6; 95% CI: 1.1, 2.1; Ischemic Heart Disease SMR = 1.4; 95%CI: 0.8 to 2.0). 5 Iribarren et al reported multivariate adjusted relative rates of CHD of 1.22 (95% CI: 1.14 to 1.31) among asthmatic women and 0.99 (95% CI: 0.93 to 1.05) among asthmatic men in a large managed care population4. In another study using the ARIC cohort, the rate ratios for stroke differed substantially among men (HR = 0.72, 95% CI: 0.26 to 1.95) and women (HR = 2.20, 95% CI: 1.25 to 3.90) although the authors reported that effect modification on the asthma/stroke association by gender was statistically non-significant6.
To our knowledge, our study is the first to examine asthma age of onset subtypes with respect to atherosclerotic risk. The fact that the association was limited to adult-onset asthma was an unexpected finding. The lack of association between child onset asthma could potentially be due to the fact that many such participants were no longer affected by asthma and were primarily affected only as children. However this seems unlikely as supplemental analysis revealed that women with current and former child onset asthma did not differ in regards to IMT. Another possibility is that child-onset asthmatics did not experience increased atherosclerosis due to different duration of corticosteroid use. However, literature suggests that use of corticosteroids may have either beneficial or detrimental cardiovascular effects depending on the dosage and administration route 18, 25, Thus, it is difficult to predict what impact these drugs might have on the observed association.
Although the mechanism behind the observed differential association of IMT and asthma based on age of onset is unknown, distinct inflammatory mechanisms in these two types of asthma may explain our findings8,9. Adult-onset asthmatics have higher bronchial tissue eosinophil counts 9 and twice as high urinary leukotriene E4 levels compared to childhood-onset asthmatics8, despite the fact that the prevalence of allergen specific immunoglobulin E (IgE) decreases with increasing age of asthma onset 9. The number of polymorphonuclear (PMN) cells in bronchial secretions, which produce leukotrienes, is also higher among adult-onset asthmatics while childhood-onset asthmatics have a greater number of CD3(+) lymphocytes8. Taken together, these data indicate a larger role of leukotriene-mediated inflammatory response in adult-onset asthma than the childhood-onset subtype, which in turn may lead to greater atherosclerotic risk.
The mechanism of the gender specificity reported in this study and in others is also unknown. Our analysis suggests that lower lung function does not appear to be associated with greater increases in IMT among women than among men, therefore lung function should not be the underlying mechanism for the stronger association of asthma history in women than in men. There is literature to suggest a role of female hormones. The human uterus is capable of synthesizing leukotrienes and uterine leukotriene release peaks during menstruation, suggesting hormone-dependent leukotriene production 26. It is possible that this hormonal effect on leukotriene production in the uterus may also affect inflammation elsewhere in the body and therefore predispose asthmatic women to atherosclerosis. Indeed, 30–40% of asthmatic women seen at allergy clinics report worsening of asthma during the premenstrual and menstrual phase of their menstrual cycles27. Women’s potentially greater vulnerability to the inflammatory consequences of asthma may parallel their distinct susceptibility to other inflammatory and immunologic diseases. For example, rheumatoid arthritis and lupus disproportionately affect women and are associated with substantially higher risk of coronary heart disease28–30.
A major limitation of this study is self reporting of asthma status. Asthma self-reporting, especially self-reporting of physician diagnosed asthma, has low sensitivity but high specificity31. Because of the number of nonasthmatics in this study is very large compared to the number of asthmatics, misclassification with poor sensitivity but high specificity should have little or no effect on the validity of the point estimates. Nonetheless, although we only included asthmatic subjects who reported that their asthma diagnosis was confirmed by a physician, some of these patients may actually have chronic obstructive pulmonary disease (COPD) rather than asthma. COPD is associated with systemic inflammation and atherosclerosis32. Even the gender specificity of the association we observed could potentially be explained by such misclassification as physicians may be more likely to misdiagnosis COPD as asthma in women than in men33. To investigate the impact of such misdiagnosis, we examined whether the association differed significantly according to smoking status because COPD is rare among non-smokers. We found that the interaction with smoking was not statistically significant among women. Nonetheless, it is still possible that some of the association we detected among women with adult-onset asthma is attributable to the mixing of misdiagnosed COPD cases with adult-onset asthmatics.
Self reporting of asthma may also result in differential reporting rates according to the perceived health status of participants. We included subjects with symptomatic coronary heart disease in our study population, which is valid if it is assumed that the asthma-IMT association does not change substantially following diagnosis of CHD, regardless of treatment regimen. However, it is possible that subjects who perceive themselves to be in poor cardiovascular health would be more likely to recall other comorbidities such as asthma. To account for this, we controlled for prevalent coronary heart disease in the analysis. Furthermore, the gender specificity of the results would also seem to contradict a large role of such reporting differences as an explanation to the results, since there is no reason to suspect that recall bias occurs differently by gender.
Because the study is observational, confounding is also potential source of bias. Women with adult onset asthma had significantly higher prevalences of hypertension and diabetes compared to other women. However, the association of adult onset asthma with IMT in women persisted after controlling for these factors in multivariable models. This association remained even when we excluded subjects with hypertension and diabetes from analysis. One might also expect smoking, physical activity, lung function, and asthma medication use to be potentially strong confounders of the association between asthma and atherosclerosis. While physical activity did not differ meaningfully according to asthma history, men and women in our study with adult-onset asthma did report greater pack years of smoking, had lower FEV1 measurements, and had a greater prevalence of asthma medication use than either non-asthmatics or those with childhood-onset asthma. In particular, women with adult-onset asthma reported almost 50% greater pack years of smoking compared to non-asthmatic women. Although we were able to control for these variables, we cannot discount the possibility of residual confounding as a potential source of bias.
In conclusion, adult-onset asthma is associated with carotid atherosclerosis among women but not among men. In contrast, childhood-onset asthma is not significantly associated with atherosclerosis among either women or men. This association, which should be confirmed in other cohorts, may have substantial public health importance because adult-onset asthma affects 3–4% of women and atherosclerotic disease is the leading cause of death among women. If confirmed, this association would help to identify women who could benefit from more attention to cardiovascular disease risk.
Acknowledgments
This study was supported by grants from the National Institutes of Health K24 HL077506 and K23 HL07295.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 2.Jala VR, Haribabu B. Leukotrienes and atherosclerosis: new roles for old mediators. Trends Immunol. 2004;25:315–22. doi: 10.1016/j.it.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Votava Z. Are the leukotrienes involved in the bronchial asthma? Biomed Biochim Acta. 1984;43:S434–7. [PubMed] [Google Scholar]
- 4.Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33:743–8. doi: 10.1093/ije/dyh081. [DOI] [PubMed] [Google Scholar]
- 5.Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol. 1996;25:617–20. doi: 10.1093/ije/25.3.617. [DOI] [PubMed] [Google Scholar]
- 6.Schanen JG, Iribarren C, Shahar E, Punjabi NM, Rich SS, Sorlie PD, Folsom AR. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60:633–8. doi: 10.1136/thx.2004.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–8. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Hsu JY, King SL, Kuo BI, Chiang CD. Age of onset and the characteristics of asthma. Respirology. 2004;9:369–72. doi: 10.1111/j.1440-1843.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 10.Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88:587–90. doi: 10.1136/adc.88.7.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikstrand J, Wendelhag I. Methodological considerations of ultrasound investigation of intima-media thickness and lumen diameter. J Intern Med. 1994;236:555–9. doi: 10.1111/j.1365-2796.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 12.ARIC Investigators. ARIC Manual 1. General Description and Study Management. Chapel Hill, NC: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 13.ARIC Investigators. ARIC Protocol 10. Clinical Chemistry Determinations. Version 1.0. Chapel Hill, NC: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 14.ARIC Investigators. ARIC Protocol 7. Blood Collection and Processing. Chapel Hill, NC: National Heart, Blood, and Lung Institute; 1987. [Google Scholar]
- 15.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 16.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 17.Au DH, Udris EM, Fan VS, Curtis JR, McDonell MB, Fihn SD. Risk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunction. Chest. 2003;123:1964–9. doi: 10.1378/chest.123.6.1964. [DOI] [PubMed] [Google Scholar]
- 18.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–70. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 19.ARIC Investigators. ARIC Protocol 4. Pulmonary Function Assessment. Version 7. Chapel Hill, NC: National Heart Lung and Blood Institute, National Institutes of Health; 1987. [Google Scholar]
- 20.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–81. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 21.High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. The ARIC Study Group. J Neuroimaging. 1991;1:168–72. [PubMed] [Google Scholar]
- 22.High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC) The ARIC Study Group. J Neuroimaging. 1991;1:68–73. [PubMed] [Google Scholar]
- 23.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–87. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 24.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 25.Huiart L, Ernst P, Ranouil X, Suissa S. Low-dose inhaled corticosteroids and the risk of acute myocardial infarction in COPD. Eur Respir J. 2005;25:634–9. doi: 10.1183/09031936.05.00079004. [DOI] [PubMed] [Google Scholar]
- 26.Rees MC, DiMarzo V, Tippins JR, Morris HR, Turnbull AC. Leukotriene release by endometrium and myometrium throughout the menstrual cycle in dysmenorrhoea and menorrhagia. J Endocrinol. 1987;113:291–5. doi: 10.1677/joe.0.1130291. [DOI] [PubMed] [Google Scholar]
- 27.Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol. 2003;112:271–82. doi: 10.1067/mai.2003.1676. [DOI] [PubMed] [Google Scholar]
- 28.Petri M. Long-term outcomes in lupus. Am J Manag Care. 2001;7:S480–5. [PubMed] [Google Scholar]
- 29.Van Doornum S, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 2002;46:862–73. doi: 10.1002/art.10089. [DOI] [PubMed] [Google Scholar]
- 30.Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124–6. doi: 10.1161/01.cir.100.21.2124. [DOI] [PubMed] [Google Scholar]
- 31.Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. 1993;104:600–8. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 33.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691–5. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]