Abstract
In cerebellar granule neurons of neonatal rats micromolar concentrations of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA-21) and cyclothiazide, two negative modulators of the spontaneous agonist-dependent rapid desensitization of α-amino-3-hydroxy-5-methylisoxazolepropionic acid (AMPA)-gated ion channels, facilitate AMPA receptor function by increasing the content of free cytosolic Ca2+ as measured by single-cell fura-2 acetoxymethyl ester (Fura-2) Ca2+-dependent fluorescence and intracellular Na+ measured with the sodium-binding bezofuran isophthalate acetoxymethyl ester fluorescence indicator. IDRA-21 increases intracellular Na+ transient with a threshold (5 μM) that is ≈10 times higher and has an intrinsic activity significantly lower than that of cyclothiazide. By virtue of its low intrinsic activity, IDRA-21 elicits a free cytosolic Ca2+ transient increase that is shorter lasting than that elicited by cyclothiazide even when the drug is left in contact with cultured granule cells for several minutes. Additionally, while dose dependently, 5–25 μM cyclothiazide in the presence of AMPA is highly neurotoxic, IDRA-21 (up to 100 μM) is devoid of neurotoxicity. The neurotoxicity elicited by cyclothiazide persists in the presence of dizocilpine (an antagonist of N-methyl-d-aspartate-selective glutamate receptors) but is blocked by 2,3-dihydroxy-6-nitrosulfamoylbenzo[f]quinoxaline (a competitive AMPA receptor antagonist) and the 1-(aminophenyl)-4-methyl-7,8-methylendioxy-5H-2,3-benzodiazepine (GYKI 52466; a noncompetitive AMPA receptor antagonist). Since the doses of IDRA-21 that enhance cognitive processes in rats and monkeys are several orders of magnitude lower than those required to elicit marginal neurotoxicity in cultured neurons, it can be surmised that IDRA-21 is a potent cognition-enhancing drug virtually devoid of neurotoxic liability because it acts as a partial negative allosteric modulator of AMPA receptor desensitization.
The discovery that the rapid spontaneous agonist-dependent desensitization of α-amino-3-hydroxy-5-methylisoxazolepropionic acid (AMPA)-gated ion channels can be reduced by drugs that act on specific allosteric sites present within or in the proximity of the flip-flop 38-amino acid region of the AMPA receptor subunits (1–5) has become an interesting model to assist discovery of drugs that might ameliorate learning, memory, and attention deficits via a facilitation of AMPA-selective glutamatergic synaptic function (6–8). Pyrrolidinone derivatives, such as aniracetam (9); benzoylpiperidine derivatives, such as BDP-12 or BDP-20 (6, 10); and benzothiadiazine derivatives such as cyclothiazide, diazoxide, and 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA-21) (11–13) attenuate the desensitization rate of AMPA-gated Na+ currents.
Among the benzothiadiazine derivatives, IDRA-21 has attracted particular interest because when given orally in low (μmol/kg) doses to rats it improves their performance in water maze and passive avoidance tests when they are impaired by drugs that amplify γ-aminobutyric acid (GABA)-gated Cl− current intensity (benzodiazepines), or inhibit muscarinic receptor function (scopolamine), or competitively antagonize glutamate action at AMPA/kainate receptors [2,3-dihydroxy-6-nitrosulfamoylbenzo[f]quinoxaline (NBQX) (7, 8, 14)]. Moreover, in monkeys IDRA-21 antagonizes the cognitive deficit elicited by alprazolam, a benzodiazepine with full positive allosteric modulatory activity on GABAA receptors (8), by a mechanism independent from the benzodiazepine action on GABAA receptors (6, 8). In addition, IDRA-21 facilitates the induction of long-term potentiation by lowering its threshold in rat hippocampal slices (15).
Thus, IDRA-21 might represent a prototype that enhances cognitive function presumably by acting with a limited intrinsic activity and high potency as an inhibitor of agonist-dependent AMPA receptor rapid desensitization. Because of this pharmacological profile, this drug could be tested as a remedy for attention disorders in children and as a treatment of senile dementias, including early stages of Alzheimer disease.
Concerns have been raised, however, regarding the therapeutic use of inhibitors of the rapidly occurring agonist-dependent AMPA receptor desensitization because of their potential neurotoxic liability (16, 17). Moudy et al. (16) showed that in primary cultures of hippocampal neurons prepared from rat embryos, the increase in duration of AMPA-gated channel function by cyclothiazide or diazoxide resulted in a strong amplification of the Ca2+ transients, leading to excitotoxicity. Thus, we considered it important to compare in dissociated primary neuronal cultures of neonatal rats the potency and intrinsic activity of cyclothiazide and IDRA-21 on AMPA-mediated Na+ and Ca2+ influx with their respective neurotoxic liabilities. By measuring free cytosolic Ca2+ ([Ca2+]i) and Na+ [Na+]i transients monitored with fura-2 acetoxymethyl ester (Fura-2) and with sodium-binding benzofuran isophthalate acetoxymethyl ester (SBFI), respectively, we have studied the kinetics of the AMPA-induced [Ca2+]i and [Na+]i transient increase elicited by IDRA-21 and cyclothiazide. The results obtained indicate that IDRA-21, in concentrations two orders of magnitude greater than those achieved in the brain with administration of doses that virtually block drug-induced cognition impairment in rats (7, 14) and monkeys (8), increased AMPA-induced [Na+]i and [Ca2+]i transients with a low intrinsic activity. Because of its partial allosteric modulatory activity, IDRA-21 is virtually devoid of neurotoxic liability even in doses that are one order of magnitude greater than those antagonizing the cognitive deficit elicited by benzodiazepines in rats (7, 14) and monkeys (8).
MATERIALS AND METHODS
Cultures of Cerebellar Granule Neurons.
Dissociated neurons enriched in granule cells (about 95%) were prepared from cerebella of 8-day-old Sprague–Dawley rats and cultured for 8–9 days in medium containing 25 mM K+ as described (18).
Estimation of Cell Viability.
Neuronal cell death was estimated by cell counts after intravital staining of the cultures for 3 min at 22°C with a mixture of fluorescein-diacetate (15 μg/ml) and propidium iodide (4.6 μg/ml). The stained cells were then counted at ×200 magnification using a standard epi-illumination-fluorescence microscope as described by Favaron et al. (18). The same field was counted simultaneously by two blinded observers. Counts that did not differ by >10% were used for the final calculations. Cell counts for each experimental session consisted of four to six fields per dish and three to five dishes per each data point.
Single-Cell [Na+]i.
imaging. To monitor [Na+]i, cells were loaded for 1 hr at 37°C with SFBI (10 μM) dissolved in culture medium supplemented with 0.1% Pluronic F-127 (BASF Bioresearch, Cambridge, MA) (19). The cells were then washed four times with 1 ml of Locke’s solution (154 mM NaCl/5.6 mM KCl/3.6 mM NaHCO3/2.5 mM CaCl2/1 mM MgCl2/5.6 mM glucose/5 mM Hepes, adjusted to a final pH of 7.4) and allowed to equilibrate for 30 min at room temperature in the above buffer. Using an Attofluor digital microscopy system (Atto Instrument, Potomac, MD) the fluorescence of up to 35 cells was monitored simultaneously at room temperature in each experimental session. Digital imaging of SBFI was carried out in individual cells at excitation wavelengths of 334 nm and 380 nm with an emission wavelength at 520 nm from 3.5 mm2 squares positioned in the middle of each cell. Calibration of [Na+]i was performed in situ after each experiment. Cells were treated with a calibration medium made from the appropriate mixture of high-concentration solutions of Na+ and K+. The Na+ high-concentration solution contained 134.2 mM sodium gluconate, 25.4 mM NaCl, 1 mM MgCl2, 3.6 mM NaHCO3, 1.3 mM CaCl2, 5.6 mM glucose, 200 mM ouabain, 5 μM gramicidin, and 5 mM Hepes, pH 7.2; the K+ high-concentration solution was identical except for complete replacement of Na+ with K+. The partial Cl− 50 replacement with gluconate in the calibration experiments prevented excessive cell swelling during calibration (19). The ratio of the fluorescence intensities produced by 334 nm and 380 nm excitation at four different [Na+]i concentrations (5 mM, 25 mM, 50 mM, and 100 mM) were used to calculate the sodium calibration curve for each individual cell, as described by Harootunian et al. (20).
Single-Cell [Ca2+]i Imaging.
To monitor changes in [Ca2+]i, cells were loaded for 30 min at 37°C with Fura-2 (5 μM) applied to the culture medium. After washing four times with Locke’s solution, the fluorescence emitted at >500 nm after excitation at 334 nm (F334) or 380 nm (F380) was averaged from each cell using the Attofluor digital microscopy system as described above. The [Ca2+]i was calculated from the F334/F380 ratio as described by DeErausquin et al. (21) using the Kd of Fura-2 for Ca2+ (264 nM) after correction for room temperature. Because the fluorescent properties of Fura-2 are strongly affected by the intracellular milieu, calibration of [Ca2+]i was performed by an in situ assay in the presence of 10 μM ionomycin as described by Kiedrowski and Costa (22). The experimental chamber was 0.5 ml, and each drug was applied by replacement of the medium including the drug. To remove a previously applied drug, the chamber was washed four times with 0.5 ml of Locke’s solution before the next drug addition.
Drugs.
Dizocilpine and AMPA were both purchased from Research Biochemicals; NBQX from Tocris Neuramin (Bristol, U.K.) Cyclothiazide and the 1-(aminophenyl)-4-methyl-7,8-methylendioxy-5H-2,3-benzodiazepine (GYKI 52466) were a gift from Lilly Research Laboratories. IDRA-21 was kindly synthesized by A. Kozikowski (Georgetown University, Washington, DC) and found chemically pure by gas chromatographic mass fragmentographic analyses). Fura-2, and SBFI were obtained from Molecular Probes. All other drugs were purchased from Sigma. Stock solutions of IDRA-21 and cyclothiazide were prepared in dimethyl sulfoxide at concentrations of 100 mM; dilutions were made before every experiment. The highest concentration of dimethyl sulfoxide was 0.1%. We made sure that this concentration of dimethyl sulfoxide did not affect the parameters to be measured in the cells.
Statistical Analyses.
Data on neuronal death are presented as the mean ± SEM. Statistical comparisons were made using analyses of variance followed by Duncan’s multiple range test using the computer-assisted program of Tallarida and Murray (23). Data on [Na+]i were analyzed using Student’s t test II: pair data (23). Regression lines and differences in the slopes were evaluated statistically according to the analysis of variance (23).
RESULTS
Cyclothiazide But Not IDRA-21 Increases AMPA Excitotoxicity.
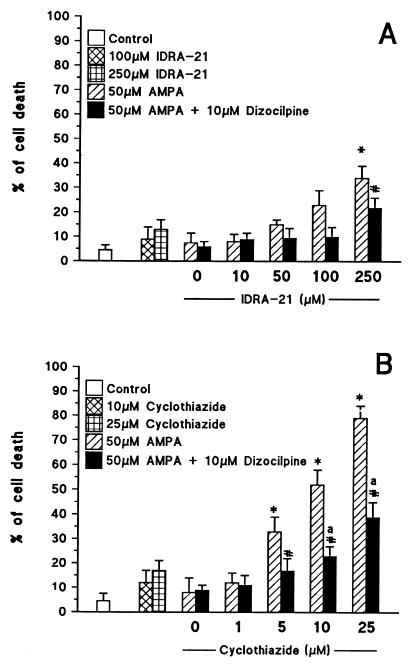
Exposure of cerebellar granule neurons for 1 hr to 50 μM AMPA (Fig. 1) failed to produce detectable neurotoxicity during the following 24 hr. Though cyclothiazide and IDRA-21 in the absence of AMPA failed to induce neurotoxicity in doses up to 25 or 250 μM, respectively (Fig. 1), 1-hr exposure to a combination of 50 μM AMPA with increasing cyclothiazide concentrations (up to 25 μM) resulted in a dose-related incidence of cell death during the following 24 hr. In contrast, the association of 50 μM AMPA with 10–100 μM IDRA-21 failed to elicit significant neurotoxic effects (Fig. 1B). A small but significant neurotoxic effect was observed only when neurons were exposed to 50 μM AMPA in the presence of 250 μM IDRA-21 (Fig. 1A) suggesting that the IDRA-21 neurotoxic liability is about 1/50 that of cyclothiazide.
Figure 1.
Differences in the neurotoxicity of simultaneous application of AMPA and IDRA-21 (A) or AMPA and cyclothiazide (B) in primary cultures of cerebellar granule cells. The ordinate shows % neuronal death (mean ± SEM = 6) measured 24 hrs after a 60 min exposure. Where indicated dizocilpine was added 5 min prior to the simultaneous application of AMPA and one of the two modulators. ∗, P < 0.01 when AMPA/IDRA-21- or AMPA/cyclothiazide-treated neurons were compared with control treated neurons or with neurons treated with IDRA-21 or cyclothiazide without AMPA. #, P < 0.01 when AMPA/IDRA-21- or AMPA/cyclothiazide-treated neurons in the presence of dizocilpine were compared with neurons receiving the same drug treatment combination without dizocilpine. a, P < 0.05 when AMPA/cyclothiazide/dizocilpine-treated neurons were compared with neurons treated with AMPA or with cyclothiazide without AMPA. Note that 50 μM AMPA, 10 or 25 μM cyclothiazide, and 100–250 μM IDRA-21 per se failed to produce a significant increase of neuronal death. Results similar to those reported in A and B were obtained in at least four other experiments with different batches of cerebellar granule cells.
The neurotoxicity elicited by the combination of 50 mM AMPA with 10 mM cyclothiazide [% cell death varied from 6.0 ± 1.2 (vehicle-treated cells) to 52 ± 8.5 (cyclothiazide-treated cells), n = 5] was counteracted by the application of either NBQX [% cell death 9.5 ± 2.0, P < 0.01] or GYKI 52466 [% cell death 7.8 ± 1.8, P < 0.01], a competitive (24) and a noncompetitive (25) AMPA receptor antagonist, respectively. The neurotoxicity of such association was antagonized only partially by dizocilpine, a specific N-methyl-d-aspartate (NMDA) receptor blocker (26) (Fig. 1B). In contrast, the association of AMPA + 250 μM IDRA-21 in the presence of dizocilpine was virtually devoid of toxicity and was identical to the effect of 250 μM IDRA-21 alone (Fig. 1A). Similar results were obtained if 100 μM AMPA was used instead of 50 μM AMPA or if 1 mM glutamate plus 5 μM dizocilpine were used in combination with 10 μM cyclothiazide or 250 μM IDRA-21 (% neuronal death by glutamate 14 ± 2.5; glutamate + 10 μM cyclothiazide 43 ± 5; glutamate + 25 μM cyclothiazide 62 ± 2, glutamate + 250 μM IDRA-21 17 ± 1.8).
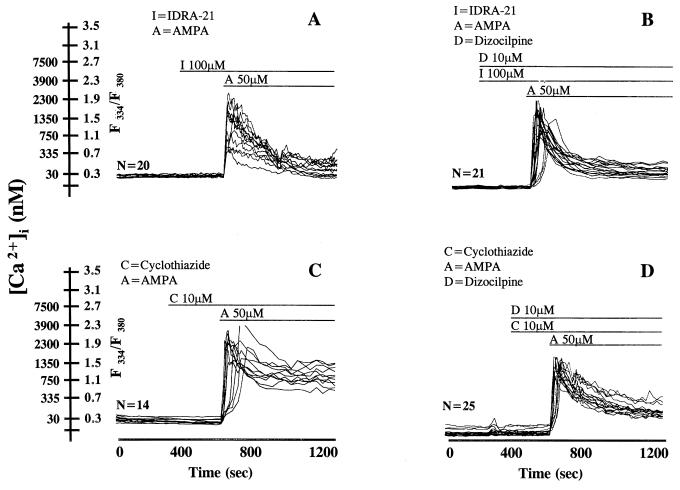
Duration of [Ca2+]i Homeostasis Destabilization Elicited by a Protracted Exposure to AMPA/IDRA-21 or AMPA/Cyclothiazide.
The addition to the cerebellar cell culture of 50 μM AMPA with 100 or 250 μM IDRA-21 resulted in a rapid increase of neuronal [Ca2+]i from ≈30 nM to 4,000–5,000 nM (Fig. 2A), but this [Ca2+]i elevation declined in 4–5 min to ≈10% of its peak value (300–500 nM) even though IDRA-21 was continuously present in the incubation buffer (see Fig. 2A). The time-course of the [Ca2+]i transients elicited by IDRA-21 was almost identical in the presence or the absence of 10 μM dizocilpine (Fig. 2 A and B). The exposure of neurons to 50 μM AMPA in the presence of 10 μM cyclothiazide resulted in a rapid increase of [Ca2+]i to concentrations similar to those observed in cells treated with IDRA-21 (Fig. 2B); however, [Ca2+]i in the presence of IDRA-21 declined to 10% of the peak value in 200 sec, whereas [Ca2+]i in the presence of cyclothiazide persisted at a high steady-state level (80% of peak value) for >200 sec (Fig. 2C). Both the peak and the prolonged increase in [Ca2+]i elicited by 10 μM cyclothiazide were reduced in 200 sec to 30% of the peak level by a dizocilpine pretreatment (Fig. 2D).
Figure 2.
Changes in the time course of [Ca2+]i transients and in granule cerebellar neurons in primary cultures elicited by IDRA-21 (I)/AMPA (A) or cyclothiazide (C)/AMPA (A). Experimental conditions as reported in Materials and Methods. The experiments were repeated 3–5 times with different batches of cerebellar granule cells, with similar results.
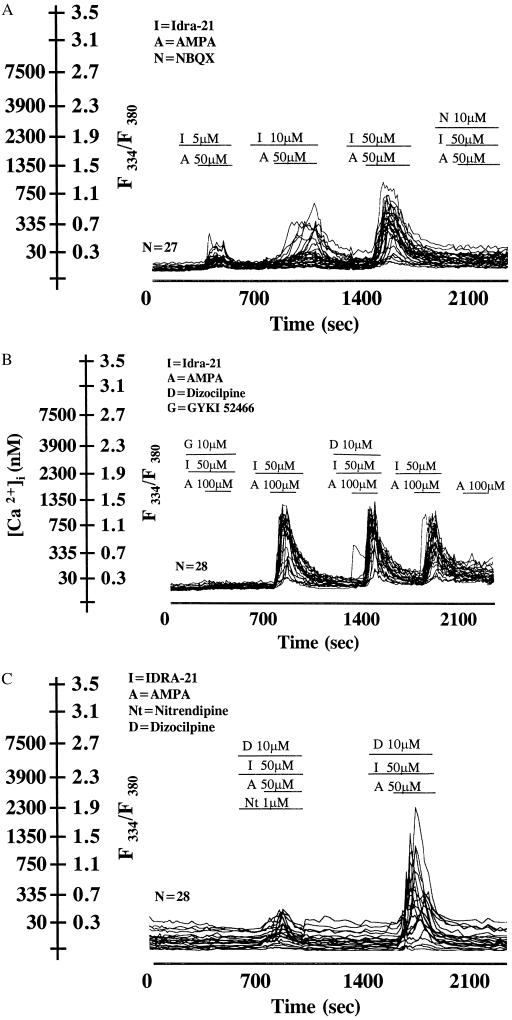
The fleeting increase of the Ca2+ transient elicited by IDRA-21 and the long lasting increase elicited by cyclothiazide could not be attributed to the use of threshold doses of IDRA-21 because virtually every neuron responded with a maximal [Ca2+]i increase when 50 μM IDRA-21 was applied in the presence of either 50 or 100 μM AMPA (Fig. 3 A and B). Interestingly, this response, virtually identical in the absence or in the presence of dizocilpine (Fig. 3B), was completely blocked by NBQX (Fig. 3A) and GYKI 52466 (Fig. 3B). The increase in the [Ca2+]i transient elicited by combined AMPA/IDRA-21 (Fig. 3) or AMPA/cyclothiazide treatments (data not shown) were also considerably reduced by nitrendipine, a blocker of L-type-voltage-sensitive calcium channels (VSCC) (27). Such nitrendipine pretreatment also reduced by ≈50% the neuronal death induced by 25 μM cyclothiazide in the presence of 50 μM AMPA.
Figure 3.
[Ca2+]i transients in cerebellar granule neurons exposed to increasing concentrations of IDRA-21 (I) prior to the application of AMPA (A) or A/10 μM NBQX (N) (A), A/GYKI (G) and A/dizocilpine (D) (B), A/nitrendipine (Nt) (C). Duration of drug application is indicated by a solid line. Note in B that A fails to increase [Ca2+]i when applied alone. [Ca2+]i calibration at the end of the experiment was carried out in cells treated with ionomycin (not shown). Each line represents data from a single cell in the same culture dish. n = number of neurons analyzed. Similar results were obtained three times with different batches of cerebellar granule cells. I or N alone was ineffective.
[Na+]i Transients after Application of AMPA/IDRA-21 or AMPA/Cyclothiazide.
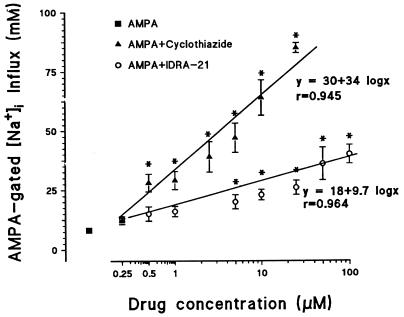
AMPA applied to granule cells in the presence of 10 μM dizocilpine, 1 μM tetrodotoxin (TTX), to block the voltage-sensitive Na+ channels, and 1 μM nitrendipine produced a modest increase in [Na+]i (≈10 mM) (Fig. 4) without changing [Ca2+]i (Fig. 3B). This increase in [Na+]i, which was identical for doses of 10 and 100 μM AMPA, reached a peak in 1–2 min and then decreased toward control levels even if AMPA was maintained in the incubation medium. In contrast, in the presence of IDRA-21 and cyclothiazide the increase in [Na+]i elicited by the application of AMPA reached a peak in 1–2 min but then persisted unabated for the duration of the drug application. The increase in [Na+]i transients elicited by 10 μM AMPA was facilitated in a dose-related manner by IDRA-21 or cyclothiazide (Fig. 4). However, the slope of the increase in [Na+]i elicited by 0.25–100 μM IDRA-21 (9.6 ± 0.82) was significantly lower (P > 0.01) than that elicited by 0.25 to 10 μM cyclothiazide (34 ± 2.1) (Fig. 4). Only 250 μM IDRA-21 produced an increase in [Na+]i that was equivalent to that of 5 μM cyclothiazide. Moreover, threshold doses of IDRA-21 (5–10 μM) and cyclothiazide (0.5–1 μM) which potentiated the AMPA-elicited increase in [Na+]i (Fig. 4). also increased [Ca2+]i (Fig. 3). It is important to note that 250 μM was the neurotoxic threshold dose of IDRA-21, whereas 5 μM was that of cyclothiazide. Thus, the therapeutic index (ratio between the threshold concentration for excitotoxicity and the threshold concentration for [Na+]i increase) is ≈50 for IDRA-21 and 10 for cyclothiazide. This finding suggests that when the positive allosteric modulation of AMPA action by IDRA-21 is compared with that of cyclothiazide, IDRA-21 has the characteristics expected of a partial allosteric modulator of AMPA action.
Figure 4.
Potentiation of AMPA-evoked [Na+]i transients in cultured cerebellar granule neurons exposed to increasing doses of either cyclothiazide or IDRA-21. [Na+]i is measured by SFBI-fluorescence as described in the Materials and Methods section. The SFBI-loaded cells were incubated for 3 min in the presence of 1 μM nitrendipine, 10 μM dizocilpine, and 1 μM tetrodotoxin prior to the application of AMPA (10 μM). The level of [Na+]i in the cells before the application of AMPA is 15 ± 1.2 mM. This basal value is subtracted from the values obtained after application of AMPA (▪) or AMPA plus modulators (○ = IDRA-21; ▴ = cyclothiazide). The dose-response relationship for IDRA-21 and cyclothiazide was constructed on the same culture dish by applying for 2 min AMPA to cells preincubated (1–2 min) with increasing concentrations of the modulators. After reaching the maximal fluorescence intensity, the cells were washed sufficient time (≈2 min) for the complete return of the fluorescence intensity ratio to baseline values. At the end of each experimental session, the cells were washed with Locke’s solution, and after 10, min the [Na+]i calibration was performed as described in Materials and Methods. Each value is the mean ± SEM of the last six experiments. ∗, P < 0.01 when values for AMPA/cyclothiazide and AMPA/IDRA-21 were compared with values of AMPA alone. The slopes of the two regression curves are significantly different (P < 0.01).
DISCUSSION
In cerebellar granule neurons of neonatal rats maintained in culture for 8–9 days, IDRA-21 and cyclothiazide are potent (low micromolar range) negative modulators of agonist-induced rapid desensitization of the AMPA receptor as monitored by measurements of SFBI/Na+- and Fura-2/Ca2+-dependent fluorescence. The mechanisms by which IDRA-21 and cyclothiazide increase [Na+]i and [Ca2+]i transients are presumably similar because they require the presence of AMPA in the incubation medium and are blocked by NBQX and GYKI 52466. However, IDRA-21 possesses an intrinsic activity that is about one-fourth lower than that of cyclothiazide. This is reflected by the duration of the [Ca2+]i transient increase elicited by IDRA-21, which is shorter lasting than that elicited by cyclothiazide when the drug is left in contact with the granule cells for an equal period of time (Fig. 2). These data suggest that IDRA-21 is a partial modulator of agonist-dependent rapid desensitization of the AMPA receptor for the range of doses in which one can presume it exerts a beneficial effect on cognitive processes in rats and monkeys (7, 8).
Moudy et al. (16) have reported that in cultures of rat hippocampal neurons cyclothiazide dramatically potentiated the neurotoxic action of glutamate even when the experiment was conducted in the presence of a large dose of dizocilpine to exclude the role of NMDA receptors in the neurotoxic action of glutamate. We have confirmed that in cultures of cerebellar granule neurons, 5 to 10 μM cyclothiazide applied together with glutamate or AMPA and in the presence of dizocilpine is neurotoxic. In contrast, despite the ability of 50–250 μM IDRA-21 to reduce the spontaneous and rapid desensitization of the AMPA receptor (13) and to increase significantly [Na+]i and [Ca2+]i transients, no neurotoxicity was observed when these doses of IDRA-21 were administered with dizocilpine. Only when a dose of 250 μM IDRA-21 is administered together with AMPA, but in absence of dizocilpine, does a modest neurotoxicity ensue. Since cerebellar granule cells are glutamatergic neurons, the neurotoxicity of a 250 μM dose of IDRA-21 can be accounted for by the action of the released glutamate on AMPA receptors.
Most likely the reason for the greater neurotoxic effect of cyclothiazide as compared with IDRA-21 may be due to the higher intrinsic activity of cyclothiazide that completely inhibits the spontaneous agonist-dependent AMPA receptor desensitization via an increase of Na+ influx through the AMPA receptor open channel, which in turn causes a long lasting increase of the [Ca2+]i transient via the voltage-dependent Ca2+ channels. In contrast, during the co-application of IDRA-21/AMPA, the increase in [Na+]i and the level of depolarization are less pronounced, and the associated increase in [Ca2+]i is short lasting even though the peak of the [Ca2+]i transient is similar. We have previously reported (21) that the duration of [Ca2+]i homeostasis destabilization is more relevant than the peak values as a predictor of AMPA excitotoxicity and that the rapidity of the return to a normal value of the [Ca2+]i transient following the application of neurotoxic doses of AMPA in the presence of allosteric modulators of the spontaneous desensitization depends on the magnitude of the [Na+]i increase (19). The present data confirm this conclusion.
At least theoretically, in cultured cerebellar granule cells four mechanisms (activation of NMDA receptors, participation of VSCC, reversal of the Na+/Ca2+ exchange function, and AMPA-operated channels) may contribute to destabilize Ca2+ homeostasis.
We observed that the [Ca2+]i transient but not the [Na+]i transient increase elicited by application of AMPA/IDRA-21 is reduced (by 60–70%) when the cells are exposed to the blocker of VSCC nitrendipine (27). At a dose of 1 μM, nitrendipine reduces by ≈50% AMPA plus cyclothiazide-induced neurotoxicity, suggesting that the time-course of the [Ca2+]i transient increase elicited by application of AMPA/IDRA-21 or AMPA/cyclothiazide is at least in part indirectly mediated by the opening of L-type VSCC triggered by the neuronal depolarization caused by a Na+ influx occurring via AMPA receptor activation. In addition to the VSCC, the experiments with dizocilpine indicate a contribution of NMDA receptor function to the increase of [Ca2+]i transients and to the neurotoxicity elicited by cyclothiazide, suggesting that AMPA receptor-mediated depolarization caused by an increase in intracellular Na+ (Fig. 4) facilitates the release of glutamate from the granule neurons in the medium, which in turn will act at NMDA receptors in which the Mg2+ blockade is presumably reduced by the membrane depolarization via AMPA receptor stimulation. It is noteworthy that dizocilpine lacks significant effects on the increase in [Na+]i elicited by the co-administration of AMPA and one of the two allosteric modulators under investigation.
Finally, we have demonstrated (19) that in cultured cerebellar granule cells a large increase of Na+, as occurs after cyclothiazide application, decreasing the efficacy of the Na+/Ca2+ exchanger, prolongs neuronal exposure to toxic concentrations of Ca2+. Taken together, these data and the evidence that cultured cerebellar granule cells express GLUR2 subunits in the edited form (P.L., unpublished work), suggest that only a small amount of Ca2+ may enter the cells via the AMPA-operated channels, directly.
An important question to be addressed concerns the possible difference between the mechanism of action of cyclothiazide and IDRA-21 on AMPA receptors. The negative allosteric modulation of AMPA receptor desensitization by cyclothiazide is achieved by greatly slowing the rate constant of desensitization and by significantly increasing the agonist’s affinity for the “non-desensitized closed receptor state” (4) by a preferential action at the flip splice variant of the receptor. In contrast, the aniracetam modulation at the AMPA receptor is believed to be due to a prolongation of the channel closing time by a preferential action of aniracetam on the flop splice variant of the receptor (1, 4).
With the exception of cyclothiazide and aniracetam, very little is known about the modifications induced by other inhibitors of AMPA receptor desensitization, including IDRA-21, on structurally different AMPA receptor subtypes. However, given that IDRA-21 has a low intrinsic activity, as expected for a partial modulator, we may suggest that it elicits a prolongation of the AMPA channel closing time due to a preferential action at the flop variant of the AMPA receptor. Although this hypothesis cannot be substantiated by the present studies, it is noteworthy to mention that in preliminary experiments with neuronal cultures of the hippocampus from neonatal rats, IDRA-21 has a lower neurotoxicity than cyclothiazide (not shown here). It is important to note that IDRA-21 in very low doses (1–3 μmol/kg given orally) improves memory tasks in rats (7) and monkeys (8) for a period lasting at least 3–4 hr.
It can be estimated that IDRA-21 brain concentrations in monkeys receiving 1–3 μmol/kg orally of this drug are at least 5 times lower than those required to inhibit AMPA receptor desensitization in vitro and at least 250 times lower than the threshold dose to elicit neurotoxicity. Although we cannot explain the difference in IDRA-21 potency between the in vitro and the in vivo results, one might surmise that IDRA-21 elicits its modification of drug-induced cognitive deficit in vivo either via a metabolite more active than IDRA-21 itself on AMPA receptor desensitization or by a preferential metabolism of one of the enantiomeric forms of IDRA-21, which we have shown has the inhibitory activity in vivo. We have reported that the (+) enantiomer of IDRA-21 is more active than the racemic form in increasing cognitive function in rats (14). Thus, it is not inconceivable that (−) IDRA-21 may even act as an antagonist of the (+) IDRA-21 enantiomer. Interestingly, racemic IDRA-21 in doses >10× those (22 μmol/kg orally) that are maximally active on cognitive tests fails to potentiate the frequency and the intensity of kainic acid-induced seizures in rats (8). Thus, two alternative interpretations can be offered to explain the differences between in vitro and in vivo potency of IDRA-21: (i) it may act on cognitive processes by mechanisms other than an inhibition of agonist-dependent AMPA receptor rapid and spontaneous desensitization; (ii) a minimal modification of the agonist-dependent AMPA receptor desensitization is required to obtain an action in vivo.
Discrepancies similar to those observed for IDRA-21 between in vitro and in vivo potency have been reported for the chemically unrelated AMPA receptor modulators of the benzoylpiperidine class (6, 10). Thus, one alternative interpretation could be that the summation of the minimal changes in the duration or in the intensity of the Na+ current elicited by low concentrations of IDRA-21 at any given AMPA receptor unit leads to a physiologically important modification of neuronal excitability due to the recruitment of many AMPA receptors expressed in high density clusters in selected neuronal populations. Another interpretation (10, 15) could be that threshold concentrations of IDRA-21 for the inhibition of AMPA receptor spontaneous desensitization in isolated neurons can become fully active during in vivo functional tests involving a chain of excitatory (glutamate, acetylcholine) or inhibitory (GABA) synaptic amplification steps, such as those involved in the somatosensory information processing in the neocortex. Unlike in vitro, the efficacy of IDRA-21 in vivo may become progressively amplified as somatosensory signals reaching the cortex from the thalamus are serially processed through successive stages of the excitatory or inhibitory feed-forward or feedback circuitries involved in the complex neuronal mechanisms underlying acquisition and learning expressed in the various behavioral tests used to evaluate this drug’s potency.
Acknowledgments
We thank Drs. S. M. Paul (Lilly Research Laboratories, Indianapolis) and S. M. Rothman (Department of Pediatric Neurology, Washington University, St. Louis) for their constructive criticisms and suggestions in the preparation of the manuscript. This work was supported in part by National Institutes of Health Grant PO1NS-28130-08
ABBREVIATIONS
- [Ca2+]i
free cytosolic Ca2+, Fura-2, fura-2 acetoxymethyl ester
- [Na+]i
intracellular Na+
- SFBI
sodium-binding bezofuran isophthalate acetoxymethyl ester
- IDRA-21
7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide
- AMPA
α-amino-3-hydroxy-5-methylisoxazolepropionic acid
- NBQX
2,3-dihydroxy-6-nitrosulfamoylbenzo[f]quinoxaline
- VSCC
voltage-sensitive calcium channels
- NMDA
N-methyl-d-aspartate
- GYKI 52466
1-(amino-phenyl)-4-methyl-7,8-methylendioxy-5H-2,3-benzodiazepine
References
- 1.Johansen T H, Chaudhary A, Verdoorn T A. Mol Pharmacol. 1995;48:946–955. [PubMed] [Google Scholar]
- 2.Partin K M, Patneau D K, Mayer M L. Mol Pharmacol. 1994;46:129–138. [PubMed] [Google Scholar]
- 3.Partin K M, Bowie D, Mayer M L. Neuron. 1995;14:833–843. doi: 10.1016/0896-6273(95)90227-9. [DOI] [PubMed] [Google Scholar]
- 4.Partin K M, Fleck M W, Mayer M L. J Neurosci. 1996;16:6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stern-Bach Y, Bettler B, Hartley M, Sheppard P O, O’Hara P J, Heinemann S F. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 6.Staubli U, Perez Y, Xu F, Rogers G, Ingwar M, Stone-Elander S, Lynch G. Proc Natl Acad Sci USA. 1994;91:11158–11162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zivkovic I, Thompson D M, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A. J Pharmacol Exp Ther. 1995;272:300–309. [PubMed] [Google Scholar]
- 8.Thompson D M, Guidotti A, DiBella M, Costa E. Proc Natl Acad Sci USA. 1995;92:7667–7671. doi: 10.1073/pnas.92.17.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito I, Tanabe S, Kohda A, Sugiyama H. J Physiol (London) 1990;424:533–543. doi: 10.1113/jphysiol.1990.sp018081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai A, Kessler M, Rogers G, Lynch G. J Pharmacol Exp Ther. 1996;278:627–638. [PubMed] [Google Scholar]
- 11.Yamada K A, Rothman S M. J Physiol (London) 1992;458:385–407. [Google Scholar]
- 12.Yamada K A, Tang C M. J Neurosci. 1993;13:3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertolino M, Baraldi M, Parenti C, Braghiroli D, DiBella M, Costa E. Recept Channels. 1993;1:267–278. [PubMed] [Google Scholar]
- 14.Uzunov D P, Zivkovich I, Pirkle W H, Costa E, Guidotti A. J Pharm Sci. 1995;84:937–942. doi: 10.1002/jps.2600840807. ,. [DOI] [PubMed] [Google Scholar]
- 15.Arai A, Guidotti A, Costa E, Lynch G. NeuroReport. 1996;7:2211–2215. doi: 10.1097/00001756-199609020-00031. [DOI] [PubMed] [Google Scholar]
- 16.Moudy A M, Yamada K A, Rothman S M. Neuropharmacology. 1994;33:953–962. doi: 10.1016/0028-3908(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 17.Hack N J, Sluiter A A, Balazs R. Dev Brain Res. 1995;87:55–61. doi: 10.1016/0165-3806(95)00054-h. [DOI] [PubMed] [Google Scholar]
- 18.Favaron M, Manev H, Alho H, Bertolino M, Ferret B, Guidotti A, Costa E. Proc Natl Acad Sci USA. 1988;85:7351–7355. doi: 10.1073/pnas.85.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiedrowski L, Wroblewski J T, Costa E. Mol Pharmacol. 1994;45:1050–1054. [PubMed] [Google Scholar]
- 20.Harootunian A C, Kao J P Y, Echert B K, Tsien R Y. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- 21.DeErausquin G A, Manev H, Guidotti A, Costa E, Brooker G. Proc Natl Acad Sci USA. 1990;87:8017–8021. doi: 10.1073/pnas.87.20.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiedrowski L, Costa E. Mol Pharmacol. 1995;47:140–147. [PubMed] [Google Scholar]
- 23.Tallarida R, Murray R B. Manual of Pharmacologic Calculations with Computer Programs. Philadelphia: Micro Computer Specialist; 1987. [Google Scholar]
- 24.Sheardown M J, Nielsen E O, Hansen A J, Jacobsen P, Honore T. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- 25.Donevan SD, Rogawski M A. Neuron. 1993;10:51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- 26.Collingridge G L, Lester R A J. Pharmacol Rev. 1989;40:143–210. [PubMed] [Google Scholar]
- 27.Janis R A, Triggle D J. J Med Chem. 1983;26:775–785. doi: 10.1021/jm00360a001. [DOI] [PubMed] [Google Scholar]