Abstract
For whole-body CT images of small rodents, a voxel resolution of at least 10−3 mm3 is needed for scale-equivalence to that currently achieved in clinical CT scanners (∼1 mm3) in adult humans. These “mini-CT” images generally require minutes rather than seconds to complete a scan. The radiation exposure resulting from these mini-CT scans, while higher than clinical CT scans, is below the level resulting in acute tissue damage. Hence, these scans are useful for performing clinical-type diagnostic and monitoring scans for animal models of disease and their response to treatment. “Micro-CT”, with voxel size <10−5 mm3, has been useful for imaging isolated, intact organs at an almost cellular level of resolution. Micro-CT has the great advantage over traditional microscopic methods in that it generates detailed 3D images in relatively large, opaque, volumes such as an intact rodent heart or kidney. The radiation exposure needed in these scans results in acute tissue damage if used in living animals.
Experience with micro-CT is contributing to exploration of new applications for clinical CT imaging by providing insights into different modes of x-ray image formation as follows:
Spatial resolution should be sufficient to detect an individual Basic Functional Unit (BFU, the smallest collection of diverse cells, such as hepatic lobule, that behaves like the organ), which requires voxels ∼10−3 mm3 in volume, so that the BFUs can be counted.
-
Contrast resolution sufficient to allow quantitation of:
New microvascular growth, which manifests as increased tissue contrast due to x-ray contrast agent in those vessels' lumens during passage of injected contrast agent in blood.
Impaired endothelial integrity which manifests as increased opacification and delayed washout of contrast from tissues.
Discrimination of pathological accumulations of metals such as Fe and Ca, which occur in the arterial wall following hemorrhage or tissue damage.
Micro-CT can also be used as a test bed for exploring the utility of several modes of x-ray image formation, such as the use of dual energy x-ray subtraction, x-ray scatter, phase delay and refraction-based imaging for increasing the contrast amongst soft tissue components. With the recent commercial availability of high speed, multi-slice CT scanners which can be operated in dual energy mode, some of these micro-CT scanner capabilities and insights are becoming implementable in those CT scanners. As a result, the potential diagnostic spectrum that can be addressed with those scanners is broadened considerably.
Keywords: X-ray, Synchrotron, Contrast agents, Three-dimensional, Small animal
Basic Components for High-Resolution Computed Tomography
All micro-CT scanners follow the same general plan: an x-ray source, a specimen that is to be imaged, an x-ray-to-electronic signal-converting imaging array, and a device that either rotates the specimen within a stationary scanner or rotates the scanner around the stationary specimen. The method for providing high resolution is illustrated in Fig. 1.
Fig. 1.
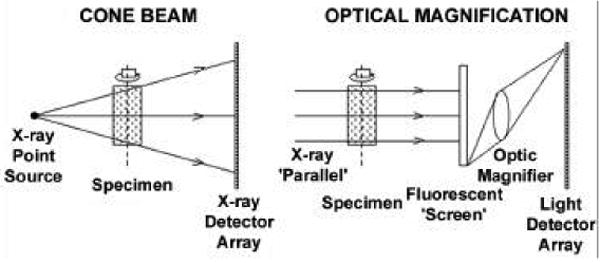
The left panel shows an x-ray cone-beam, which projects a magnified x-ray image onto a large-area x-ray imaging system. The right panel shows how a nonmagnified x-ray image can be magnified by subsequent optical means either by a lens or by a tapered fiber-optic coupling (not shown here). This method can use both cone beam and parallel beam (synchrotron) geometries. (Reproduced with permission from Ann. Rev. Biomed. Eng. 6:185-208, 2004).
The volume of a mammal's internal organs scales with its body weight [1]. Hence, if we wish to merely scale clinical images of an adult human to a mouse, we need to have a scanner that has voxels of the order of (100 μm)3 for a 25 g adult mouse. In addition to dimensional scaling, which, broadly speaking, is proportional to body mass as M0.3, the functional aspects, such as heart and respiratory cycle durations, scale as M−0.25 so that scan aperture durations should be reduced from <300 ms in humans to <75 ms in mice if, for instance, the heart is to be imaged.
The impact of the different resolutions is illustrated in Fig. 2 [2].
Fig. 2.
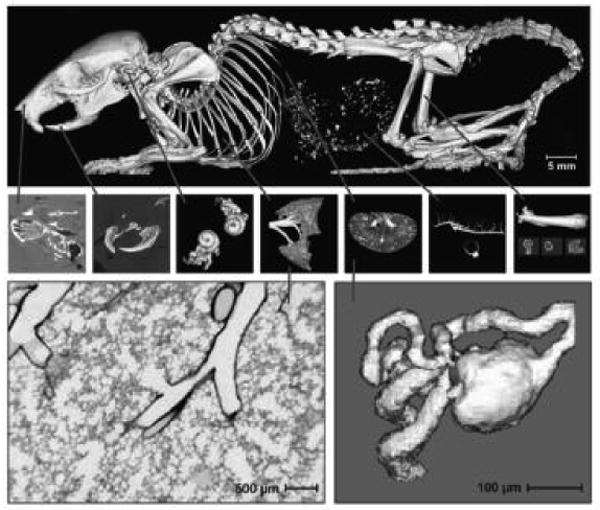
Computer-generated displays of mini- and micro-CT image data. The upper set of panels depict a voxel resolution (40 μm) that might be expected from a mini-CT scanner, which is a clinical CT scanner scaled down to match the mouse. The lower two panels depict higher voxel resolution (5 μm) micro-CT. These images clearly resolve the basic functional units (approximately 0.001 mm3), the terminal bronchiole and its alveoli of the lung (left) and the glomerulus and associated arterioles and tubule in the kidney (right). (Reproduced with permission from Reference [2]).
Although bench-top micro-CT scanners can achieve meaningful cubic voxel sizes as small as 5 μm, the synchrotron radiation-based scanners provide the highest resolution. This is because of the narrow bandwidth of the energy-selective x-ray and intensity is sufficient to permit rapid scanning [3]. In addition, there are structures, such as pores, trabeculi, ductules, and blood vessels, that have a range of dimensions. Kinney et al. [4] showed how CT voxel size plays a role in the ability to accurately measure such features. With voxel sizes less than (10 μm)3, even cellular dimensions, such as osteoclast erosions in the surface of bone [5] can be imaged, and with voxel sizes less than (1 μm)3, sub-cellular dimensions can be imaged [6].
In an attempt to generate quasi-monochromatic radiation in bench-top micro-CT scanners, some x-ray source anode materials can be selected for their characteristic Ka emission radiation (e.g., 17.5 keV for Mo). This radiation output can then be filtered through a thin metal foil selected to have its K absorption edge at an energy just above the Ka of the respective anode material (e.g., 18.0 keV for Zr), which predominantly attenuates the x-ray photons with energies above and below the Ka characteristic radiation energy.
For application to living animals, the speed of scanning is critically important. This can be achieved by several mechanisms [7,8]. One is by use of higher x-ray photon energies (e.g., >50 keV). Increased speed can also be enhanced by bringing the x-ray source closer to the object, thus increasing the cone-beam angle together with all the difficulties that result (e.g., penumbral blurring owing to focal spot diameter and the reconstruction algorithm needed). Scan speed can be further increased by using multi x-ray source/detector array scanners so that the scan duration is proportionately decreased.
For the micro-CT scanners, specimens are generally rotated around a vertical axis within a fixed x-ray/detector system. However, anesthetized animals would be physiologically challenged if held in this vertical orientation for any extended period of time. Rotation of the animal horizontally around its cephalo-caudal axis would result in gross distortions and movements of the animal during the scan. Hence, the torso of intact animals requires a fixed horizontal support table for the animal, and the x-ray/detector system should rotate around the animal, resulting in a true mini-clinical CT scanner.
With slower bench-top scanners, some cyclic motions owing to the heartbeat could be accommodated by acquiring the necessary scan data incrementally at the same phase of the cardiac cycle of sequential heart beats. However, noncyclic transient events, such as the progressive accumulation or washout of a contrast agent into or out of a physiological space, cannot be scanned fast enough, even by use of high-intensity synchrotron radiation. The transient process can, however, be literally snap-frozen within the tissue of interest, and that frozen specimen can then be scanned at leisure while frozen [9].
Ongoing Developments of X-Ray Micro-CT
There will likely be continued incremental improvements of the technology of the scanners, and there are several potential physics aspects that are being explored for the purposes of broadening the spectrum of contrast mechanisms that can be utilized [10]. The synchrotron radiation source, with its brilliance and energy-selective and narrow bandwidth photon generation capabilities, provides a convenient and effective means to study nonattenuation-based x-ray imaging, such as phase delay [11], fluorescence [12] and K-edge subtraction [13], and x-ray scatter-based [14] micro-CT imaging. Much technological development is needed to make such approaches suitable for routine bench-top CT imaging use. A particular advantage of the fluorescent and scatter-based approaches is that the detected x-ray is the signal of interest (so-called “dark field” acquisition) as compared to attenuation-based methods in which the signal is a reduction in the “bright-field” acquisition.
A major limitation of x-ray attenuation imaging is its ionizing effect, which can result in immediate radiation damage and long-term genetic damage [15]. The signal-to-noise ratio of x-ray attenuation imaging depends largely on the number of x-ray photons detected per voxel of the 3-D image (i.e., the x-ray exposure has to increase with increasing spatial resolution [16]); therefore, its sensitivity is ultimately limited by its potential for radiation damage. Grodzins showed that for constant signal-to-noise ratio, the radiation dose necessary for small samples was approximately proportional to the specimen diameter to the third power. An exposure of 6 Gy is generally considered lethal for a small rodent; therefore, exposures must be much less in animals required to undergo multiple sequential scans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calder WA., III . Cambridge: Harvard Univ Press; Cambridge, MA: 1984. [Google Scholar]
- 2.Ritman EL. J Cell Biochem. 2002;39:116. doi: 10.1002/jcb.10415. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, DeCarlo F, Foster I, Insley J, Kesselman C, Lane P, von Laszewski G, Mancini D, McNulty I, Su MH, Tieman B. Proc SPIE. 1999;3772:318. [Google Scholar]
- 4.Kinney JH, Haupt DL, Nichols MC, Breunig TM, Marshall GW, Jr, Marshall SJ. Nucl Instr Methods Phys Res. 1994;A 347:480. [Google Scholar]
- 5.Ritman EL, Bolander ME, Fitzpatrick LA, Turner RT. Technol Health Care. 1998;6:403. [PubMed] [Google Scholar]
- 6.Haddad WS, McNulty GI, Trebes JE, Anderson EH, Levesque RA, Yang L. Science. 1994;266:1213. doi: 10.1126/science.266.5188.1213. [DOI] [PubMed] [Google Scholar]
- 7.Kohlbrenner A, Koller B, Hammerle S, Rüegsegger P. Adv Exp Med Biol. 2001;496:213. doi: 10.1007/978-1-4615-0651-5_20. [DOI] [PubMed] [Google Scholar]
- 8.Sasov A. Proc IEEE Int Symp Biomed Imaging. 2002. p. 377. [Google Scholar]
- 9.Kantor B, Jorgensen SM, Lund PE, Chmelik MS, Reyes DA, Ritman EL. Scanning. 2002;24:186. doi: 10.1002/sca.4950240405. [DOI] [PubMed] [Google Scholar]
- 10.Bonse U. Proc SPIE. 2006;5535 In Press. [Google Scholar]
- 11.Beckman F, Bonse U, Busch F, Gunnewig O. J Comput Assist Tomogr. 1997;21:539. doi: 10.1097/00004728-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Takeda T, Yu Q, Yashiro T, Yuasa T, Hasegawa Y, Itai Y, Akatsuka T. Proc SPIE. 1999;3772:258. [Google Scholar]
- 13.Torikoshi M, Tsunoo T, Sasaki M, Endo M, Noda Y, Ohno Y, Kohno T, Hyodo K, Uesugi K, Yagi N. Phys Med Biol. 2003;7:673. doi: 10.1088/0031-9155/48/5/308. [DOI] [PubMed] [Google Scholar]
- 14.Schlomka JP, Delfs J, Barschdorf H, Thran A, van Stevendaal U. Proc SPIE. 2004;5535:410. [Google Scholar]
- 15.Dowseth DJ, Kenny PA, Johnston RE. London: Chapman Hall Med; 1998. [Google Scholar]
- 16.Chesler DA, Riederer SJ, Pelc NJ. J Comput Assist Tomogr. 1997;1:64. doi: 10.1097/00004728-197701000-00009. [DOI] [PubMed] [Google Scholar]


