Abstract
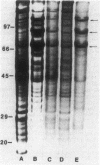
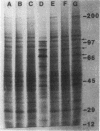
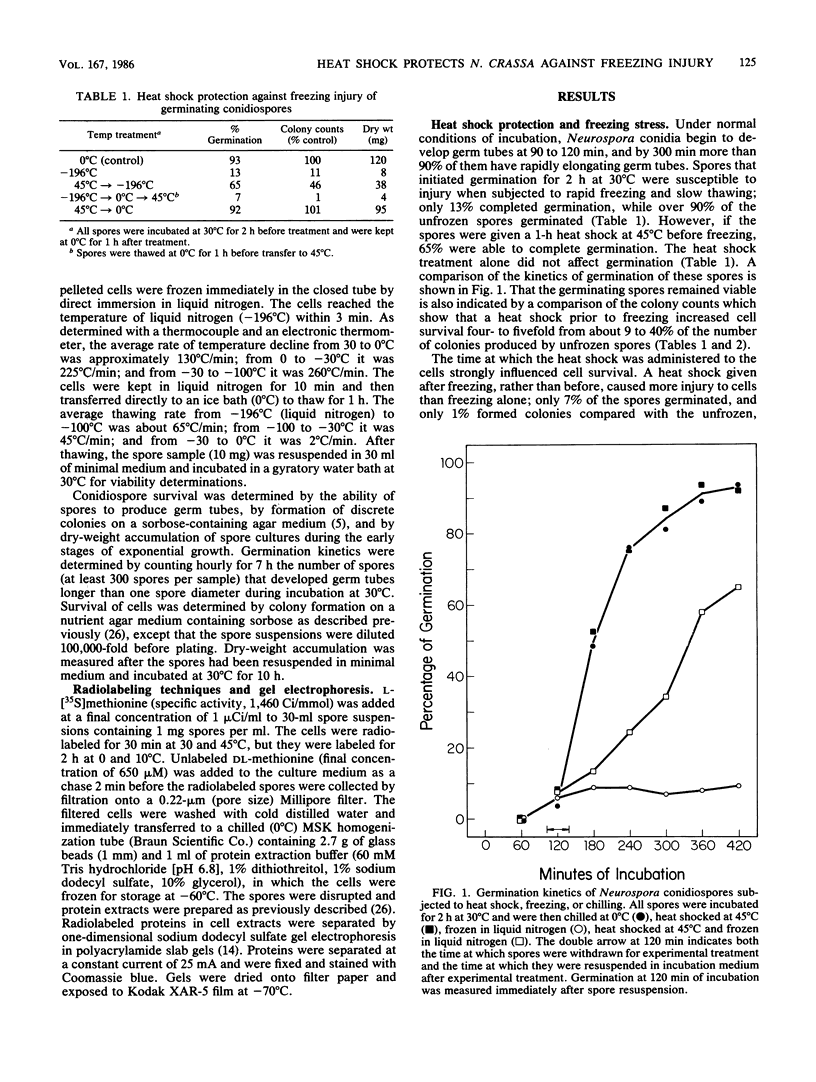
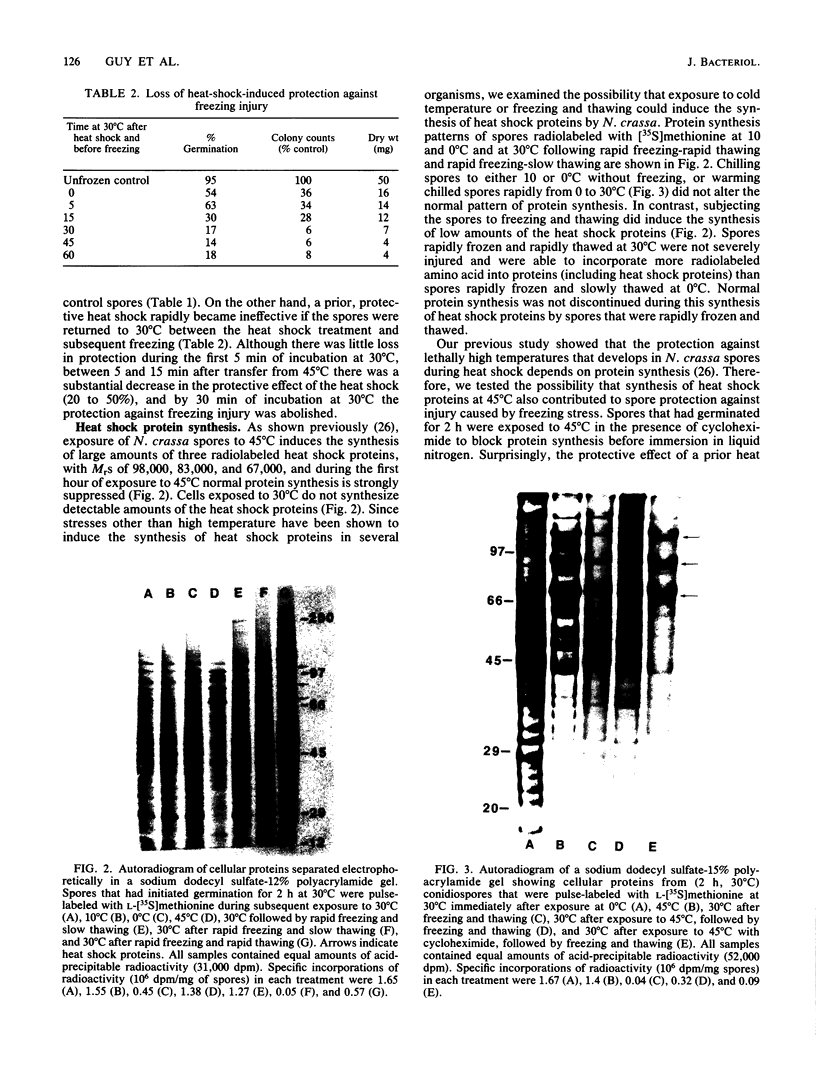
Germinating conidiospores of Neurospora crassa that were exposed to 45 degrees C, a temperature that induces a heat shock response, were protected from injury caused by freezing in liquid nitrogen and subsequent thawing at 0 degrees C. Whereas up to 90% of the control spores were killed by this freezing and slow thawing, a prior heat shock increased cell survival four- to fivefold. Survival was determined by three assays: the extent of spore germination in liquid medium, the number of colonies that grew on solid medium, and dry-weight accumulation during exponential growth in liquid culture. The heat shock-induced protection against freezing injury was transient. Spores transferred to normal growth temperature after exposure to heat shock and before freezing lost the heat shock-induced protection within 30 min. Spores subjected to freezing and thawing stress synthesized small amounts of the heat shock proteins that are synthesized in large quantities by cells exposed to 45 degrees C. Pulse-labeling studies demonstrated that neither chilling the spores to 10 degrees C or 0 degrees C in the absence of freezing nor warming the spores from 0 degrees C to 30 degrees C induced heat shock protein synthesis. The presence of the protein synthesis inhibitor cycloheximide during spore exposure to 45 degrees C did not abolish the protection against freezing injury induced by heat shock. Treatment of the cells with cycloheximide before freezing, without exposure to heat shock, itself increased spore survival.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Barnhart E. R., Terry C. E. Cryobiology of Neurospora crassa. II. Alteration in freeze response of Neurospora crassa conidia by additives. Cryobiology. 1971 Aug;8(4):328–332. doi: 10.1016/0011-2240(71)90126-x. [DOI] [PubMed] [Google Scholar]
- Barnhart E. R., Terry C. E. Cryobiology of neurospora crassa. I. Freeze response of Neurospora crassa conidia. Cryobiology. 1971 Aug;8(4):323–327. doi: 10.1016/0011-2240(71)90125-8. [DOI] [PubMed] [Google Scholar]
- Cosgrove J. W., Brown I. R. Heat shock protein in mammalian brain and other organs after a physiologically relevant increase in body temperature induced by D-lysergic acid diethylamide. Proc Natl Acad Sci U S A. 1983 Jan;80(2):569–573. doi: 10.1073/pnas.80.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Kapoor M. A study of the heat-shock response in Neurospora crassa. Int J Biochem. 1983;15(5):639–649. doi: 10.1016/0020-711x(83)90188-x. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leef J. L., Mazur P. Physiological response of Neurospora conidia to freezing in the dehydrated, hydrated, or germinated state. Appl Environ Microbiol. 1978 Jan;35(1):72–83. doi: 10.1128/aem.35.1.72-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- MAZUR P. Studies on the effects of subzero temperatures on the viability of spores of Aspergillus flavus. I. The effect of rate of warming. J Gen Physiol. 1956 Jul 20;39(6):869–888. doi: 10.1085/jgp.39.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P., Schmidt J. J. Interactions of cooling velocity, temperature, and warming velocity on the survival of frozen and thawed yeast. Cryobiology. 1968 Jul-Aug;5(1):1–17. doi: 10.1016/s0011-2240(68)80138-5. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. A response of protein synthesis to temperature shift in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5222–5225. doi: 10.1073/pnas.76.10.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Brambl R. Heat shock response of Neurospora crassa: protein synthesis and induced thermotolerance. J Bacteriol. 1985 Jun;162(3):1083–1091. doi: 10.1128/jb.162.3.1083-1091.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesset J., Palm C., McLaughlin C. S. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1340–1345. doi: 10.1016/0006-291x(82)92147-7. [DOI] [PubMed] [Google Scholar]
- Silver J. C., Andrews D. R., Pekkala D. Effect of heat shock on synthesis and phosphorylation of nuclear and cytoplasmic proteins in the fungus Achlya. Can J Biochem Cell Biol. 1983 Jun;61(6):447–455. doi: 10.1139/o83-060. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984 Mar;36(3):655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Vincent M., Tanguay R. M. Different intracellular distributions of heat-shock and arsenite-induced proteins in Drosophila Kc cells. Possible relation with the phosphorylation and translocation of a major cytoskeletal protein. J Mol Biol. 1982 Dec 5;162(2):365–378. doi: 10.1016/0022-2836(82)90532-0. [DOI] [PubMed] [Google Scholar]
- Watson K., Dunlop G., Cavicchioli R. Mitochondrial and cytoplasmic protein syntheses are not required for heat shock acquisition of ethanol and thermotolerance in yeast. FEBS Lett. 1984 Jul 9;172(2):299–302. doi: 10.1016/0014-5793(84)81145-x. [DOI] [PubMed] [Google Scholar]
- Wellman A. M., Pendyala L. Permeability changes in membranes of Neurospora crassa after freezing and thawing. Cryobiology. 1979 Apr;16(2):184–195. doi: 10.1016/0011-2240(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Tobe T., Ito K., Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]