Abstract
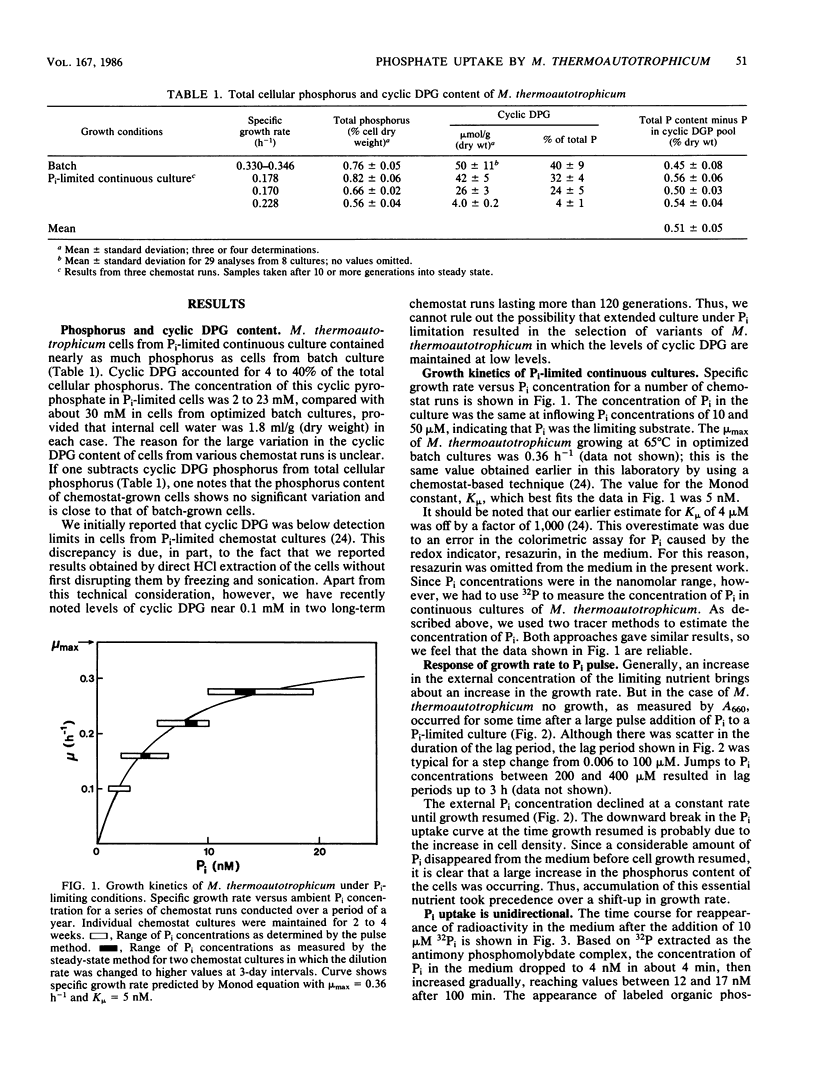
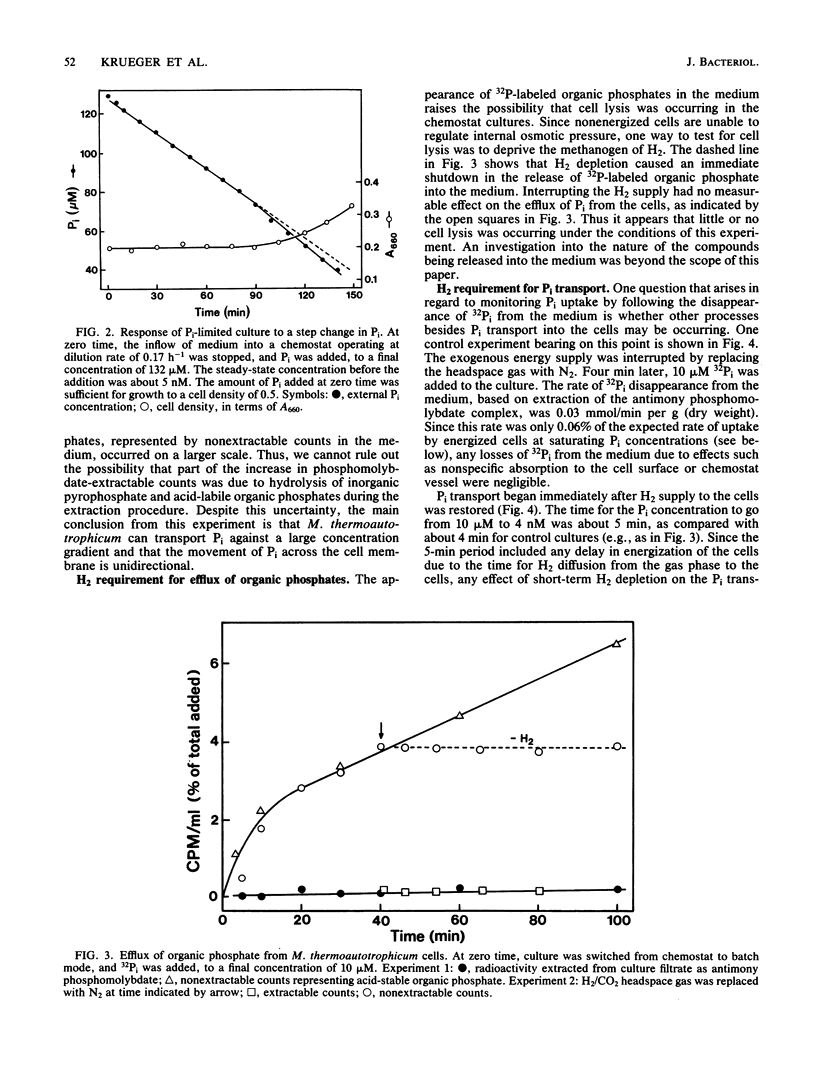
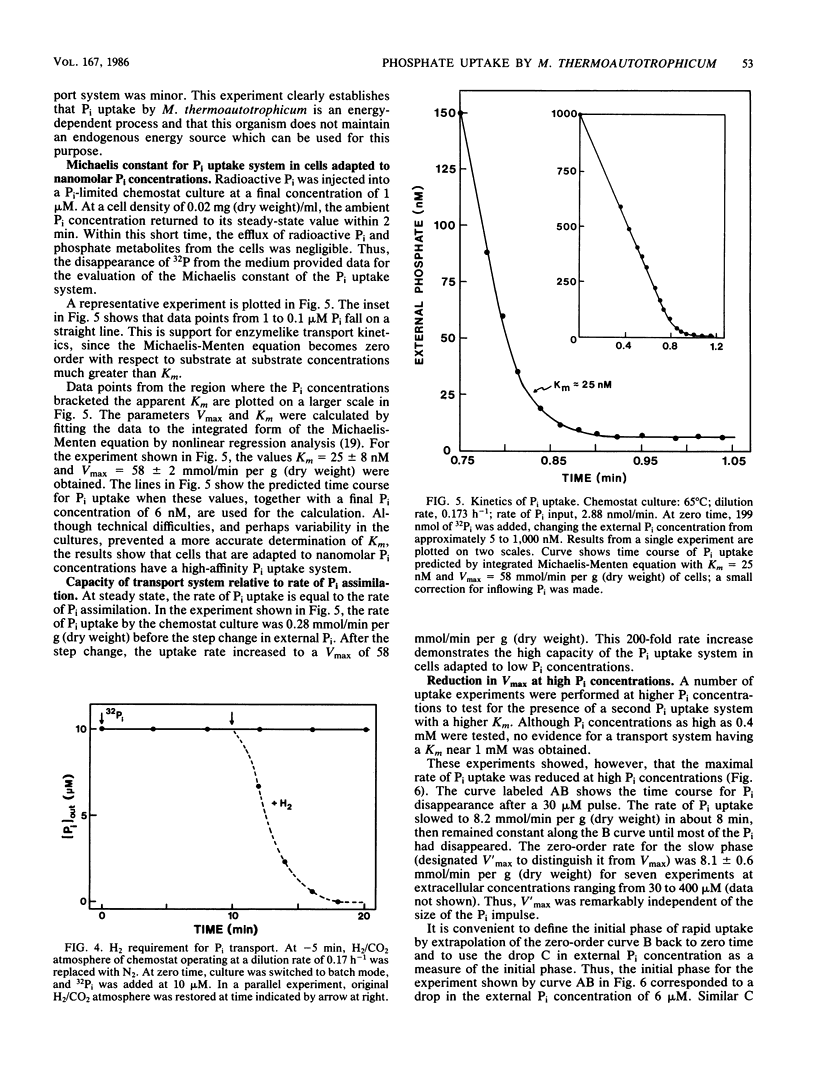
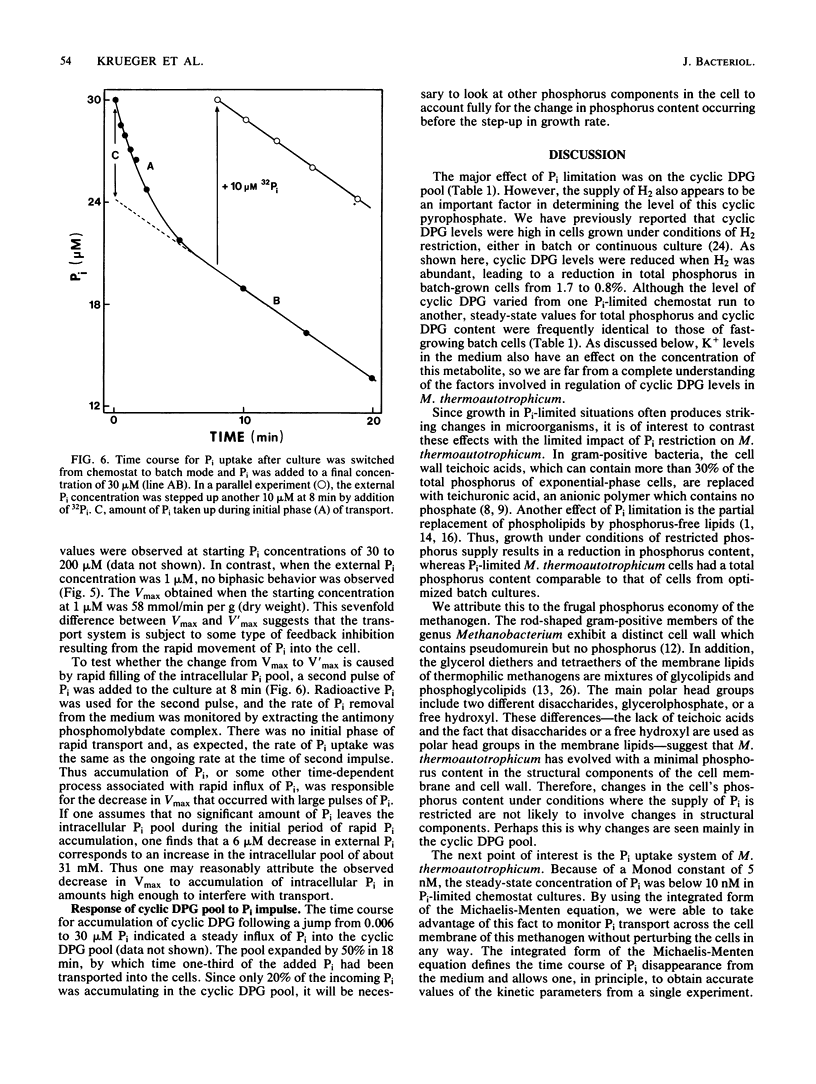
The archaebacterium Methanobacterium thermoautotrophicum was grown in continuous culture at 65 degrees C in a phosphate-limited medium at specific growth rates from 0.06 to 0.28 h-1 (maximum growth rate [mu max] = 0.36 h-1). Cyclic-2,3-diphosphoglycerate (cyclic DPG) levels ranged from 2 to 20 mM in Pi-limited cells, compared with about 30 mM in batch-grown cells. The Monod constant for Pi-limited growth was 5 nM. Pi uptake rates were determined by following the disappearance of 32Pi from the medium. Interrupting the H2 supply stopped the uptake of Pi and the release of organic phosphates. Little or no efflux of Pi occurred in the presence or absence of H2. Pi uptake of cells adapted to nanomolar Pi concentrations could be accounted for by the operation of one uptake system with an apparent Km of about 25 nM and a Vmax of 58 nmol of Pi per min per g (dry weight). Uptake curves at 30 microM Pi or above were biphasic due to a sevenfold decrease in Vmax after an initial phase of rapid movement of Pi into the cell. Under these conditions the growth rate slowed to zero and the cyclic DPG pool expanded before growth resumed. Thus, three properties of M. thermoautotrophicum make it well adapted to live in a low-P environment: the presence of a low-Km, high-Vmax uptake system for Pi; the ability to accumulate cyclic DPG rapidly; and a growth strategy in which accumulation of Pi and cyclic DPG takes precedence over a shift-up in growth rate when excess Pi becomes available.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batrakov S. G., Bergelson L. D. Lipids of the Streptomycettes. Structural investigation and biological interrelation a review. Chem Phys Lipids. 1978 Apr;21(1-2):1–29. doi: 10.1016/0009-3084(78)90052-x. [DOI] [PubMed] [Google Scholar]
- Blaut M., Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984 May 15;141(1):217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- Button D. K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985 Sep;49(3):270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Sparling R., Sprott G. D. The bioenergetics of methanogenesis. Biochim Biophys Acta. 1984 Sep 6;768(2):113–163. doi: 10.1016/0304-4173(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Grant W. D. Cell wall teichoic acid as a reserve phosphate source in Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):35–43. doi: 10.1128/jb.137.1.35-43.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo J. F., Gibson J. Regulation of phosphate accumulation in the unicellular cyanobacterium Synechococcus. J Bacteriol. 1979 Nov;140(2):508–517. doi: 10.1128/jb.140.2.508-517.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O., König H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch Microbiol. 1978 Aug 1;118(2):141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Sprott G. D., Smith I. C. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim Biophys Acta. 1981 Apr 23;664(1):156–173. doi: 10.1016/0005-2760(81)90038-2. [DOI] [PubMed] [Google Scholar]
- Laine R. A., Griffin P. F., Sweeley C. C., Brennan P. J. Monoglucosyloxyoctadecenoic acid--a glycolipid from Aspergillus niger. Biochemistry. 1972 Jun 6;11(12):2267–2271. doi: 10.1021/bi00762a009. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. Replacement of acidic phosphates by acidic glycolipids in Pseudomonas diminuta. Nature. 1974 May 17;249(454):268–269. doi: 10.1038/249268a0. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely R. J., Fahrney D. E. A novel diphospho-P,P'-diester from Methanobacterium thermoautotrophicum. J Biol Chem. 1983 Sep 25;258(18):10835–10838. [PubMed] [Google Scholar]
- Seely R. J., Fahrney D. E. Levels of cyclic-2,3-diphosphoglycerate in Methanobacterium thermoautotrophicum during phosphate limitation. J Bacteriol. 1984 Oct;160(1):50–54. doi: 10.1128/jb.160.1.50-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene T. G., Langworthy T. A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science. 1979 Jan 5;203(4375):51–53. doi: 10.1126/science.758677. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



