Abstract
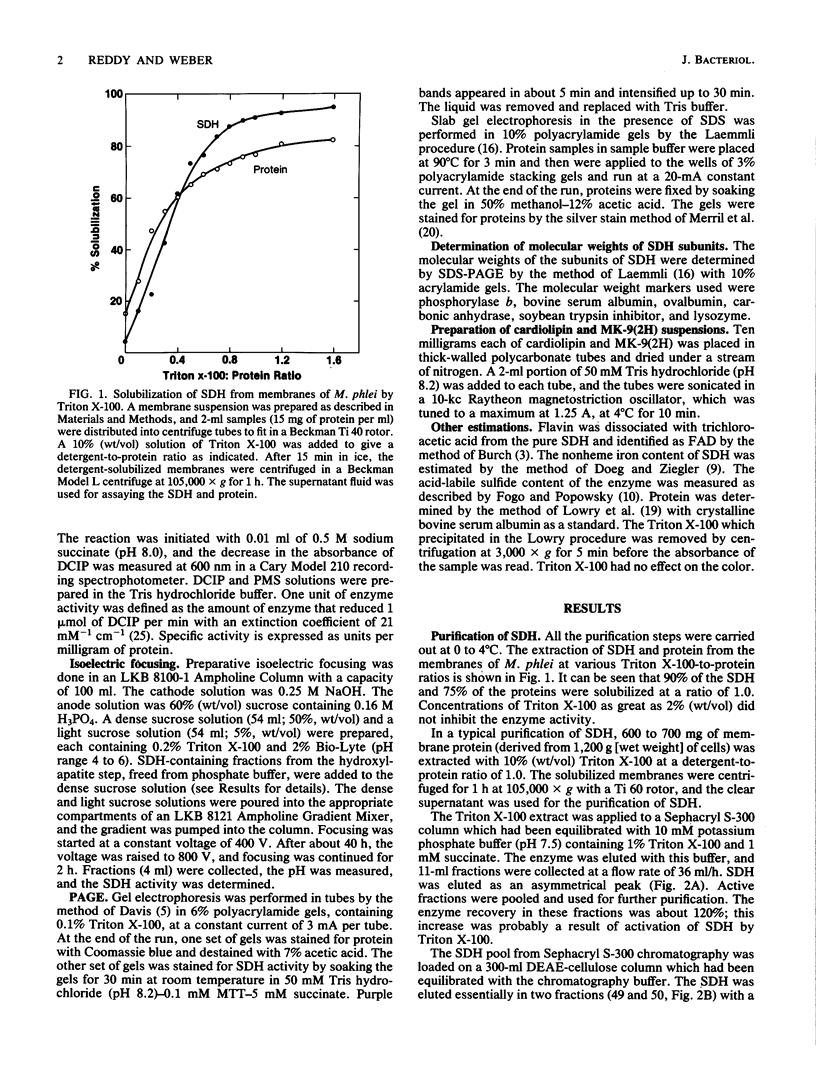
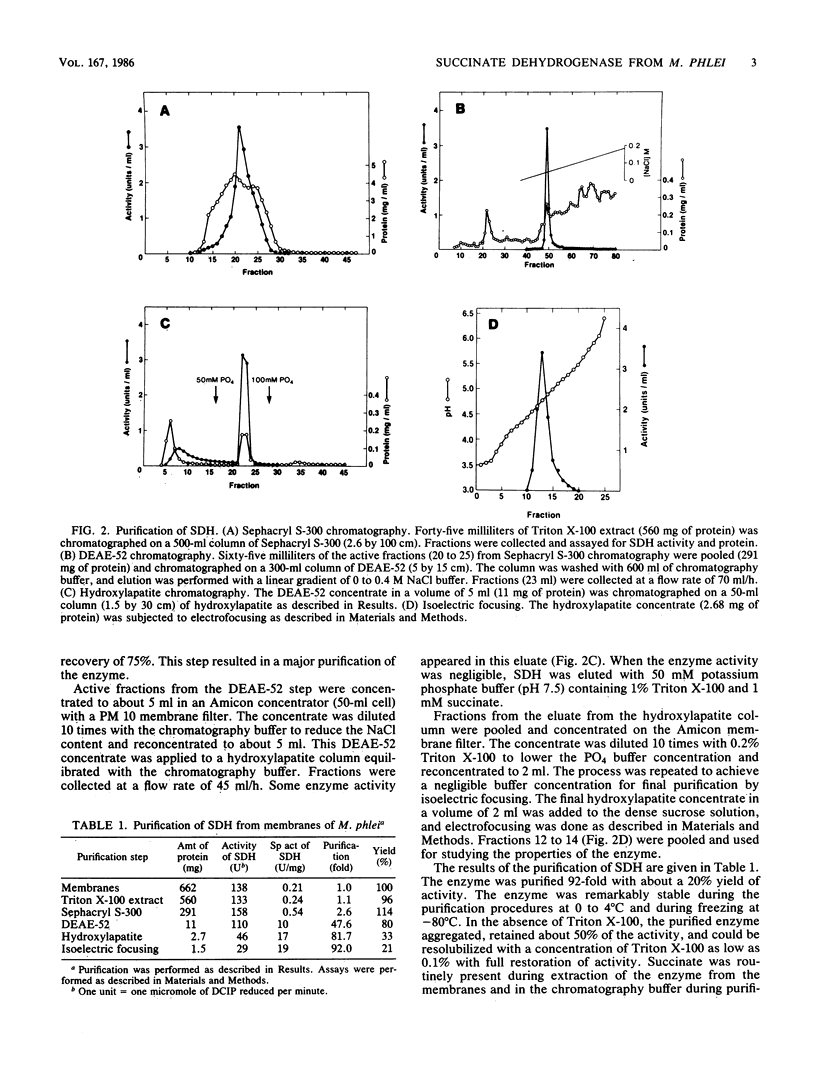
Succinate dehydrogenase (SDH) was solubilized from membranes of Mycobacterium phlei by Triton X-100 with a recovery of about 90%. The solubilized SDH was purified about 90-fold by Sephacryl S-300, DEAE-cellulose, hydroxylapatite, and isoelectric focusing in the presence of Triton X-100 with a 20% recovery. SDH was homogeneous, as determined by polyacrylamide gel electrophoresis in nondenaturing gels containing Triton X-100. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the enzyme revealed two subunits with molecular weights of 62,000 and 26,000. SDH is a flavoprotein containing 1 mol of flavin adenine dinucleotide, 7 to 8 mol of nonheme iron, and 7 to 8 mol of acid-labile sulfide per mol of protein. Using phenazine methosulfate and 2,6-dichloroindophenol as electron acceptors, the enzyme had an apparent Km of 0.12 mM succinate. SDH exhibited a sigmoidal relationship of rate to succinate concentration, indicating cooperativity. The enzyme was competitively inhibited by fumarate with a Ki of 0.15 mM. In the absence of Triton X-100, the enzyme aggregated, retained 50% of the activity, and could be resolubilized with Triton X-100 with full restoration of activity. Cardiolipin had no effect on the enzyme activity in the absence of Triton X-100, but it stimulated the activity by about 30% in the presence of 0.1% Triton X-100 in the assay mixture. Menaquinone-9(2H), isolated from M. phlei, had no effect on the enzyme activity either in the presence or absence of Triton X-100.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAMATSU Y., NOJIMA S. SEPARATION AND ANALYSES OF THE INDIVIDUAL PHOSPHOLIPIDS OF MYCOBACTERIA. J Biochem. 1965 Mar;57:430–439. doi: 10.1093/oxfordjournals.jbchem.a128097. [DOI] [PubMed] [Google Scholar]
- BRODIE A. F., GRAY C. T. Phosphorylation coupled to oxidation in bacterial extracts. J Biol Chem. 1956 Apr;219(2):853–862. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOEG K. A., ZIEGLER D. M. Simplified methods for the estimation of iron in mitochondria and submitochondrial fractions. Arch Biochem Biophys. 1962 Apr;97:37–40. doi: 10.1016/0003-9861(62)90041-3. [DOI] [PubMed] [Google Scholar]
- Dancey G. F., Shapiro B. M. Specific phospholipid requirement for activity of the purified respiratory chain NADH dehydrogenase of Escherichia coli. Biochim Biophys Acta. 1977 May 25;487(2):368–377. doi: 10.1016/0005-2760(77)90013-3. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Crawford I. P., Baltscheffsky H. Purification, molecular properties, and amino acid composition of the subunits of Rhodospirillum rubrum succinate dehydrogenase. Arch Biochem Biophys. 1977 Apr 30;180(2):459–464. doi: 10.1016/0003-9861(77)90060-1. [DOI] [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Fractionation of the electron-transport chain of Escherichia coli. Biochim Biophys Acta. 1974 Aug 23;357(2):215–230. doi: 10.1016/0005-2728(74)90062-0. [DOI] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XII. SOME OBSERVATIONS ON THE RECONSTITUTION OF THE SUCCINATE OXIDASE SYSTEM FROM HEART MUSCLE. J Biol Chem. 1963 Dec;238:4037–4051. [PubMed] [Google Scholar]
- Kalra V. K., Murti C. R., Brodie A. F. Resolution and reconstitution of the succinoxidase pathway of Mycobacterium phlei. Arch Biochem Biophys. 1971 Dec;147(2):734–743. doi: 10.1016/0003-9861(71)90433-4. [DOI] [PubMed] [Google Scholar]
- LARA F. J. The succinic dehydrogenase of Propionibacterium pentosaceum. Biochim Biophys Acta. 1959 Jun;33(2):565–567. doi: 10.1016/0006-3002(59)90153-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER A. M., Jr, HOWLAND J. L., MILLER P. A. Electron-transport systems in Corynebacterium diphtheriae. Biochim Biophys Acta. 1962 Oct 22;64:229–242. doi: 10.1016/0006-3002(62)90734-5. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Linder R., Salton M. R. Characterization of the membrane-bound succinic dehydrogenase of Micrococcus lysodeikticus. J Bacteriol. 1971 Jul;107(1):230–238. doi: 10.1128/jb.107.1.230-238.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy T. L., Hendler R. W. Reconstitution of escherichia coli succinoxidase from soluble components. J Biol Chem. 1978 Nov 10;253(21):7972–7979. [PubMed] [Google Scholar]
- STEYN-PARVE E. P., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. VI. Isolation and properties of stable enzyme-substrate complexes. J Biol Chem. 1958 Oct;233(4):843–852. [PubMed] [Google Scholar]
- Singer T. P., Kearney E. B., Kenney W. C. Succinate dehydrogenase. Adv Enzymol Relat Areas Mol Biol. 1973;37:189–272. doi: 10.1002/9780470122822.ch4. [DOI] [PubMed] [Google Scholar]
- WEBER M. M., BRODIE A. F., MERSELIS J. E. Possible role for vitamin K in electron transport. Science. 1958 Oct 17;128(3329):896–898. doi: 10.1126/science.128.3329.896-a. [DOI] [PubMed] [Google Scholar]
- WEBER M. M., HOLLOCHER T. C., ROSSO G. THE APPEARANCE AND GENERAL PROPERTIES OF FREE RADICALS IN ELECTRON TRANSPORT PARTICLES FROM MYCOBACTERIUM PHLEI. J Biol Chem. 1965 Apr;240:1776–1782. [PubMed] [Google Scholar]
- Yamashita S., Racker E. Reconstitution of the mitochondrial oxidation chain from individual components. J Biol Chem. 1968 May 10;243(9):2446–2447. [PubMed] [Google Scholar]



