Abstract
Collagen binds to a receptor protein present on the surfaces of Staphylococcus aureus cells. Binding of 125I-labeled type II collagen to its bacterial receptor is reversible, and Scatchard plot analysis indicates the presence of one class of receptor that occurs on an average of 3 X 10(4) copies per cell and binds type II collagen with a Kd of 10(-7) M. Studies on the specificity of collagen cell binding indicate that the receptor does not recognize noncollagenous proteins but binds all of the different collagen types tested (types I to VI). Furthermore, isolated collagen alpha chains and peptides generated by cyanogen bromide cleavage of type I collagen alpha chains are recognized by the receptor as indicated by the ability of these polypeptides to inhibit binding of 125I-labeled type II collagen to staphylococcal cells. Synthetic collagen analogs were tested as inhibitors of type II collagen binding to bacterial cells. The peptides (Pro-Gly-Pro)n, (Pro-Pro-Gly)10, and (Pro-OH-Pro-Gly)10 were recognized by the receptor, whereas the peptides (Pro-Ala-Gly)n and polyproline showed no inhibitory activity.
Full text
PDF
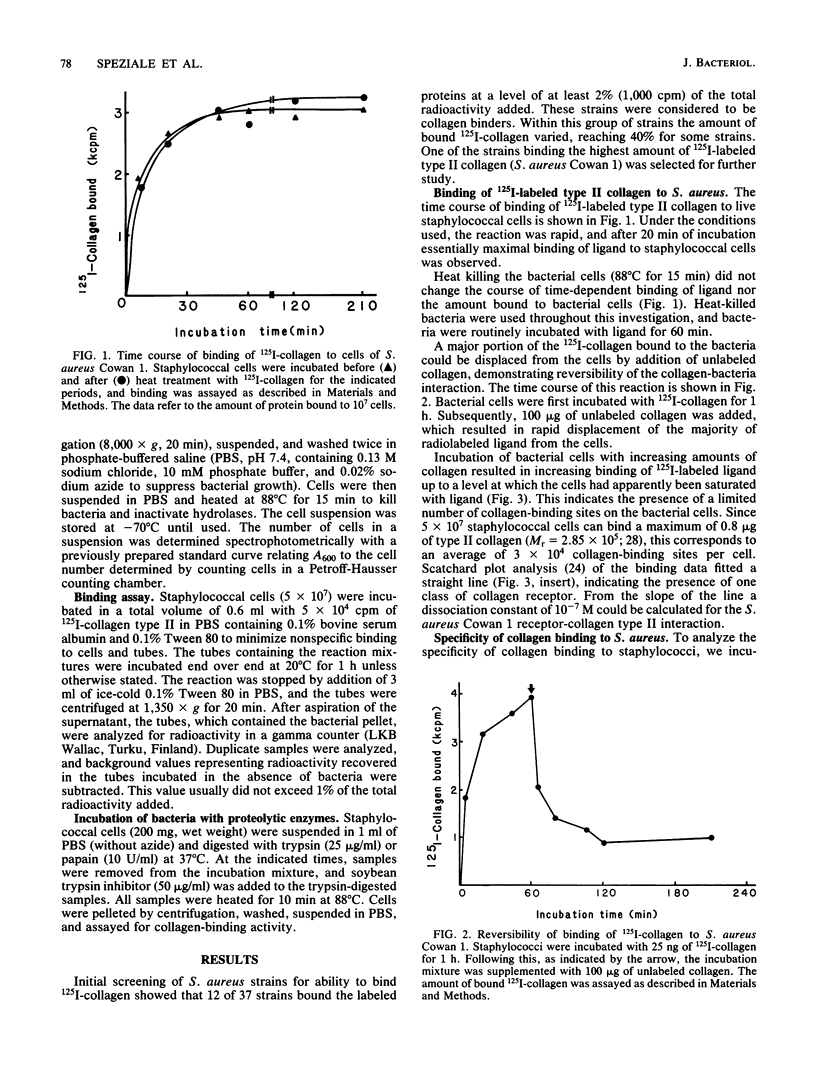
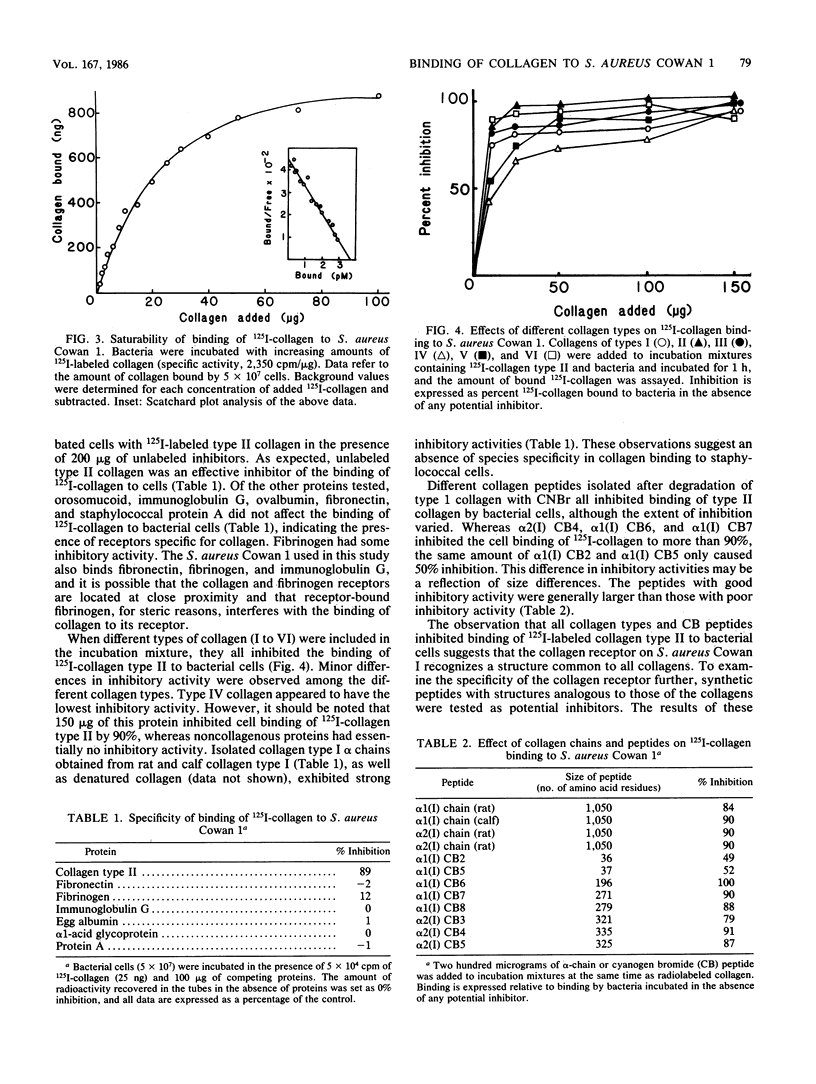
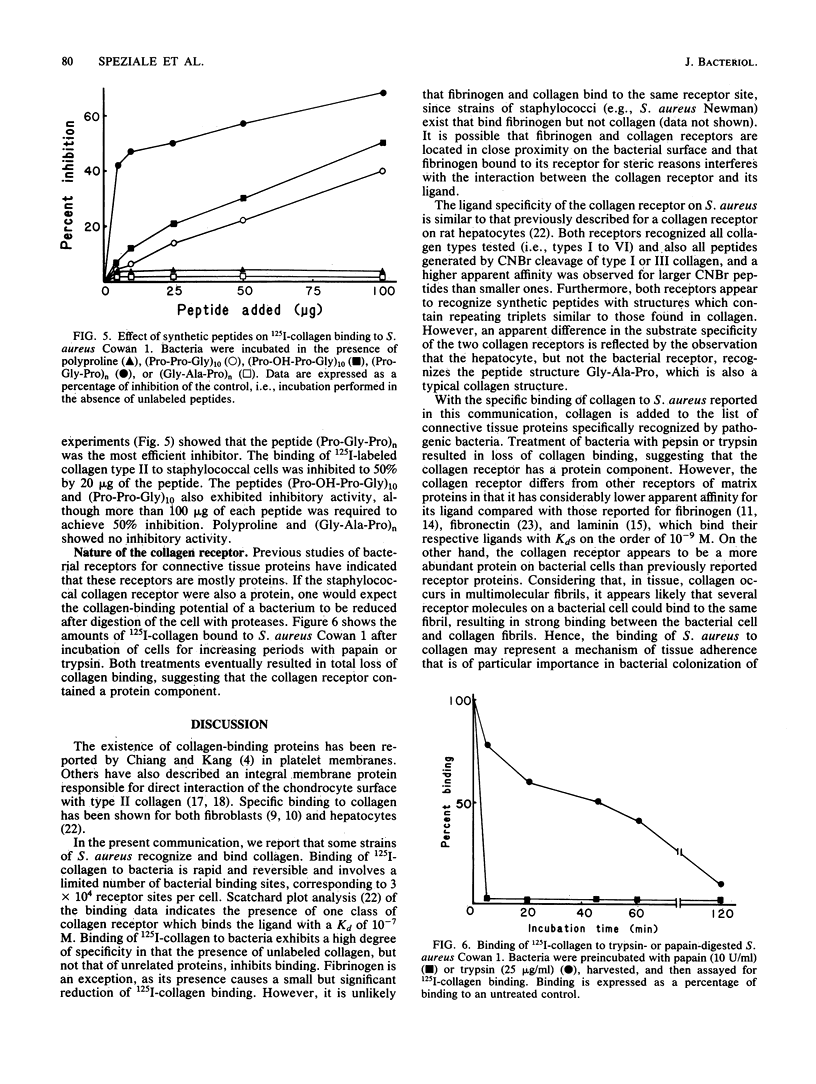

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentz H., Bächinger H. P., Glanville R., Kühn K. Physical evidence for the assembly of A and B chains of human placental collagen in a single triple helix. Eur J Biochem. 1978 Dec;92(2):563–567. doi: 10.1111/j.1432-1033.1978.tb12778.x. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- Carret G., Emonard H., Fardel G., Druguet M., Herbage D., Flandrois J. P. Gelatin and collagen binding to Staphylococcus aureus strains. Ann Inst Pasteur Microbiol. 1985 Mar-Apr;136A(2):241–245. doi: 10.1016/s0769-2609(85)80063-6. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H. Isolation and purification of collagen alpha 1(I) receptor from human platelet membrane. J Biol Chem. 1982 Jul 10;257(13):7581–7586. [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Piez K. A. Isolation and characterization of the cyanogen bromide peptides from the alpha 2 chain of rat skin collagen. Biochemistry. 1969 May;8(5):2129–2133. doi: 10.1021/bi00833a052. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Goldberg B. D., Burgeson R. E. Binding of soluble type I collagen to fibroblasts: specificities for native collagen types, triple helical structure, telopeptides, propeptides, and cyanogen bromide-derived peptides. J Cell Biol. 1982 Dec;95(3):752–756. doi: 10.1083/jcb.95.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Binding of soluble type I collagen molecules to the fibroblast plasma membrane. Cell. 1979 Feb;16(2):265–275. doi: 10.1016/0092-8674(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Hawiger J., Timmons S., Strong D. D., Cottrell B. A., Riley M., Doolittle R. F. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982 Mar 16;21(6):1407–1413. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- Holderbaum D., Spech R. A., Ehrhart L. A. Specific binding of collagen to Staphylococcus aureus. Coll Relat Res. 1985 Jun;5(3):261–271. doi: 10.1016/s0174-173x(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Hawiger J. Recognition site for the platelet receptor is present on the 15-residue carboxy-terminal fragment of the gamma chain of human fibrinogen and is not involved in the fibrin polymerization reaction. Thromb Res. 1983 Jan 15;29(2):249–255. doi: 10.1016/0049-3848(83)90147-0. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J., Bee J. A., Lizarbe M. A., von der Mark K. Role of anchorin CII, a 31,000-mol-wt membrane protein, in the interaction of chondrocytes with type II collagen. J Cell Biol. 1984 Apr;98(4):1572–1579. doi: 10.1083/jcb.98.4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer J., von der Mark K. Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J. 1983;2(1):45–50. doi: 10.1002/j.1460-2075.1983.tb01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Odermatt E., Risteli J., van Delden V., Timpl R. Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J. 1983 May 1;211(2):295–302. doi: 10.1042/bj2110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Strawich E., Nimni M. E. Properties of a collagen molecule containing three identical components extracted from bovine articular cartilage. Biochemistry. 1971 Oct 12;10(21):3905–3911. doi: 10.1021/bi00797a017. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel F. A., Papageorgiou P. S. Osteomyelitis: the past decade. N Engl J Med. 1980 Aug 14;303(7):360–370. doi: 10.1056/NEJM198008143030703. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]



