Abstract
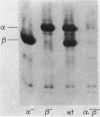
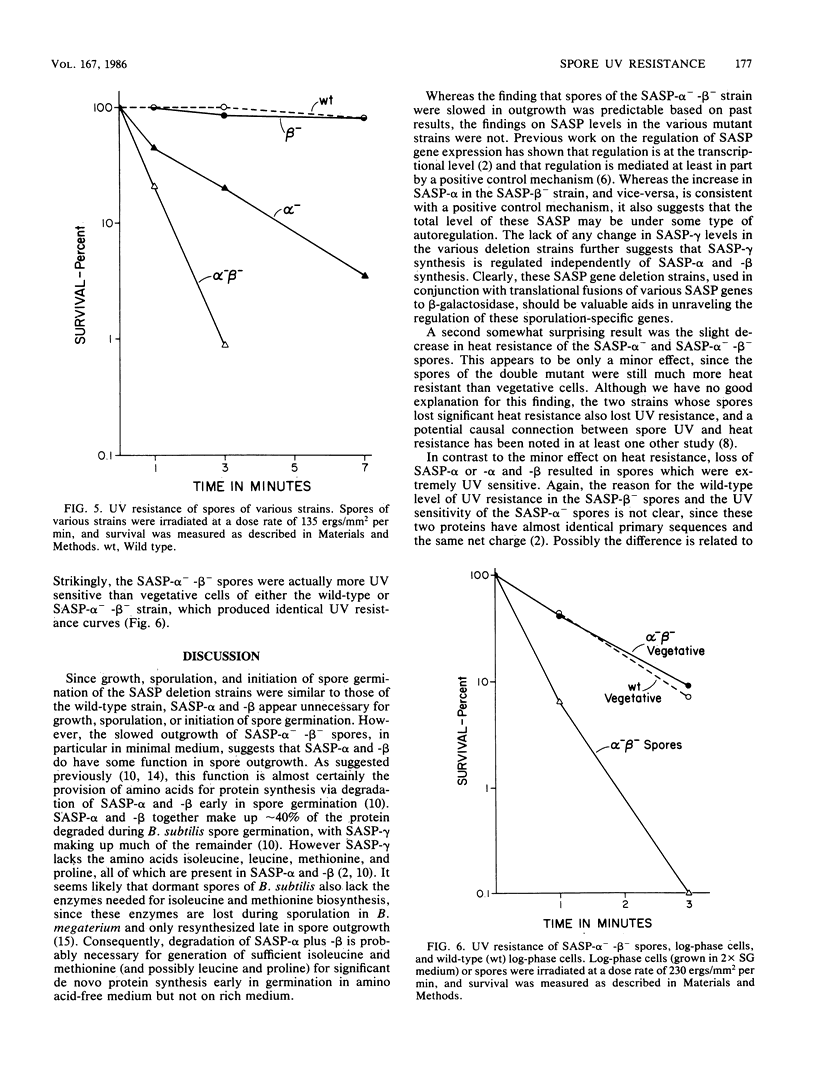
Bacillus subtilis strains containing deletions in the genes coding for one or two of the major small, acid-soluble spore proteins (SASP; termed SASP-alpha and SASP-beta) were constructed. These mutants sporulated normally, but the spores lacked either SASP-alpha, SASP-beta, or both proteins. The level of minor SASP did not increase in these mutants, but the level of SASP-alpha increased about twofold in the SASP-beta- mutant, and the level of SASP-beta increased about twofold in the SASP-alpha- mutant. The growth rates of the deletion strains were identical to that of the wild-type strain in rich or poor growth media, as was the initiation of spore germination. However, outgrowth of spores of the SASP-alpha(-)-beta- strain was significantly slower than that of wild-type spores in all media tested. The heat resistance of SASP-beta- spores was identical to that of wild-type spores but slightly greater than that of SASP-alpha- and SASP-alpha(-)-beta- spores. However, the SASP-alpha- and SASP-alpha(-)-beta- spores were much more heat resistant than vegetative cells. The UV light resistances of SASP-beta- and wild-type spores were also identical. However, SASP-alpha(-)-beta- spores were slightly more sensitive to UV light than were log-phase cells of the wild-type or SASP-alpha(-)-beta- strain (the latter have identical UV light resistances); SASP-alpha- spores were slightly more UV light resistant than SASP-alpha(-)-beta- spores. These data strongly implicate SASP, in particular SASP-alpha, in the UV light resistance of B. subtilis spores.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baillie E., Germaine G. R., Murrell W. G., Ohye D. F. Photoreactivation, photoproduct formation, and deoxyribonucleic acid state in ultraviolet-irradiated sporulating cultures of Bacillus cereus. J Bacteriol. 1974 Oct;120(1):516–523. doi: 10.1128/jb.120.1.516-523.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M. J., Mason J. M., Setlow P. Cloning and nucleotide sequencing of genes for three small, acid-soluble proteins from Bacillus subtilis spores. J Bacteriol. 1986 May;166(2):417–425. doi: 10.1128/jb.166.2.417-425.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel-Quesada E., Setlow B., Setlow P. Cloning of the gene for C protein, a low molecular weight spore-specific protein from Bacillus megaterium. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3250–3254. doi: 10.1073/pnas.80.11.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Murrell W. G. Effect of dipicolinic acid on the ultraviolet radiation resistance of Bacillus cereus spores. Photochem Photobiol. 1973 Mar;17(3):145–153. doi: 10.1111/j.1751-1097.1973.tb06344.x. [DOI] [PubMed] [Google Scholar]
- Goldrick S., Setlow P. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1459–1462. doi: 10.1128/jb.155.3.1459-1462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlin J. H., Lombardi S. J., Slepecky R. A. Heat and UV light resistance of vegetative cells and spores of Bacillus subtilis Rec-mutants. J Bacteriol. 1985 Aug;163(2):774–777. doi: 10.1128/jb.163.2.774-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Mahler I., Phillips K., Tipper D. J. Transcriptional control of synthesis of acid-soluble proteins in sporulating Bacillus subtilis. J Bacteriol. 1985 Aug;163(2):543–551. doi: 10.1128/jb.163.2.543-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Tipper D. J. Acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):972–982. doi: 10.1128/jb.146.3.972-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- Setlow B., Hackett R. H., Setlow P. Noninvolvement of the spore cortex in acquisition of low-molecular-weight basic proteins and UV light resistance during Bacillus sphaericus sporulation. J Bacteriol. 1982 Feb;149(2):494–498. doi: 10.1128/jb.149.2.494-498.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Localization of low-molecular-weight basic proteins in Bacillus megaterium spores by cross-linking with ultraviolet light. J Bacteriol. 1979 Aug;139(2):486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford R. S., Donnellan J. E., Jr Photochemical evidence for conformation changes in DNA during germination of bacterial spores. Proc Natl Acad Sci U S A. 1968 Mar;59(3):822–828. doi: 10.1073/pnas.59.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Ferrari E. Replacement of the Bacillus subtilis subtilisin structural gene with an In vitro-derived deletion mutation. J Bacteriol. 1984 May;158(2):411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese A. J. 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun. 1970 Feb 6;38(3):484–490. doi: 10.1016/0006-291x(70)90739-4. [DOI] [PubMed] [Google Scholar]
- Young M. Gene amplification in Bacillus subtilis. J Gen Microbiol. 1984 Jul;130(7):1613–1621. doi: 10.1099/00221287-130-7-1613. [DOI] [PubMed] [Google Scholar]