Introduction
Obstructive sleep apnea (OSA) is a chronic condition characterized by repetitive collapse of the upper airway during sleep leading to significant hypoxemia and recurrent arousals from sleep. It is a prevalent disorder particularly among middle-aged, obese men, although its existence in women, as well as in lean individuals, is increasingly recognized (1-5). Four percent of adult men and 2% of adult women in general population random samples meet the current clinical and polysomnographic criteria for the diagnosis of sleep apnea warranting immediate therapeutic intervention (2-4). A much larger group, 17–24% of men and 5–9% of women, demonstrate an apnea/hypopnea index of more than five events per hour of sleep (2-4), which was the originally proposed criterion for sleep apnea (6).
OSA is associated with considerable morbidity and mortality. Various studies indicate a causal relationship between OSA and hypertension, cardiovascular disease and diabetes mellitus, independently of obesity (7). Obesity is the most important reversible risk factor for OSA (8). Furthermore, OSA is associated with excessive daytime sleepiness which results in declines in quality of life (9) and increased risk for crashes while driving (10). The first line of therapy is continuous positive airways pressure (CPAP) but its efficacy is limited, especially in mild to moderate OSA, and the compliance is poor (1, 7).
In this chapter, we review knowledge accumulated during the last 10 years about sleep apnea and its association with the stress system, inflammation, insulin resistance and visceral obesity.
Sleep apnea and the Stress System
The hypothalamic-pituitary-adrenal (HPA) axis mediates the reaction to acute physical and psychological stress. HPA and sleep interact in multiple ways. Sleep, in particular deep sleep, has an inhibitory influence on the HPA axis (11), whereas activation of the HPA axis or administration of glucocorticoids can lead to arousal and sleepiness (12, 13).
The sequence of events in OSA – breathing cessation, nocturnal hypoxia, continuous brief arousals and sleep fragmentation - could activate both the systemic sympathetic/adrenomedullary and the HPA axis limbs of the stress system (14). Nocturnal awakenings are associated with pulsatile cortisol release (15) and autonomic activation. Plasma and urinary catecholamines measured during the nighttime, and surge of sympathetic nerve activity determined by microneurography, are elevated in patients with OSA compared to obese controls (16, 17). However, the limited existing literature has failed to detect any differences in plasma cortisol levels between sleep apneics and controls (18). Most studies have focused on the effects of CPAP on cortisol with conflicting data (19-23). Two studies have reported that CPAP does not reduce cortisol levels (19, 20). One study reported that acute withdrawal of CPAP therapy does not result in an increase in cortisol levels (22). In contrast, another study reported that CPAP corrected pre-existing hypercortisolemia, particularly after prolonged use (21). In several of these studies cortisol was measured at a single time point and appropriate controls were not selected.
According to our unpublished data cortisol levels were slightly higher in obese apneic patients, compared to obese controls, and both groups had lower plasma levels of cortisol compared to nonobese controls (24). In the sleep apneic patients, CPAP lowered significantly diastolic and mean blood pressure (p<0.05) and tended to reduce cortisol levels. In another study, corticotropin-releasing hormone (CRH) administration resulted in a higher corticotropin (ACTH) response in both obese apneic and non-apneic groups compared to non-obese controls, whereas there were no differences of cortisol response to CRH. These results suggest, that in non-distressed obese, HPA axis activity is rather low due to hyposecretion of hypothalamic CRH, whereas apnea causes a mild activation of the axis that is corrected with the use of CPAP.
Sleep apnea and inflammation
Proinflammatory cytokines, particularly IL-1 and TNFa, have been studied extensively in animal studies and have been found to meet the criteria of sleep regulating substances (for a thorough review see article by Dr. Krueger et al. in this issue).
In normal people the inflammatory cytokines tumor necrosis factor-a (TNF-a), interleukin-1b (IL-1b), and interleukin-6 (IL-6) are involved in physiological sleep regulation with a circadian pattern of secretion (25-28). Decreased overall secretion of IL-6 is associated with a good night's sleep and a good sense of well-being the next day (27). Increased secretion or exogenous administration of Il-6 to humans is associated with excessive daytime sleepiness (EDS) and fatigue (29). EDS occurs in about 5% to 10% of the general population (30-31) and is the chief complaint of the majority of patients evaluated in sleep disorders centers. EDS is one of the major physiological consequences of obstructive sleep apnea.
We evaluated cytokine levels in three patient populations with EDS; sleep apneics (n=12), narcoleptics (n=11) and idiopathic hypersomniacs (n=8) (32). TNFa was significantly elevated in sleep apneics and narcoleptics and IL-6 was elevated only in sleep apneics (Fig. 1). Both TNFa and IL-6 plasma concentrations were positively correlated with the presence of EDS. Furthermore, TNFa was positively correlated with the degree of nocturnal sleep disturbance, and the degree of hypoxia, whereas IL-6 was positively correlated with the degree of nocturnal sleep disturbance, degree of hypoxia, and body mass index (BMI).
Fig 1.
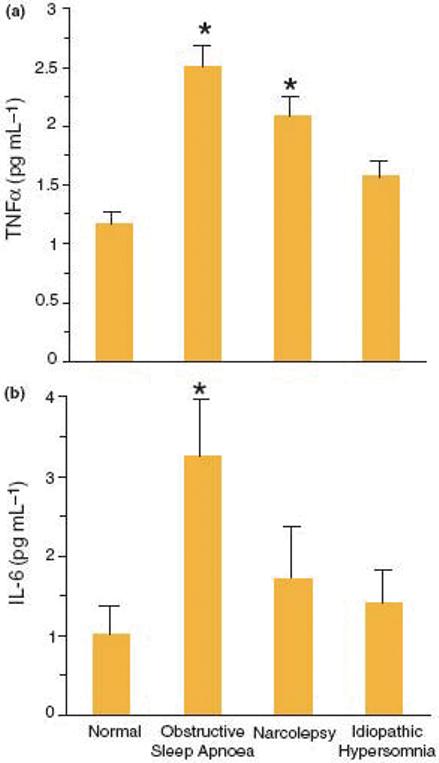
Plasma TNFa and IL-6 levels in normal subjects and patients with EDS. (a) *P < 0.001 versus normal; (b) *P = 0.028 versus normal.
In a new study that we controlled for obesity, the sleep apneic men had higher plasma concentrations of TNFa, IL-6, and leptin than nonapneic, obese men who had intermediate values or lean men who had the lowest values (33). These findings suggested that TNFa and IL-6 were elevated in sleep apnea independently of obesity. BMI correlated positively with both cytokines and leptin.
IL-6 induces C-reactive protein (CRP) production (34). CRP is a biomarker of low-grade systemic inflammation which plays an important role in arterial plaque formation, plaque rupture, and vascular thrombosis thereby increasing the susceptibility to myocardial ischemia and infarction (35). Low grade systemic inflammation may be a possible mechanism linking OSA to cardiovascular disease. Prior studies examining the association between OSA and CRP levels have produced conflicting results, with some studies verifying an independent association with disease severity (36-45) and others showing no relationship (46-51). Potential limitations of previous work are the confounding effects of obesity and medical comorbidity. A recent, well designed study demonstrated that, in the absence of confounding medical conditions, sleep disordered breathing (SDB) was associated with elevated levels of CRP with a dose-response relationship, independent of age, BMI, waist circumference, and percent body fat, suggesting that a state of low-grade inflammation is present in SDB and may act as an intermediary in the causal pathway to cardiovascular disease (52).
Sleep apnea and leptin
Leptin is an adipocyte-derived hormone that regulates body weight through control of appetite and energy expenditure (53). Leptin levels correlate with BMI and insulin levels, and its secretion is further modulated by the stress system and cytokines (53).
Several studies have shown that sleep apnea is associated with hyperleptinemia that correlates to insulin levels (33, 54-56). In studies that included primarily obese patients, the higher levels of leptin have been accounted for by obesity and/or excessive visceral fat (33), whereas in others that included primarily non-obese patients, elevated leptin levels were reported independently of obesity.
In our study, apnea/hypopnea index (AHI) did not make an additional contribution to leptin levels, and we suggested that the increase in leptin levels in sleep apnea may be related to the higher amount of visceral fat and/or cytokines (33). Leptin levels were relatively higher in sleep apneics compared to normal weight controls when measured at two time periods. Further research with serial 24-h sampling is needed to evaluate if sleep apnea is associated with a change of the circadian secretion of leptin (33, 56).
Sleep apnea and insulin resistance
Because of the association between inflammation and insulin resistance, a condition of increased insulin levels associated with normoglycemia (for detailed description see article by Drs. Aurora and Punjabi in this issue), we examined whether OSA acts as an independent risk factor for insulin resistance. We evaluated 14 obese men with symptomatic sleep apnea versus 11 BMI- and age-matched, obese, non-apneic controls (33). Mean fasting blood glucose levels were higher in the apneics than in obese controls (106.2±4.1 vs. 85.4±4.4, P<0.01). Mean plasma insulin levels were also higher in sleep apneics than in obese controls (25.7±4.2 vs. 14.6±2.5, P<0.05).
Our findings were confirmed by three relatively large studies: 1) a sleep center population in Hong Kong (57) 2) a community-based sample in the Baltimore area (58) and 3) most recently, in a large sample from the Sleep Heart Health Study (59). Importantly, one study observed that the association between OSA and insulin resistance was present even in non obese subjects (57), whereas the other study reported insulin resistance even in mild forms of sleep apnea (58).
Previous studies reported inconsistent results in terms of an association between sleep apnea and insulin resistance. A large study showed a modest relation (r2 = 0.10) between the A/HI and fasting insulin levels, but not fasting blood glucose levels (60). Two other studies showed an association between severity of sleep apnea and indices of insulin resistance (61) and that sleep apnea occurred commonly in obese patients with diabetes type II who had excessive daytime sleepiness or heavy snoring (62). In contrast, two other controlled studies suggested that the relation between sleep apnea and plasma insulin levels (63) or insulin resistance (64) reflected the known effects of obesity. However, in one of these studies, the apneics were otherwise healthy normotensive individuals (64), whereas in the second one, the apneics were lean and less symptomatic (63). The weak correlations between sleep apnea and insulin levels in clinical samples and the absence of insulin resistance in otherwise asymptomatic apneics reported in some studies may be due to the possibility that sleep apnea is a heterogeneous disorder in terms of its association with insulin resistance and/or that sleep apnea without symptoms has a weak association with insulin resistance.
Sleep apnea and visceral fat
Visceral fat is closely associated with insulin resistance and insulin resistance is associated with sleep apnea independently of obesity. Waist circumference is a better predictor of OSA than BMI (65). In a study we examined whether sleep apnea correlates with visceral, subcutaneous (SC) or total fat by using computed tomographic (CT) scanning to asses body fat distribution (33). Male patients with OSA had a greater amount of CT-determined visceral adipose tissue into the abdomen than a group of BMI-matched men without SDB (P<0.05). Interestingly, BMI correlated significantly with total body fat (measured at L3: rxy = 0.83; P<0.01) and SC fat (rxy = 0.88; P<0.01), but not with visceral fat. Importantly, visceral, but not SC fat, was significantly correlated with indices of sleep apnea (rxy = 0.70; P<0.01 for A/HI and rxy = −0.60; P<0.01 for minimum SaO2) (Fig 2). Our findings are consistent with reports that visceral fat accumulation is an important risk factor for OSA in obese subjects (66), and the AHI is significantly correlated with intra-abdominal fat but not with subcutaneous fat in the neck region or parapharyngeal fat (67).
Fig 2.
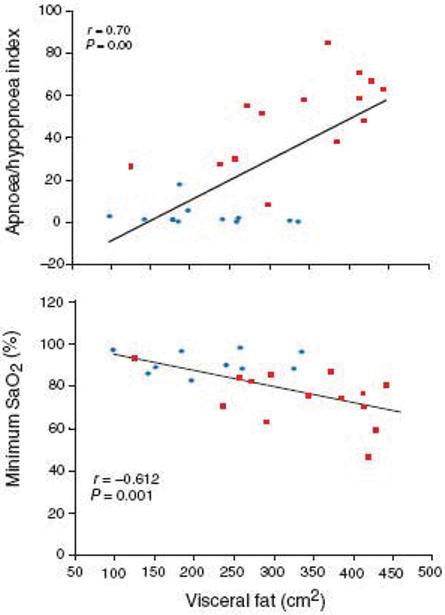
Visceral fat significantly correlated with indexes of sleep apnoea. sleep apnoeics;
sleep apnoeics;  obese controls.
obese controls.
According to these results, we proposed that visceral obesity and insulin resistance determined by both genetic and environmental factors, progressively leads to worsening metabolic syndrome manifestations and sleep apnea (33). Progressive deterioration of sleep apnoea may then accelerate the worsening of visceral obesity and the metabolic syndrome by providing a stress stimulus and causing nocturnal elevations of hormones, such as cortisol and insulin, that promote visceral adiposity, metabolic abnormalities and cardiovascular complications (33, 68) (Fig 3). One other factor may be the sleep loss experienced by these patients, as this has recently been found to increase insulin resistance in normal subjects (69).
Fig 3.
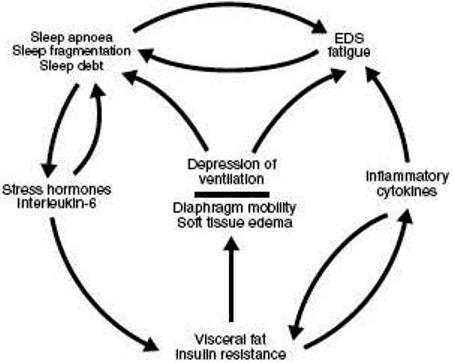
A model of the complex feed forward associations between visceral fat/insulin resistance, inflammatory cytokines, stress hormones, excessive daytime sleepiness and sleep apnoea.
Sleep apnea in disorders characterized by insulin resistance as the primary pathophysiologic mechanism
If sleep apnea is associated with insulin resistance independently of obesity, then sleep apnea should be more prevalent in disorders in which insulin resistance is a primary abnormality, such as the polycystic ovary syndrome (PCOS) (70).
In collaboration with the Department of Obstetrics and Gynecology at Hershey Medical Center, we conducted a study that included 53 women with PCOS and 452 pre-menopausal women as controls (71). The diagnosis of PCOS was made by the presence of chronic anovulation (six or fewer menstrual periods per year) in association with elevated circulating androgen levels (70). Obstructive sleep apnea was diagnosed using Sleep Disorders Clinic criteria, which employed sleep laboratory (AHI ≥ 10) plus clinical findings (3, 4). PCOS women were 30 times more likely to suffer from sleep disordered breathing (SDB) than controls [OR=30.6, 95% CI (7.2, 139.4), P<0.0001]. Even when we controlled for BMI, the difference between the two groups remained significant. Potential predictive factors, such as age, BMI, free and total testosterone, fasting insulin levels and glucose to insulin ratio were included in a logistic regression analysis. The backward conditional analysis eliminated all variables but insulin and glucose to insulin ratio, suggesting that insulin resistance was a stronger predictor for sleep apnea than age, BMI, or testosterone.
In a new study, we evaluated 42 obese women with PCOS, 17 body mass index–comparable obese controls, and 15 normal-weight controls free from apnea for single morning cytokine plasma concentrations, and insulin resistance indices (72). Women with PCOS exhibited higher plasma concentrations of IL-6 than obese controls, who had intermediate values, or normal-weight controls, who had the lowest values (4.75±0.5 vs 3.65±0.4 vs 1.84±0.3 pg/mL, P<0.01) (Fig 4). TNFa values were higher in PCOS and obese controls compared with normal-weight controls, but the difference was not statistically significant. Based on backward regression analysis, IL-6 levels had a stronger association with the PCOS group than with the obese group, and the sleep or hypoxia variables did not make a significant contribution to either IL-6 or TNF-a. Both, IL-6 and TNF-a, correlated positively with body mass index (P<0.01) in obese controls but not in women with PCOS. Within the PCOS group, IL-6 and TNF-a, correlated more strongly with indices of insulin resistance than obesity.
Fig 4.
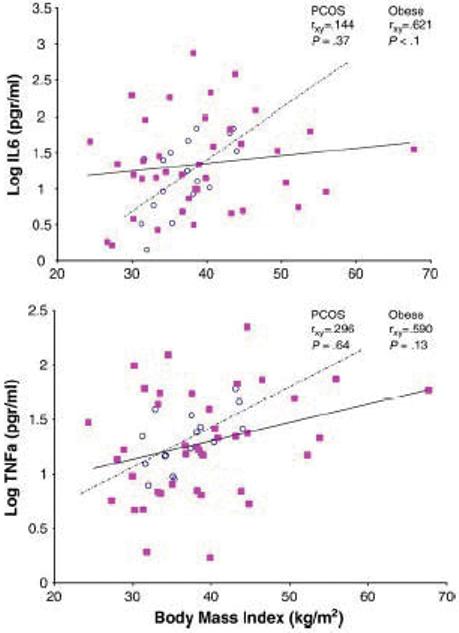
Plasma IL-6 (top) and TNFa (bottom) are positively correlated with BMI in obese controls, but not in obese women with PCOS. (■) women with PCOS; (○), obese controls.
Our findings on the association of PCOS and OSA were confirmed in three other studies (73-75) suggesting that visceral obesity and insulin resistance, which is frequently associated with PCOS (70), is a primary pathogenetic mechanism leading to sleep apnea (76).
Sleep apnea, menopause and hormone replacement therapy
The prevalence of sleep apnea is quite low in pre-menopausal women (0.6%) as well as post-menopausal women on hormone therapy (HT) (4), and the presence of sleep apnea appears to be associated exclusively with obesity (BMI>32.3%). Post-menopausal women without HT have a prevalence of sleep apnea that is close, although still lower, to the prevalence in men.
Loss of estrogen after menopause is associated with elevated IL-6, and with an increase in obesity (primarily central) and cardiovascular diseases (77). It is possible that the elevations of inflammatory cytokines, central obesity and/or insulin resistance are risk factors for increased prevalence of OSA and cardiovascular disease in post-menopausal women. In a recent study from the Women's Health Initiative Hormone Trial, estrogen plus progestin decreased diabetes and insulin resistance in post-menopausal women, which might be a mechanism through which HT protects women from sleep apnea (78). Furthermore, the adverse effect of menopause and the protective role of gonadal hormones in sleep apnea in women was confirmed in the Sleep Heart Health Study as well as in a Wisconsin cohort (79-80).
Sleep apnea and diabetes
Several studies have shown an increased prevalence of sleep apnea and sleep disordered breathing in patients with diabetes mellitus type II (62, 81). Mondini and Guilleminault (82) reported increased frequency of abnormal breathing during sleep in lean and obese diabetics. Brooks and colleagues (62) demonstrated that 70% of obese diabetics had moderate or severe OSA (62). In a Chinese population with OSA, diabetes mellitus was the second most common medical condition (about 10%) next to hypertension, associated with sleep apnea (83). Two large prospective studies, the one from Sweden and the other from the US (Nurses' Health Study Cohort) showed that regular snoring is associated with a 2- to 7-fold risk for type II diabetes over a 10-year period (84-85). These studies collectively suggest that diabetes is associated with OSA and, along with hypertension, should be added to the signs and symptoms of this prevalent sleep disorder (for a more extensive review of the issue, see chapter by Drs. Aurora and Punjabi in this issue).
Age distribution of sleep apnea and metabolic syndrome
Large epidemiologic studies have shown that the prevalence of significant apneic activity as measured in the sleep laboratory largely not associated with clinical symptoms (non-symptomatic apnea) is much higher than that of sleep apnea based on the presence of both sleep laboratory and clinical findings (symptomatic apnea) (2, 3, 4). According to these data researchers propose that there are at least two different types of apnea (3, 86). The first type of apnea has an age-related distribution with a peak around age 55 years for men and 65 years for women and accounts for the symptomatic apnea, and the second type occurs primarily in the elderly and has not the clinical consequences of the first type.
The age distribution of symptomatic sleep apnea is similar to the age distribution of the metabolic syndrome (87). Specifically, in a study on the prevalence of the metabolic syndrome in the US population from the Third National Health and Nutrition Examination Survey 1988–1994, it was demonstrated that the prevalence of the metabolic syndrome that is closely linked to insulin resistance rose with age, reached peak levels between ages 50 and 70, and then declined. Also, menopause increased the risk for metabolic syndrome in women. The similarities in age distribution between symptomatic sleep apnea and metabolic syndrome support our proposal that insulin resistance and visceral adiposity are more strongly linked to symptomatic apnea.
Sleep apnea and Continuous Positive Airway Pressure (CPAP) Treatment
CPAP is the treatment of choice, especially in moderate to severe OSA, with proven efficacy on daytime sleepiness and high blood pressure (88-89). Randomized trials have shown benefit in severe OSA in subjective sleepiness, objective tests of sleepiness, quality of life, driving performance, and depression scores (88). On the other hand no benefits of therapy were observed in severe OSA if subjects were not sleepy (90). In mild to moderate disease, CPAP ameliorated only nighttime symptoms (e.g. snoring) in all studies (91-95), whereas in most studies subjective sleepiness did not change (92, 94, 95).
The effects of CPAP on the metabolic alterations associated with OSA have not been studied systematically, and the results are inconsistent (33, 96). No improvement in insulin sensitivity was observed in 9 studies. The beneficial effect confirmed in one study, was observed primarily in non-obese patients with OSA (97). Also, in non-obese patients with OSA, visceral fat decreased after 6 months of CPAP use, even without change in BMI (98).
Inconsistent were also the results on the effects of CPAP on inflammation. A significant decrease in TNFa was reported after the use of CPAP (99-100). IL-6 increased in one study (101) and decreased in another study after the use of CPAP (38).
Studies to date have primarily evaluated short-term outcomes of CPAP use, typically 1 to 2 months, in both obese and non-obese patients with OSA.
According to our unpublished data, the therapeutic use of CPAP for three months although ameliorated blood pressure and objective and subjective sleepiness, did not improve low-grade inflammation, insulin resistance, or visceral adiposity in obese men with severe sleep apnea. From the studies reviewed, it appears that CPAP has no effect on insulin resistance, inflammation, or visceral obesity in obese apneics. Besides methodological limitations inherent in human studies, the differential response of obese vs. nonobese apneics to CPAP may suggest that the two groups of apneics are different in terms of the underlying pathophysiology and symptom profile. Indeed, nonobese apneics are characterized by less daytime sleepiness and more frequent presence of anatomic abnormalities than obese apneics (31, 102). In turn, the metabolic/inflammatory aberrations in obese apneics may be primary to the pathogenesis of apnea, whereas in the nonobese they may be secondary phenomena to the apnea per se. From a clinical standpoint current evidence suggests that additional therapeutic measures, e.g. weight loss, exercise, pharmacological agents such as antibodies that neutralize cytokines, and drugs that improve insulin sensitivity, and reduce visceral fat, should be included in the management of sleep apnea.
The role of diet, exercise, and cytokine antagonists in the management of sleep apnea
Even modest weight loss results in a clinically significant improvement of metabolic complications and cardiovascular risk profile of obesity which are closely associated with OSA (103). Weight loss in surgically treated morbidly obese patients improved the symptoms of OSA (104) and reduced AHI (105). In patients with OSA and severe visceral obesity (baseline BMI of 54.7±9.5 kg/m2) treatment with intragastric balloon reduced the number of apneic episodes during sleep and the daytime symptoms of OSA, even with a relatively modest level of weight loss and despite the fact the patients still remained affected by severe obesity after treatment (106). The improvements of OSA observed after a modest weight loss may be a part of the better responsiveness of visceral than subcutaneous fat to caloric restriction (107).
In a recent study in 1104 men and women enrolled in the Wisconsin Sleep Cohort Study, ≥7 h of exercise per week compared to 0 h of exercise per week was associated with a significant reduction of AHI (5.3 vs. 2.8) independent of BMI, age, gender, and other covariates (108). According to our unpublished data, among sleep apneics regular exercise is the strongest predictor of excessive daytime sleepiness in men, while depression, metabolic syndrome and AHI are the strongest predictors of EDS in women. Exercise improves insulin resistance and visceral adiposity independently of body weight and this can be the mechanism positively affecting nighttime and daytime symptoms of OSA.
Finally, in order to test our hypothesis that the proinflammatory cytokines TNFa and IL-6 are mediators of excessive daytime sleepiness in humans, we proceeded with a pilot study during which we administered etanercept, a medication that neutralizes TNF-a, or placebo, in eight male, obese apneics (109). There was a significant and marked decrease of sleepiness by etanercept, which increased sleep latency during the multiple sleep latency test (MSLT) by about 3.1 min compared to placebo. Also, the number of apneas/hypopneas per hour was reduced significantly by the drug compared to placebo (52.8±9.1 vs. 44.3±10.3; adjusted difference—8.4±2.3; P<0.05) (Fig 5). We concluded that neutralizing TNF-a activity is associated with a significant reduction of objective sleepiness in obese patients with OSA and that this effect suggests that pro-inflammatory cytokines contribute to the pathogenesis of OSA and sleepiness.
Fig 5.
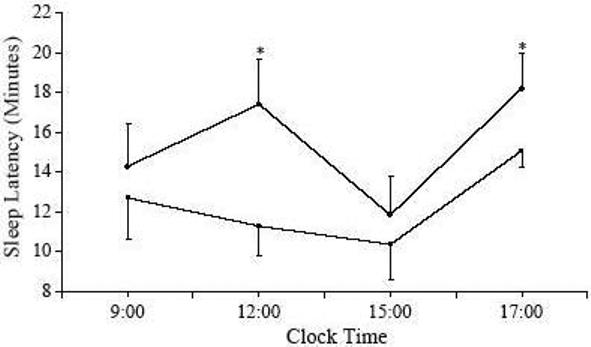
Sleep latencies during daytime testing with MSLT in the placebo (■) and etanercept (○) conditions. Each data point represents the mean±SE, P<0.05, adjusted change between placebo and etanercept.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vgontzas AN, Kales A. Sleep and its disorders. Annu Rev Med. 1999;50:387–400. doi: 10.1146/annurev.med.50.1.387. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-age adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Lin H-M, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 6.Guilleminault C, van den Hoed J, Mitler MM. Clinical overview of the sleep apnea syndromes. In: Guilleminault C, Dement WC, editors. Sleep apnea syndromes. Alan R. Liss, Inc.; New York: 1978. pp. 1–12. [Google Scholar]
- 7.Allan I. Pack Advances in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2006;173:7–15. doi: 10.1164/rccm.200509-1478OE. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 9.Jenkinson C, Stradling J, Petersen S. Comparison of three measures ofquality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 10.George CF, Nickerson PW, Hanly PJ, Millar TW, Kryger MH. Sleep apnoea patients have more automobile accidents. Lancet. 1987;2:447. doi: 10.1016/s0140-6736(87)90974-3. [DOI] [PubMed] [Google Scholar]
- 11.Weitzman ED, Zimmerman JC, Czeisler CA. Cortisol secretion is inhibited during sleep in normal man. J Clin Endocrinol Metab. 1983;56:352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- 12.Opp M. Corticotropin-releasing hormone involvement in stressor-induced alterations in sleep and in the regulation of waking. Adv Neuroimmunol. 1995;5:127–143. doi: 10.1016/0960-5428(95)00004-l. [DOI] [PubMed] [Google Scholar]
- 13.Chrousos CA, Kattah JC, Beck RW, Cleary PA. Side effects of glucocorticoid treatment. JAMA. 1993;269:2110–2112. [PubMed] [Google Scholar]
- 14.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 15.Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10:35–44. doi: 10.1093/sleep/10.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Waradckar NV, Sinoway LI, Zwillich CW, Leuenberger UA. Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med. 1996;153:1333–1338. doi: 10.1164/ajrccm.153.4.8616563. [DOI] [PubMed] [Google Scholar]
- 18.Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. 1996;153:1080–1086. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- 19.Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, Sullivan CE. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airway pressure therapy. J Clin Endocrinol Metab. 1989;68:352–358. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- 20.Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sulivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep. 1996;19:774–782. doi: 10.1093/sleep/19.10.774. [DOI] [PubMed] [Google Scholar]
- 21.Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP) Respir Med. 1999;93:1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- 22.Cooper BG, White JE, Ashworth LA, Alberti KG, Gibson GJ. Hormonal and metabolic profiles in subjects with obstructive sleep apnea syndrome and the acute effects of nasal continuous positive airway pressure (CPAP) treatment. Sleep. 1995;18:172–179. [PubMed] [Google Scholar]
- 23.Meston N, Davies RJO, Mullins R, Jenkinson C, Wass JAH, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med. 2003;254:447–454. doi: 10.1046/j.1365-2796.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Pejovic S, Lin H-L, Zoumakis E, Bixler EO, Calhoun S, Trakada G, Chrousos GP. In the Quiet and Acclimatized State of a Sleep Laboratory, Obesity Is Associated with Decreased Hypothalamic-Pituitary-Adrenal (HPA) Axis Activity Consistent with the Hypoarousal and Daytime Sleepiness and Fatigue of Obese Patients: Role of Sleep Apnea and CPAP Therapy. ENDO. 2005 [Google Scholar]
- 25.Opp MR, Kapas L, Toth LA. Cytokine involvement in the regulation of sleep. Proc Soc Exp Biol Med. 1992;201:16–27. doi: 10.3181/00379727-201-43474. [DOI] [PubMed] [Google Scholar]
- 26.Kapas L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer JM, Krueger JM. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-a and TNF-a fragments. Am J Physiol. 1992;263:708–15. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quality and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Bixler EO, Lin H-M, Prolo P, Trakada G, Chrousos GP. IL-6 and its Circadian Secretion in Humans: Fact or Artifact? NeuroImmunomodulation. 2005;12(3):131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 29.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic–pituitary–adrenal axis in humans. J Clin Endocrinol Metab. 1993;77:1690–4. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 30.Bixler EO, Kales A, Soldatos CR, Kales JD, Healay S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;1136:1256–62. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 31.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 33.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 34.Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 35.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 36.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 37.Teramoto S, Yamamoto H, Ouchi Y. Increased C-reactive protein and increased plasma interleukin-6 may synergistically affect the progression of coronary atherosclerosis in obstructive sleep apnea syndrome. Circulation. 2003;107(5):E40. doi: 10.1161/01.cir.0000053956.46188.5f. [DOI] [PubMed] [Google Scholar]
- 38.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 39.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113(6):564–569. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 40.Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J. 2005;46(5):801–809. doi: 10.1536/ihj.46.801. [DOI] [PubMed] [Google Scholar]
- 41.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111(15):1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 42.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi M, Fujimoto K, Urushibata K, Takamizawa A, Kinoshita O, Kubo K. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology. 2006;11(1):24–31. doi: 10.1111/j.1440-1843.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 44.Kageyama N, Nomura M, Nakaya Y, Watanabe T, Ito S. Relationship between adhesion molecules with hs-CRP and changes therein after ARB (Valsartan) administration in patients with obstructive sleep apnea syndrome. J Med Invest. 2006;53(12):134–139. doi: 10.2152/jmi.53.134. [DOI] [PubMed] [Google Scholar]
- 45.Zouaoui BK, Van Meerhaeghe A, Doumit S, et al. Sleep apnoea/hypopnoea index is an independent predictor of high-sensitivity Creactive protein elevation. Respiration. 2006;73(2):243–246. doi: 10.1159/000090201. [DOI] [PubMed] [Google Scholar]
- 46.Barcelo A, Barbe F, Llompart E, et al. Effects of obesity on C-reactive protein level and metabolic disturbances in male patients with obstructive sleep apnea. Am J Med. 2004;117(2):118–121. doi: 10.1016/j.amjmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep. 2004;27(8):1507–1511. doi: 10.1093/sleep/27.8.1507. [DOI] [PubMed] [Google Scholar]
- 48.Akashiba T, Akahoshi T, Kawahara S, Majima T, Horie T. Effects of long-term nasal continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44(8):899–900. doi: 10.2169/internalmedicine.44.899. [DOI] [PubMed] [Google Scholar]
- 49.Kaditis AG, Alexopoulos EI, Kalampouka E, et al. Morning levels of C-reactive protein in children with obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2005;171(3):282–286. doi: 10.1164/rccm.200407-928OC. [DOI] [PubMed] [Google Scholar]
- 50.Can M, Acikgoz S, Mungan G, et al. Serum cardiovascular risk factors in obstructive sleep apnea. Chest. 2006;129:233–237. doi: 10.1378/chest.129.2.233. [DOI] [PubMed] [Google Scholar]
- 51.Saletu M, Nosiska D, Kapfhammer G, et al. Structural and serum surrogate markers of cerebrovascular disease in obstructive sleep apnea (OSA) Association of mild OSA with early atherosclerosis. J Neurol. 2006 doi: 10.1007/s00415-006-0110-6. [DOI] [PubMed] [Google Scholar]
- 52.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. SLEEP. 2007;30(1):29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantzoros C, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis D, Griveas I, Katsilambros N, Flier J. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997;82(10):3408–13. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- 54.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 55.Manzella D, Parillo M, Razzino T, Gnasso P, Buonanno S, Gargiulo A, Caputi M, et al. Soluble leptin receptor and insulin resistance as determinant of sleep apnea. Int J Obes. 2002;26:370–5. doi: 10.1038/sj.ijo.0801939. [DOI] [PubMed] [Google Scholar]
- 56.Patel SR, Palmer LJ, Larkin EK, Jenny NS, White DP, Redline S. Relationship between obstructive sleep apnea and diurnal leptin rhythms. Sleep. 2004;27(2):235–9. doi: 10.1093/sleep/27.2.235. [DOI] [PubMed] [Google Scholar]
- 57.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 58.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 59.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep Heart Health Study I. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 60.Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, Hoffstem VS. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17:614–8. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 61.Tiihonen M, Partinen M, Närvänen S. The severity of obstructive sleep apnoea is associated with insulin resistance. J Sleep Res. 1993;2:56–61. doi: 10.1111/j.1365-2869.1993.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 62.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 63.Davies RJO, Turner R, Crosby J, Stradling JR. Plasma insulin and lipid levels in untreated obstructive sleep apnoea and snoring: their comparison with matched controls and response to treatment. J Sleep Res. 1994;3:180–5. doi: 10.1111/j.1365-2869.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 64.Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996;154:170–4. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 65.Grunstein R, Wilcox I, Yang T-S, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–540. [PubMed] [Google Scholar]
- 66.Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, Kotani K, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Int Med. 1997;241:11–18. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 67.Schafer, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–39. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 68.Vgontzas AN, Vela-Bueno A, Chrousos GP. Endocrine disorders and sleep. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Hanley and Belfus, Inc.; Philadelphia: 2002. pp. 477–87. [Google Scholar]
- 69.Allen RP. Article reviewed: Sleep apnoea and daytime sleepiness and fatigue: related to visceral obesity, insulin resistance, and hypercytokinemia. Sleep Med. 2000;1:249–50. doi: 10.1016/s1389-9457(00)00037-x. [DOI] [PubMed] [Google Scholar]
- 70.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 71.Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86:517–20. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]
- 72.Vgontzasa AN, Trakada G, Bixler EO, Lin H-M, Pejovic S, Zoumakis E, Chrousos GP, Legro RS. Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metabolism Clinical and Experimental. 2006;55:1076–1082. doi: 10.1016/j.metabol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 73.Fogel RBMA, Pillar G, Pittman SD, Dunaif A, White DP. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86(3):1175–1178. doi: 10.1210/jcem.86.3.7316. [DOI] [PubMed] [Google Scholar]
- 74.Gopal M, Duntley S, Uhles M, Attarian H. The role of obesity in the increased prevalence of obstructive sleep apnea syndrome in patients with polycystic ovarian syndrome. Sleep Med. 2002;3(5):401–404. doi: 10.1016/s1389-9457(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 75.Tasali E, Van Cauter E, Ehrmann DA. Relationships between sleep disordered breathing and glucose metabolism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006 Jan;91(1):36–42. doi: 10.1210/jc.2005-1084. [DOI] [PubMed] [Google Scholar]
- 76.Vgontzas AN, Chrousos GP. Sleep-disordered breathing, sleepiness, and insulin resistance: is the latter a consequence, a pathogenetic factor, or both? Sleep Med. 2002;3:389–91. doi: 10.1016/s1389-9457(02)00067-9. [DOI] [PubMed] [Google Scholar]
- 77.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Int Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 78.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Women's Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the women's health initiative hormone trial. Diabetologia. 2004;47:1175–87. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 79.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–92. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 80.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin sleep cohort study. Am J Respir Crit Care Med. 2003;167:1181–5. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 81.Elmasry A, Lindberg E, Berne C, Janson C, Gislason T, Awad Tageldin M, Boman G. Sleep-disordered breathing and glucose metabolism in hypertensive men: a population based study. J Int Med. 2001;249:153–61. doi: 10.1046/j.1365-2796.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 82.Mondini S, Guilleminault C. Abnormal breathing patterns during sleep in diabetes. Ann Neurol. 1985;17:391–5. doi: 10.1002/ana.410170415. [DOI] [PubMed] [Google Scholar]
- 83.Ip SM, Tsang WT, Lam WK. Obstructive sleep apnoea syndrome: an experience in Chinese adults in Hong Kong. Chin Med J (Engl) 1998;111:257–60. [PubMed] [Google Scholar]
- 84.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155:387–93. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 85.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Int Med. 2000;248:13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 86.Bliwise D. Normal aging. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. WB Saunders; Philadelphia: 2000. pp. 26–42. [Google Scholar]
- 87.Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome. Prevalence and associated risk factor findings in the US population from the third national health and nutrition examination survey, 1988–1994. Arch Int Med. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel S, White D, Malhotra A, Stanchina M, Ayas N. Continuous Positive Airway Pressure Therapy for Treating Sleepiness in a Diverse Population with Obstructive Sleep Apnea: Results of a Meta-analysis. Arch Intern Med. 2003;163:565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 89.Pepperell J, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling J, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomized parallel trial. Lancet. 2002;359:240–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 90.Barbe F, Mayoralas LR, Duran J, Masa JF, Maimo A, Montserrat JM, Monasterio C, Bosch M, Ladaria A, Rubio M, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trial. Ann Intern Med. 2001;134:1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 91.Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. 1998;157:858–865. doi: 10.1164/ajrccm.157.3.9709042. [DOI] [PubMed] [Google Scholar]
- 92.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/ hypopnoea syndrome. Thorax. 1997;52:114–119. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 94.Monasterio C, Vidal S, Duran J, Ferrer M, Carmona C, Barbe F, Mayos M, Gonzalez-Mangado N, Juncadella M, Navarro A, et al. Effectiveness of continuous positive airway pressure in mild sleep apneahypopnea syndrome. Am J Respir Crit Care Med. 2001;164:939–943. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 95.Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, Trinder J, Saunders NA, McEvoy RD, Pierce RJ. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 96.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–224. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Harsch I, Schahin S, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, et al. Continuous Positive Airway Pressure Treatment Rapidly Improves Insulin Sensitivity in Patients with Obstructive Sleep Apnea Syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 98.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 99.Ryan S, Taylor C, McNicholas W. Predictors of Elevated Nuclear Factor-? B-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–830. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 100.Minoguchi K, Tazaki T, Yokoe T, Minoguchi H, Watanabe Y, Yamamoto M, et al. Elevated production of tumor necrosis factor-a by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 101.Harsch I, Koebnick C, Wallaschofski H, Pour Schanin S, Hahn EG, Ficker JH, et al. Restin levels in patients with obstructive sleep apnea syndrome - the link to subclinical inflammation. Med Sci Monit. 2004;10:510–515. [PubMed] [Google Scholar]
- 102.Smith PL, Schwartz AR. Biomechanics of the upper airway during sleep. In: Pack AL, editor. Sleep apnea: pathogenesis, diagnosis, and treatment. Marcel Dekker, Inc.; New York: 2002. pp. 31–52. [Google Scholar]
- 103.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1991;16:397–415. [PubMed] [Google Scholar]
- 104.Dixon JB, Schachter LM, O'Brien PE. Sleep disturbance and obesity. Changes following surgically induced weight loss. Arch Intern Med. 2001;161:10–106. doi: 10.1001/archinte.161.1.102. [DOI] [PubMed] [Google Scholar]
- 105.Harman EM, Wynne JW, Block AJ. The effect of weight loss on sleep-disordered breathing and oxygen desaturation in morbidly obese men. Chest. 1982;82:291–294. doi: 10.1378/chest.82.3.291. [DOI] [PubMed] [Google Scholar]
- 106.Busetto L, Costa G, Negrin V, Peruzza S, Enzi G. Upper airways morphometry before and after weight loss in obese patients with obstructive sleep apnea (OSAS) Int J Obes Relat Metab Disord. 2003;27:S85. [Google Scholar]
- 107.Busetto L, Tregnaghi A, Bussolotto M, et al. Visceral fat loss evaluated by total body magnetic resonance in obese women operated with laparoscopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord. 2000;24:60–69. doi: 10.1038/sj.ijo.0801086. [DOI] [PubMed] [Google Scholar]
- 108.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–4. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 109.Vgontzas AN, Zoumakis E, Lin H-M, Vela-Bueno A, Trakada G, Chrousos P. Marked decrease of sleepiness in patients with sleep apnea by etanercept. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]


