Abstract
Both autism and schizophrenia feature deficits in aspects of social cognition that may be related to amygdala dysfunction, but it is unclear whether these are similar or different patterns of impairment. We compared the visual scanning patterns and emotion judgments of individuals with autism, individuals with schizophrenia and controls on a task well characterized with respect to amygdala functioning. On this task, eye movements of participants are recorded as they assess emotional content within a series of complex social scenes where faces are either included or digitally erased. Results indicated marked abnormalities in visual scanning for both disorders. Controls increased their gaze on face regions when faces were present to a significantly greater degree than both the autism or schizophrenia groups. While the control and the schizophrenia groups oriented to face regions faster when faces were present compared to when they were absent, the autism group oriented at the same rate in both conditions. The schizophrenia group, meanwhile, exhibited a delay in orienting to face regions across both conditions, although whether anti-psychotic medication contributed to this effect is unclear. These findings suggest that while processing emotional information in social scenes, both individuals with autism and individuals with schizophrenia fixate faces less than controls, although only those with autism fail to orient to faces more rapidly based on the presence of facial information. Autism and schizophrenia may therefore share an abnormality in utilizing facial information for assessing emotional content in social scenes, but differ in the ability to seek out socially relevant cues from complex stimuli. Impairments in social orienting are discussed within the context of evidence suggesting the role of the amygdala in orienting to emotionally meaningful information.
Keywords: Autism, Schizophrenia, eyetracking, social cognition, emotion, perception
Both autism and schizophrenia are characterized in part by pervasive social dysfunction that impairs the ability to initiate and maintain reciprocal interaction (DSM-IV; APA, 1994). In recent years, a great deal of attention has been devoted to uncovering specific deficits in social cognition that may contribute to this impairment. The term social cognition generally refers to the perception, processing and interpretation of information related to social interaction (Brothers, 1990). A range of social cognitive deficits have been reported for both autism and schizophrenia (Abdi & Sharma, 2004; Pinkham, Penn, Perkins, & Lieberman, 2003; Pelphrey, Adolphs, & Morris, 2005), particularly in theory of mind (Baron-Cohen, 1995; Corcoran, 2001; Yirmiya, Erel, Shaked, & Solomonica-Levi, 1998), facial affect recognition (Celani, Battacchi, & Arcidiacono, 1999; Kohler & Brennan, 2004), and the perception of social cues (Archer, Hay, & Young, 1994; Klin, Jones, Schultz, Volkmar, & Cohen, 2002a). Although reports of these impairments are often remarkably similar for both disorders, they have typically been investigated independently with little attempt to compare the groups. To this point, only three studies have contrasted social cognitive functioning in schizophrenia and autism: two reported similar impairments in autism and schizophrenia on varying measures of theory of mind (Craig, Hatton, Craig, & Bentall, 2004; Pilowski, Yirmiya, Arbelle, & Mozes, 2000), while a third found evidence for greater impairment in autism than schizophrenia for facial affect recognition (Bolte & Puskta, 2003).
Investigations aimed at characterizing the neural correlates of social cognitive dysfunction in autism and schizophrenia also tend to occur in parallel and without direct comparison, despite overlap in the neural structures that are implicated in both disorders. These studies have largely focused on a network of four areas: the fusiform gyrus, the superior temporal sulcus, the medial prefrontal cortex and the amygdala. The fusiform gyrus is implicated in face processing and identity recognition (Kanwisher, McDermott, & Chun, 1997; Puce, Allison, Gore, & McCarthy, 1995) and has been reported to be functionally abnormal in both autism (Pierce, Muller, Ambrose, Allen, & Corchesne, 2001; Schultz, et al., 2000) and schizophrenia (Quintana, Wong, Ortiz-Portillo, Marder, & Mazziotta, 2003; Streit, et al., 2001), although recent findings have challenged these assertions (Hadjikhani, et al., 2004; Hempel, et al., 2003). The posterior superior temporal sulcus is involved in the detection of gaze shifts, dynamic changes of the face and biological motion (Haxby, Hoffmann, & Gobbini, 2000; Pelphrey, Viola, & McCarthy, 2004), and while individuals with autism exhibit hypoactivation in this area relative to controls (Castelli, Frith, Happe, & Frith, 2002; Pierce, et al., 2001), individuals with schizophrenia have been reported to demonstrate normal functioning in this region (Brunet, Safarti, Hardy-Bayle, & Decety, 2003). The medial prefrontal cortex, which is implicated in theory of mind functioning and processing the mental states of others (McCabe 2001; Castelli, Happe, Frith, & Frith, 2000), has been shown to function abnormally in both autism (Baron-Cohen, et al., 1994; Happe, et al., 1996) and schizophrenia (Russell, et al., 2000).
Finally, the amygdala, a neural region that has been a primary focus of investigations regarding social cognitive impairments in autism and schizophrenia, has been identified as a critical structure for threat detection, social appraisal, and the recognition of affect, particularly negative emotions such as fear (Adolphs, Tranel, Damasio, & Damasio, 1995; reviews, see Adolphs, 2001, Amaral, 2003, and Green and Phillips, 2004). Structurally, the amygdala has been reported to be small in schizophrenia (Joyal, et al., 2003; Nelson, Saykin, Flashman, & Riordan, 1998; Wright, et al., 2000) and increased in volume in autism, although some studies find no differences in volume (for a review, see Brambilla, et al., 2003). Functionally, a number of fMRI studies demonstrate decreased amygdala activation in both disorders in response to emotionally-laden stimuli (Baron-Cohen, et al., 1999; Critchley, et al., 2000; Taylor, Liberzon, Decker, & Koeppe, 2002). Behavioral evidence is further suggestive of abnormal amygdala involvement in autism and schizophrenia, as subjects of both disorders have been shown to be impaired in the making of complex social judgments (Adolphs, Sears & Piven, 2001, Baron-Cohen, Wheelwright, & Jolliffe, 1997; Craig, et al., 2004; Oguz, Rita, Miklosne, Szabolcs, & Zoltan, 2003) and in the recognition of negative affect, particularly fear (Capps, Yirmiya, & Sigman, 1992; Evangeli & Broks, 2000; Kohler,et al. 2003; Pelphrey, et al., 2002).
Taken together, these findings suggest that amygdala dysfunction may contribute to the similar behavioral impairments exhibited by individuals with schizophrenia and autism in emotional and social processing. However, to date, no study has attempted to compare the social cognitive performance of individuals with schizophrenia to individuals with autism on a task that is well characterized with respect to amygdala functioning. Furthermore, although both autism and schizophrenia share great overlap in social cognitive dysfunction, little is known concerning the specific mechanisms that underlie these deficits. One possible mechanism contributing to this impairment includes abnormal attention to relevant social information. Using eyetracking technology, several studies have found that both autism and schizophrenia exhibit aberrant scanning patterns while viewing faces, including a reduction in attention to eye regions relative to controls and increased attention to less-salient features such as the mouth and ears (Klin et al, 2002a; Klin, Jones, Schultz, Volkmar, & Cohen, 2002b, Pelphrey, et al, 2002; Phillips & David, 1997; Williams, Loughland, Gordan, & Davidson, 1999). It is unclear, however, whether this abnormality extends to social stimuli more broadly and whether it varies depending on the type and amount of social information being processed. A need also remains to specify whether this potential mechanism of social cognitive deficit differs between the two disorders.
The present study offers a first step toward addressing these questions by comparing the visual scanning patterns of two clinical groups hypothesized to exhibit amygdala dysfunction, (i.e., autism and schizophrenia), with those of controls as they participate in an a task well-characterized with respect to amygdala functioning. In this task, the subject judges the primary emotion being portrayed in a series of static social scene images depicting complex social scenes with faces either present or digitally erased. Adolphs and Tranel (2003) first used this task with amygdala-damaged patients and found that the accuracy of their judgments for negative emotions did not improve to the same degree as controls when faces were present relative to when they were absent. Given that this task has been effectively employed with amygdala-lesioned patients, and given that both autism and schizophrenia have reported amygdala dysfunction, it was hypothesized that both disorders would exhibit similar impairments in social cognition on these stimuli, most notably in a reduced ability to process information from faces. Relative to controls, it was predicted that the two clinical groups would show a greater impairment on social stimuli when faces were included compared to when they were absent. To test this, we obtained two dependant measures: the accuracy of subjects in judging the emotional content of these stimuli, and their eye movements while doing so. Specific attention was given to fixation on faces of the scenes and latency to fixate social regions of interest. Because social attention is known to be impaired in autism from an early age (Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Dawson, et al, 2004; Swettenham, et al, 1998), it was hypothesized that rate and specificity of social orienting might differentiate the two clinical groups.
Methods
Sample
Thirty individuals (10 with autism, 10 with schizophrenia and 10 typically developing controls) participated in this study. The sample size of 10 per group is within the typical range of other eyetracking studies using these populations (Klin, et al, 2002a; Pelphrey, et al, 2002; Phillips & David, 1997). Demographic variables are summarized in Table 1. The three groups did not differ statistically in chronological age or in full-scale IQ on the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1997). Individuals with autism were recruited through referrals from local clinicians and through the North Carolina Autism Subject Registry for participation in a larger study investigating the neuropsychological characteristics of the disorder. DSM-IV diagnoses were confirmed using the Autism Diagnostic Observational Schedule (Lord, et al, 1989) and the Autism Diagnostic Interview-Revised (LeCouteur, et al, 1989). None were on psychotropic medication at the time of testing. Individuals with schizophrenia were outpatients recruited from the Schizophrenia Treatment and Evaluation Program (STEP) at UNC Hospitals. Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-P) and via chart review. All were on anti-psychotic medication (9 atypical, 1 typical) at the time of testing, with a mean Chlorpromazine equivalent dosage of 409.3mg (SD: 278.4) (dosage information was not available for one subject). All were experiencing minimal symptoms based on the Positive and Negative Syndrome Scale (PANSS; Kay, Opler, & Fiszbein, 1992) at the time of testing (mean positive, 7.5 (SD: 2.1); mean negative, 10.2 (SD: 4.2)). The group had been ill for a mean of 4.2 years (SD: 3.1). Typically-developing individuals with no history of mental illness or neurological impairment were recruited from the local community as controls for a broader study of the neuropsychological features of autism. They were matched on age and IQ with the autism subjects. The human subjects committee at the University of North Carolina at Chapel Hill approved this study, and all participants signed informed consent.
Table 1.
Demographic Variables
| Autism (n=10) | Schizophrenia (n=10) | Control (n=10) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Gender | |||
| Male | 10 | 9 | 10 |
| Female | 0 | 1 | 0 |
| Ethnicity | |||
| Caucasian | 9 | 8 | 9 |
| African American | 1 | 2 | 0 |
| Other | 0 | 0 | 1 |
| Age | 23.0 (5.27) | 28.1 (5.07) | 22.4 (6.26) |
| IQ | 107.8 (17.15) | 98.5 (12.99) | 108.1 (21.57) |
Stimuli and Task
Twelve images from the social scenes task, described elsewhere (Adolphs & Tranel, 2003), were used in this study. This subset was selected because they portrayed only a single emotion and thus eliminated the confound of processing multiple emotions contained within a scene. Modal responses from a normed population (Adolphs & Tranel, 2003) defined the emotions displayed in these images in the following way: four were angry, three were afraid, two were sad, two were happy and one was surprise.
The duration of the social scenes task lasted approximately 15 minutes and occurred within the context of a larger battery of cognitive assessment and neuropsychological measures. Subjects sat approximately 56cm from a display, with the visual angle subtending 14.2° × 10.7°. After a standard calibration procedure, each subject was then shown images one at a time, first in a block with the face region digitally removed, followed by a block in which the face region was included. Images were displayed for three seconds each, at which point the seven emotion choices (happy, surprised, afraid, angry, sad, disgusted and neutral) appeared at the bottom of the screen. The image continued to be displayed until the subject verbally selected the emotion depicted in the scene.
Eyetracking
Eye-movement data were sampled at 60Hz with a head-mounted ISCAN series RK-464 remote infrared pupil-corneal reflection eye imaging system (ISCAN Inc., Cambridge, MA, USA). In between the display of each image, a crosshair appeared at the center of the screen in order to ensure that all scanpaths began at the same point for each subject. Eye blinks resulted in missing data, but because the groups did not differ on total number of eye blinks (F (2, 29) = .08, p = .92), these were excluded from subsequent data analyses.
The use of a mobile head-mounted eyetracker and a laptop computer enabled testing to occur in either the home of the subject or in a laboratory on the campus of UNC-Chapel Hill. Measures were taken to ensure that the testing environment (e.g., lighting, etc.) was similar for each subject regardless of the testing location. To compensate for minor head movement and differences in the physical setup, the scene camera recording was later reviewed with a computer software program (PFTrack, version 2.0) for further calibration. The first author and a research assistant then manually inspected the quality of the calibration to ensure the accuracy of all eyetracking output.
Statistical Analyses
The groups were first compared on the accuracy of their emotional judgments during the face absent and face present conditions. Each emotional judgment was scored in accordance to its commonality within previously determined norms for a typically-developing population. For example, if 60% of the normed population labeled a scene as “afraid”, 30% labeled it “sad”, and 10% labeled it “surprised”, a participant in this study would receive a score of 1.0 for a response of “afraid”, a 0.5 for a response of “sad”, and a 0.167 for a response of “surprised”. A higher score therefore indicates better performance by reflecting a greater similarity to responses given by a normed population.
Next, fixation patterns to social elements of the scene (i.e., faces and bodies) were analyzed. Because the area occupied by face and body regions of interests (ROI) is different and varies across image sets, we created a ‘normalized region of interest (ROI) value’ for each image. The area inside the ROI is given an ROI value of ‘1’, while the area outside the ROI is given a ‘0’. Across all pixels, the ROI value is then z-transformed to have the 0 mean and the unit standard deviation. In this way, the larger area the ROI occupies, the smaller is the ROI value assigned for each pixel.
We also analyzed the location of the first saccade and the latency of the first fixation onto the face ROI. The differential latency across groups and conditions (i.e., face present/absent) was analyzed by looking at the temporal evolution of normalized ROI values. This process provided information about social orienting by determining when subjects in each group started to look at faces in the scenes, and the degree to which orienting was greater when the faces were present compared to when they were absent. For this analysis, we discarded all the trials where subjects’ fixation began on a face ROI; for the autism, schizophrenia and control groups, the proportion of the trials excluded was 8.8%, 4.7%, and 10.9%, respectively. We estimated the point in time the normalized ROI values diverged, using a running paired t-test. We determined the first of the three consecutive points with P < 0.05, then lowpass filtered P-values, and finally interpolated the latency as the crossing time where P-values first became p < .05.
Correlations between time spent on social regions and accuracy of emotion judgments were also analyzed.
Results
Group comparisons in the accuracy of emotional judgments
Behavioral responses were analyzed using a repeated measures ANOVA with face condition (absent vs. present) as the within-subjects factor and group (autism vs. schizophrenia vs. controls) as the between-subjects factor. The three groups did not differ significantly on the overall accuracy of their emotional judgments (F (2, 27) = 1.41, ns). All groups performed more accurately in the judgments when the face was present relative to when it was absent (F (1, 27) = 14.83, p < .01), and a non-significant interaction between face condition and group (F (2, 27) = .04, ns) suggests that the degree of improvement in emotion accuracy between the face-absent and face-present conditions did not differ between the three groups. Additionally, group behavioral responses did not significantly differ on each of the five depicted emotions (happy, surprise, angry, afraid and sad) in either the face condition. Behavioral data can be found in Table 2.
Table 2.
Accuracy of Emotional Judgments Relative to Norms
| Emotion | Autism | Schizophrenia | Controls | F |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Face-Absent Condition | ||||
| Happy | .70 (.26) | .85 (.24) | .75 (.26) | 0.90 |
| Sad | .67 (.30) | .73 (.24) | .77 (.30) | 0.37 |
| Angry | .60 (.16) | .68 (.21) | .67 (.22) | 0.43 |
| Afraid | .51 (.24) | .58 (.20) | .41 (.23) | 1.36 |
| Surprise | .38 (.31) | .33 (.18) | .44 (.25) | 0.46 |
| Total | .59 (.10) | .66 (.13) | .62 (.15) | 0.81 |
| Face-Present Condition | ||||
| Happy | 1.0 (.00) | 1.0 (.00) | 1.0 (.00) | - |
| Sad | .63 (.31) | .65 (.33) | .86 (.23) | 1.83 |
| Angry | .58 (.25) | .72 (.15) | .55 (.22) | 1.77 |
| Afraid | .73 (.17) | .75 (.33) | .67 (.26) | 0.20 |
| Surprise | .46 (.31) | .76 (.31) | .48 (.28) | 3.07 |
| Total | .69 (.10) | .76 (.14) | .70 (.10) | 1.23 |
Note. N = 30. None of these F values reached significance at p < .05.
Group comparisons in visual scanpaths
Group differences in normalized face ROI values were assessed using a repeated measures ANOVA with face condition (absent vs. present) as the within-subjects factors and group (autism vs. schizophrenia vs. controls) as the between-subjects condition. A significant main effect emerged for face condition (F (1, 27) = 177.22, p < .01), demonstrating increased fixation duration to face regions when faces were present. A significant interaction between condition and group was also found (F (2, 27) = 6.58, p < .01); Tukey post hoc tests revealed that controls spent significantly greater fixation duration on faces in the face-present condition than the autism (-.96 sec, p = .01), and a trend level effect in the same direction compared to the schizophrenia (-66 sec, p = .09). No differences, however, emerged between the schizophrenia and autism groups (-.29 sec, p = .60). Analysis on the total time spent visually inspecting the face regions of the social scenes revealed a similar pattern of results.
Despite comparable proportion of gaze time between faces and bodies for each group (see Table 3), faces constituted dramatically greater normalized ROI values relative to bodies for all groups (F (1, 27) = 502.56, p < .01, see Figure 1). An ANOVA on normalized ROI values for bodies revealed a main effect for condition (F (1, 27) = 37.69, p < .01), indicating that across all groups, fixation duration on bodies was significantly greater when faces were absent. The group X condition interaction was not significant (F (2, 27) = .732, p = .49), suggesting that fixation duration on bodies decreased to the same degree for each group in the face present condition relative to the face absent condition.
Table 3.
Scanpath Analyses
| Variable | Autism | Schizophrenia | Controls | F |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Face-Absent Condition | ||||
| Mean Fixations per Image | 6.84 (1.31) | 7.19 (2.16) | 6.80 (1.26) | 0.17 |
| Mean Saccades per Image | 6.91 (2.11) | 7.37 (2.66) | 6.67 (1.57) | 0.27 |
| % of Gaze Time on Face | 27.43 (6.58) | 28.14 (6.77) | 28.71 (4.96) | 0.11 |
| % of Gaze Time on Body | 31.37 (6.37) | 35.53 (8.29) | 35.15 (4.37) | 1.24 |
| % of 1st Fixation on Face | 35.01 (17.90) | 27.47 (24.26) | 37.50 (15.84) | 0.71 |
| Mean Latency to Face (ms) | 513 (249) | 734 (233) | 653 (213) | 2.31 |
| Face-Present Condition | ||||
| Mean Fixations per Image | 6.24 (1.51) | 6.67 (2.00) | 5.31 (1.09) | 1.95 |
| Mean Saccades per Image | 6.44 (2.08) | 7.49 (3.34) | 4.85 (1.29) | 3.10 |
| % of Gaze Time on Face | 40.35 (7.85) | 44.82 (7.86) | 52.26 (7.14) | 6.23* |
| % of Gaze Time on Body | 24.68 (7.81) | 27.83 (8.58) | 24.17 (4.48) | 0.76 |
| % of 1st Fixation on Face | 42.51 (23.70) | 24.22 (20.89) | 60.84 (10.43) | 9.09* |
| Mean Latency to Face (ms) | 500 (274) | 631 (193) | 479 (187) | 1.39 |
Note. N = 30. Values marked with an asterisk (*) are significant at p < .01.
Figure 1.
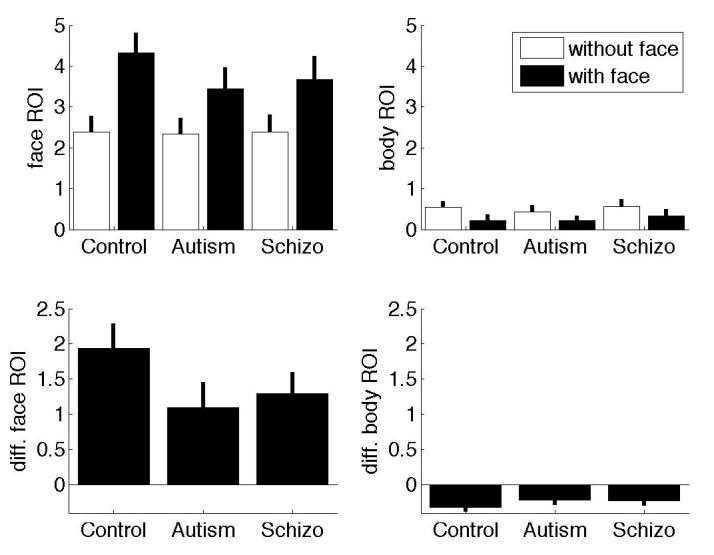
Normalized face (left column) and body (right column) ROI values for each group (sum over 3 seconds). White bars indicate the face absent condition and black bars indicate the face present condition. The bottom row shows the difference in normalized values between the face present and face absent conditions.
The groups did not differ on either total number of fixations (F (2, 27) = .86, ns) or total number of saccades (F (2, 27) = 1.55, ns).
Spatio-temporal characteristics of initial fixation patterns
Next, we concentrated on the analysis of the fixation pattern during the initial inspection period (<1 sec) because we hypothesized that the attentional orienting system would be crucially involved in the early phase of scene examination. The mean latency of the first fixation on face ROI was tabulated in Table 3 and hint at interesting group × condition differences. When faces were absent relative to when they were present, the latency to face ROI increased dramatically in controls, less so in schizophrenia, and minimally if at all in autism. Group differences in the percentage and the latency of first fixation on face regions led us to analyze the temporal evolution of normalized ROI values in order to more thoroughly investigate the timing of social orienting for each group in each condition. As can be seen in Figure 2 (top), while both the control and schizophrenia groups orient to the face region quicker when the face is present (green and blue solid lines) than when the face is absent (broken lines), the autism group orients to the face region at the same speed whether the face is present or not (red lines).
Figure 2.
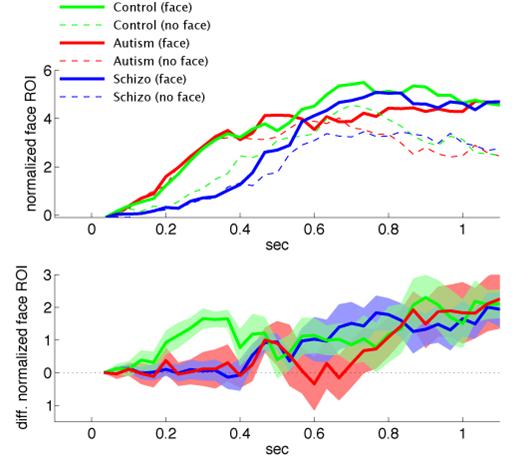
Instantaneous normalized face ROI values (dimensionless) plotted by time for each group (autism, red; normal, green; schizophrenia, blue) in the face-absent (broken lines) and the face-present (solid lines) conditions. The bottom chart shows the difference between the with-face and without-face conditions. The blur above and below the solid line indicate one standard error. Images in which fixation began on a face region were excluded.
We estimated the point in time the normalized ROI values diverged, using a running paired t-test (See methods). The latency for the differential normalized ROI values was 0.20, 0.68, and 1.03 sec for the control, schizophrenia, and autism groups (Figure 2, bottom). We also estimated when the difference in differential normalized ROI values across the groups emerged, using a running-one-way ANOVA (the same running-t-test procedure as above was performed). The differential normalized ROI values diverged at 0.22 sec across the groups (Figure 2, bottom), because the control subjects started discriminating face conditions at 0.20 sec, while the other two groups did not.
To check the robustness of our finding, we analyzed the latency without removing any trials. This resulted in values of 0.17, 0.45, and 0.80 sec for the control, schizophrenia, and autism groups, indicating no qualitative difference and the same rank order. To exclude a possibility that the difference across the groups emerged due to the accuracy of the fixation, we blurred the face ROI using a Gaussian kernel with a standard deviation of 1° visual angle. The normalized ROI values were created for each image and the differential latency was estimated. Again, there was no qualitative difference and the rank order remained the same (0.17, 0.69, and 1.03 sec for the control, schizophrenia, and autism groups).
While the autism and control groups orient to the face ROI at the same speed (mean latency ∼ 0.50 sec), the schizophrenia group does so at a much slower rate (median latency of the first fixation was > 0.63 sec). Despite this delay in overall orienting latency, the schizophrenia group begins to exhibit a fixation duration advantage for faces in the face-present condition relative to the face-absent condition much earlier (0.68 sec) than the autism group (1.03 sec). In other words, only the autism group is failing to modulate orienting based on the presence of the face. While both the control and schizophrenia groups orient to the face ROI quicker when the face is present; the autism group does not. All three groups eventually demonstrate a fixation duration advantage for faces in the face present condition relative to the face absent condition, but only the control group does so almost immediately (0.2 sec) following the onset of image presentation (Figure 2).
Correlations between visual scanning patterns and emotional judgments
Overall accuracy of emotional judgments was not significantly associated with amount of scanning time on the face region for the control and schizophrenia groups in either the face condition. This pattern of results did not differ for any of the five emotion types. A significant negative correlation, however, was found between gaze time on the face region and overall emotional accuracy for the autism group in the face-present condition only (r = -.71, p < .05), suggesting that prolonged gaze time on the face in autism may be indicative either of difficulty in decoding facial emotion or a focus on less emotionally-relevant areas of the face. Gaze time on body regions was not significantly associated with emotion accuracy for any of the three groups in either the face absent or the face present condition.
Discussion
Analysis of visual attention patterns to social scene images revealed provocative differences between the autism, schizophrenia and control groups. While all groups spent a similar proportion of their gaze time on the face region when the face was absent, controls increased their gaze time to faces in the face-present condition to a greater degree than the autism and the schizophrenia groups. This effect extended to the location of first fixation: the control group increased the percentage of time their first fixation was on a face region of a scene when the faces were included to a greater degree than the other two groups. The clinical groups, however, did not differ from each other. This similarity in abnormal scanning behavior suggests that individuals with autism and schizophrenia may not utilize facial information to the same extent as controls when assessing the emotional content of a complex social scene.
An important distinction between the autism and schizophrenia groups emerged when temporal evolution analyses were conducted to examine latencies to fixate faces in the images. While the control and schizophrenia groups oriented to face regions more rapidly when faces were present relative to when they were absent, the autism group oriented to face regions at the same speed regardless of face condition. This suggests that social orienting may not be modulated by the presence of meaningful emotional information in autism, even in the context of an emotion recognition paradigm. Alternatively, it is possible that the autism group may have oriented to face regions at the same rate regardless of the presence of emotional information, indicating an abnormal pattern of orienting to faces but not necessarily emotional information. A control condition in which the face-present condition displayed neutral expressions would be required in order to test this hypothesis. In either case, however, the fact that the autism group failed to demonstrate the typical pattern of orienting to face regions faster when faces are included suggests attentional mechanisms driven more by the general presence of a face region rather than the quality of information it contains.
This finding is consistent with impairments found in autism from a very early age in social attention and orientation (Dawson, et al, 1998; Dawson, et al, 2004; Swettenham, et al, 1998), and suggests that abnormalities in social orienting may be a primary deficit in autism that persists throughout the lifespan. In this study, the impairment was also specific to the autism group: even though the schizophrenia group demonstrated a shared impairment in fixating face regions in the social scenes, they did not exhibit the same failure to modulate orienting speed based on the presence of the face. In contrast, the schizophrenia group was slower to orient to face regions in both conditions relative to the other two groups. This result, however, should be interpreted with caution, as it is impossible for the present study to determine whether the use of anti-psychotic medication contributed to this effect.
Despite the orienting delay, the schizophrenia group mirrored the control group by modulating their latency to fixate faces in the scenes based on the presence of facial information much sooner than did the autism group. This distinction demonstrates the profitability of employing temporal evolution analyses for examining visual scanning behavior in autism and schizophrenia: while gaze time on faces did not differentiate the two clinical groups, inspection of their latency to orient to faces did. Although a number of studies independently examining social cognitive impairments in each disorder hint at significant overlap at both the behavioral and neural levels, the social orienting differences found between the groups here suggest that the underlying mechanisms subserving social cognitive dysfunction in the two disorders may differ. Examination of these mechanisms may help to identify meaningful ways in which disorder-specific developmental pathways can lead to overlapping impairments in social processing.
The autism and schizophrenia groups exhibited intact behavioral performance in the recognition of emotions portrayed in the scenes despite their marked abnormalities in visual scanning, suggesting that eyetracking may be a more sensitive measure for revealing abnormalities in social cognitive functioning than behavioral performance, at least on the task used here. The failure to find group differences in emotion recognition, albeit with a limited sample size, may suggest that the visual scanning abnormalities found in this study are less related to affect recognition than to other aspects of cognitive functioning. However, because impairments in orienting and attending to face regions within a broader social context reflect abnormal perceptual strategies for processing social information, the abnormalities in visual scanning found in the autism and schizophrenia groups may indicate a deficit in a fundamental underlying component of normative social cognitive functioning. Although irregularities in the perception of socioemotional environments should presumably hinder the detection of important social cues necessary for more sophisticated social cognitive abilities, the social scenes task used in this study was less successful than previous studies in eliciting behavioral impairments in emotion recognition. There are a number of reasons why this may have occurred. First, the duration of stimulus presentation may be too long to elicit group differences. Second, the emotional displays used here are only of simple emotions, often exaggeratedly portrayed for dramatic effect, and their depiction may not have been subtle enough to elicit group differences. Finally, the relatively small sample size of ten subjects per group raises the possibility of the occurrence of type 2 error. Although the sample size was large enough to reveal marked differences between the groups in patterns of visual scanning, a larger sample may have been needed to determine whether the social scenes task is capable of eliciting affect recognition impairment in autism and schizophrenia. To test this hypothesis, we conducted power analysis using a bootstrap method. Our simulation indicated that meaningful group difference would not have occurred even if we included ∼30 subjects in each group. The failure to find emotion recognition deficits in the two clinical groups using this task should therefore not be interpreted as conclusive, but rather should highlight the need for future work to assess the efficacy of the social scenes task for measuring affect recognition dysfunction in autism and schizophrenia.
This limitation notwithstanding, the behavioral performance by the two clinical groups in this study contrasts importantly from results previously reported for amygdala-damaged patients on the same task, whose emotional accuracy only improved minimally, if at all, when the face region was present (Adolphs & Tranel, 2003). The discrepancy in performance between the two studies emphasizes the distinction between the absence of a neural structure and impaired functioning of that region, and highlights the fact that, unlike lesioned patients who often have a clear onset of impairment, autism and schizophrenia are neurodevelopmental disorders in which transactional processes interact over time to result in neural abnormalities. An examination of developmental aspects of amygdala dysfunction with a more sensitive behavioral assay, such as eye tracking, may therefore be critical for disentangling the differential effects of lesion versus developmental impairment.
A need also exists for continued comparisons between autism and schizophrenia in order to elucidate why two heterogeneous disorders with differing etiologies and developmental pathways often, in adulthood, exhibit similar social cognitive profiles. Contrasting performance across clinical groups with overlapping features can provide greater insight into areas of similarity while simultaneously illuminating meaningful differences in phenomenology and neural circuitry between the disorders. The common practice of simply comparing clinical groups with normal populations may therefore limit the amount of information to be gained in a study by failing to highlight clinical characteristics specific to the disorder being investigated. By revealing a shared impairment in facial fixation but an important distinction in social orienting, the current study exemplifies the benefits of this approach by demonstrating how a direct comparison of autism and schizophrenia can expose both commonalities and dissociations that refine our understanding of the social cognitive deficits that characterize each disorder.
While the intact behavioral performance exhibited by the two clinical groups suggests that neither autism nor schizophrenia demonstrate the same pattern of impairment as amygdala-damaged patients, it is interesting to note that the abnormalities found for both groups in social fixation and orienting are similar to those reported for a patient with bilateral amygdala lesions (Adolphs, et al, 2005). This patient’s severe impairment in the recognition of fear in faces disappeared when specifically instructed to look only at the eye region, although the improvement was not maintained once she returned to nondirected, voluntary face viewing. This finding hints at the role of the amygdala in directing attention to emotionally-relevant information and provides a potentially provocative explanation for both the failure of the clinical groups to exhibit normal levels of facial attention, and the finding that the social orienting latency of the autism group did not differ as a function of the presence of the face. The results reported here are consistent with research indicating abnormal visual attention to social stimuli in autism (Klin, et al, 2002a; Klin, et al, 2002b; Pelphrey, et al, 2002) and schizophrenia (Phillips & David, 1997; Williams, et al, 1999), and highlight the need for further studies, particularly those using neuroimaging techniques, to assess the degree to which amygdala dysfunction may underlie deficits in social orienting in both disorders.
Acknowledgments
This project was funded by grants from the National Institutes of Mental Health (STAART grant U54 MH66418; J. Piven), the Cure Autism Now Foundation (R. Adolphs), the National Alliance for Autism Research/Autism Speaks (R. Adolphs), and Johnson and Johnson Pharmaceutical Research and Development, LLC, USA (D. Penn). Noah Sasson was supported by National Institute of Child Health and Human Development Grant T32-HD40127. We are grateful to Monica Stubbs, Ellen Cohen, Morgan Parlier, Shannon Gallagher and Todd Corl for their help collecting and coding these data, Eden Kung for his programming assistance, and Grace Baranek for her help with recruiting participants. We would also like to thank all the individuals who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi Z, Sharma T. Social cognition and its neural correlates in Schizophrenia and Autism. CNS Spectrums. 2004;9:335–343. doi: 10.1017/s1092852900009317. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinions in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. Journal of Cognitive Neuroscience. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41:1281–1289. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. The Journal of Neuroscience. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Archer J, Hay DC, Young AW. Movement, face processing, and schizophrenia: Evidence of a differential deficit in expression analysis. British Journal of Clinical Psychology. 1994;33:517–528. doi: 10.1111/j.2044-8260.1994.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness. MIT Press/Bradford Books; Boston: 1995. [Google Scholar]
- Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms: Clinical finding in children with autism and a functional neuroimaging study of normal adults. British Journal of Psychiatry. 1994;165:640–649. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Wheelwright S, Bullmore E, Brammer M, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: An fMRI study. European Journal of Neuroscience. 1999;11:1891–1998. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Jolliffe T. Is there a “language of the eyes”? Evidence from normal adults and adults with autism or Asperger syndrome. Visual Cognition. 1997;4:311–331. [Google Scholar]
- Bolte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychological Medicine. 2003;33:907–915. doi: 10.1017/s0033291703007438. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: Review of structural MRI studies. Brain Research Bulletin. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behaviour and neuropsychology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle M, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41:1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Capps L, Yirmiya N, Sigman MD. Understanding of simple and complex emotions in non-retarded children with autism. Journal of Child Psychology and Psychiatry. 1992;33:1169–1182. doi: 10.1111/j.1469-7610.1992.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms of the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Celani G, Battacchi MW, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. Journal of Autism and Developmental Disorders. 1999;29:57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Corcoran R. Theory of mind and schizophrenia. In: Corrigan PW, Penn DL, editors. Social cognition and schizophrenia. American Psychological Association; Washington, DC: 2001. pp. 149–174. [Google Scholar]
- Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: Comparison of patients with paranoid delusions, Asperger’s syndrome and healthy controls. Schizophrenia Research. 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SCR, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Evangeli M, Broks P. Face processing in schizophrenia: Parallels with the effects of amygdala damage. Cognitive Neuropsychiatry. 2000;5:81–104. doi: 10.1080/135468000395754. [DOI] [PubMed] [Google Scholar]
- Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neuroscience and Biobehavioural Reviews. 2004;28:333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C. “Theory of mind” in the brain: evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffmann EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4:223–232. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hempel A, Hempel E, Schonknecht P, Stippich C, Schroder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Research: Neuroimaging. 2003;122:115–124. doi: 10.1016/s0925-4927(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Laakso MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, et al. The amygdala and schizophrenia: A volumetric magnetic resonance imaging study in first-episode neuroleptic-naïve patients. Biological Psychiatry. 2003;54:1302–1304. doi: 10.1016/s0006-3223(03)00597-3. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Rating Manual. Multihealth Systems, Inc.; Toronto, Canada: 1992. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and Quantifying the Social Phenotype in Autism. American Journal of Psychiatry. 2002;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Brennan AR. Recognition of facial emotions in schizophrenia. Current Opinion in Psychiatry. 2004;17:81–86. [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism Diagnostic Interview: A Standardized Investigator-Based Instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Mawhood L, Schopler E. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imagin study of cooperation in two-person reciprocal exchange. PNAS. 2001;98:11832–11835. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging. Archives of General Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Oguz K, Rita E, Miklosne P, Szabolcs K, Zoltan J. The relationship between “theory of mind” and IQ in patients with schizophrenia. Psychiatria Hungarica. 2003;18:95–98. [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2005;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, David AS. Visual scanpaths are abnormal in deluded schizophrenics. Neuropsychologia. 1997;35:99–105. doi: 10.1016/s0028-3932(96)00061-9. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform “face area” in autism: Evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Arbelle S, Mozes T. Theory of mind abilities in children with schizophrenia, children with autism, and normally developing children. Schizophrenia Research. 2000;42:145–155. doi: 10.1016/s0920-9964(99)00101-2. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. American Journal of Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. Journal of Neurophysiology. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biological Psychiatry. 2003;53:1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- Russell TA, Rubia K, Bullmore ET, Soni W, Suckling J, Brammer MJ, Simmons A, Williams SCR, Sharma T. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. American Journal of Psychiatry. 2000;10:2945–2950. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides A, Sinnemann T, Wolwer W, Dammers J, Zilles K, et al. Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: A magnetoencephalographic study. American Journal of Psychiatry. 2001;158:1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A, Charman T, Rees L, Wheelwright S. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry. 1998;39:747–753. [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophrenia Research. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—3rd Edition (WAIS-3®) Harcourt Assessment; San Antonio, TX: 1997. [Google Scholar]
- Williams LM, Loughland CM, Gordon E, Davidson D. Visual scanpaths in schizophrenia. Is there a deficit in face recognition? Schizophrenia Research. 1999;40:189–199. doi: 10.1016/s0920-9964(99)00056-0. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Erel O, Shaked M, Solomonica-Levi D. Meta-analyses comparing theory of mind abilities of individuals with autism, individuals with mental retardation, and normally developing individuals. Psychological Bulletin. 1998;124:283–307. doi: 10.1037/0033-2909.124.3.283. [DOI] [PubMed] [Google Scholar]


