Abstract
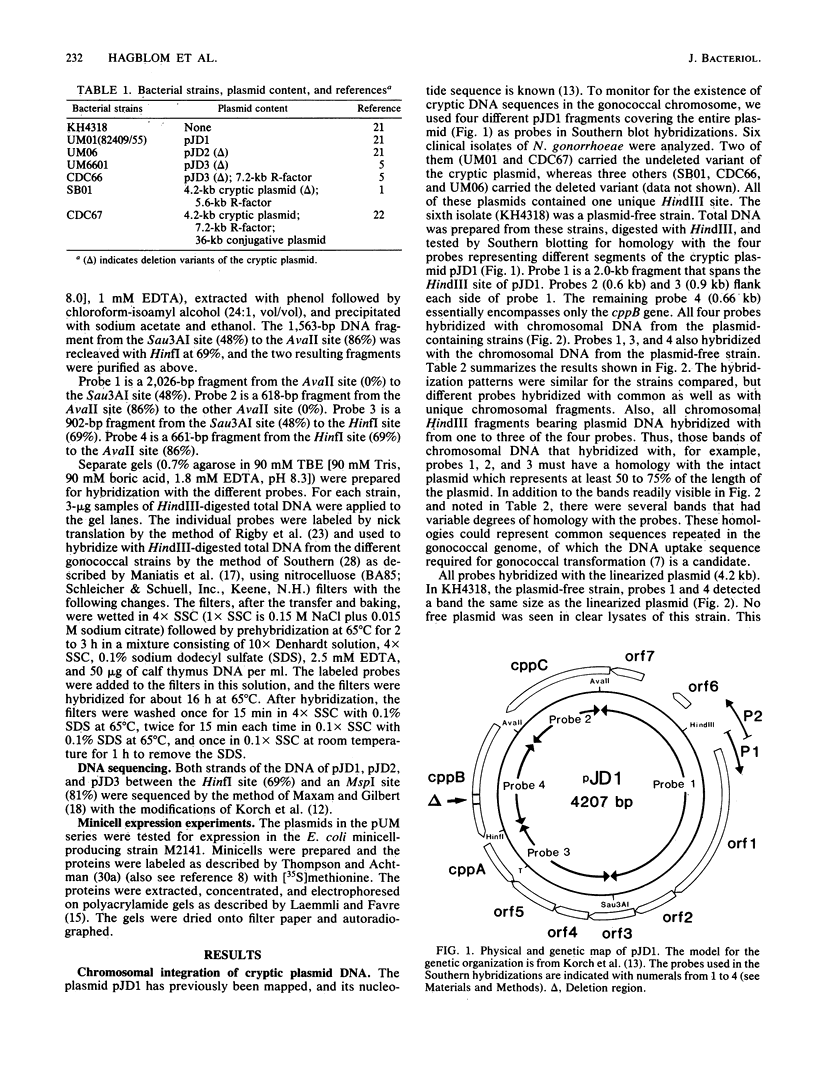
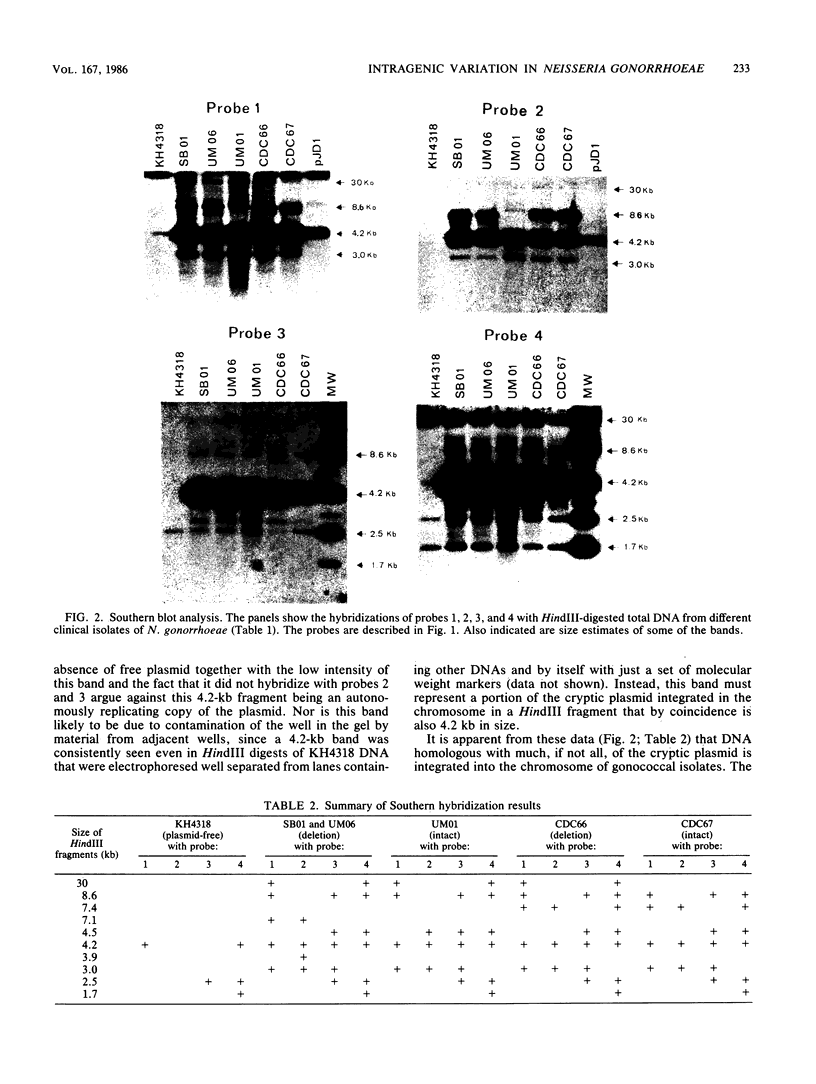
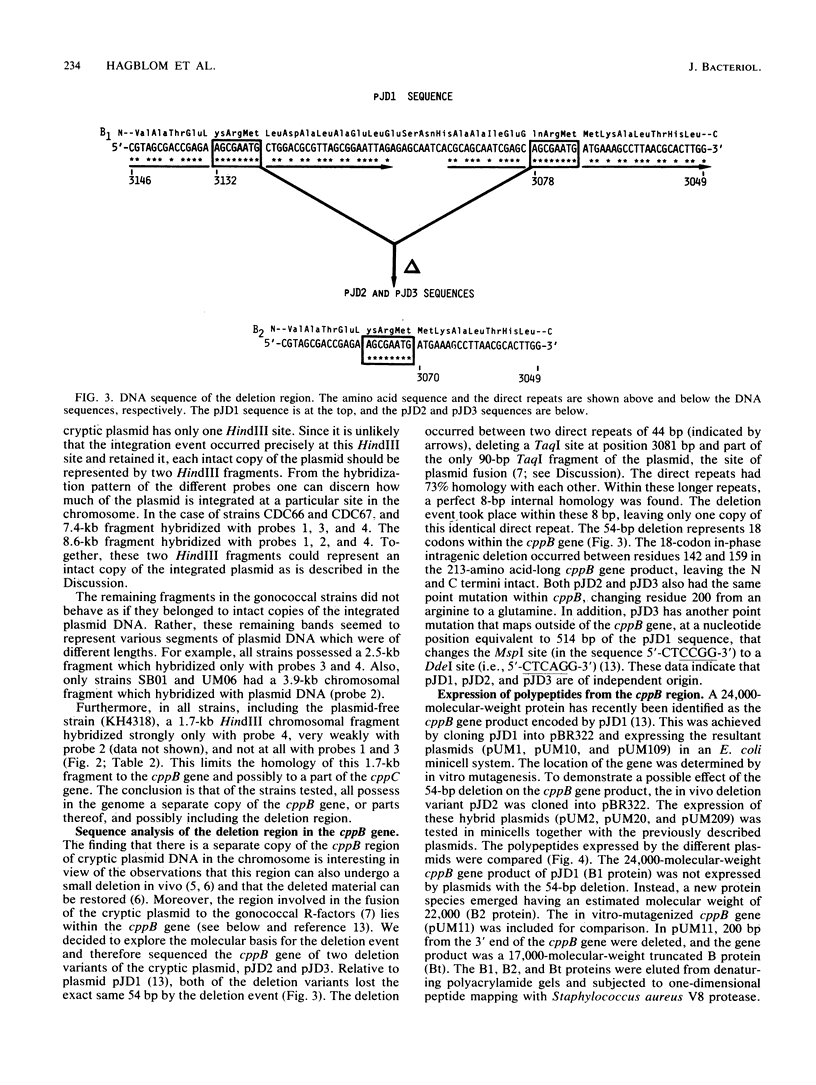
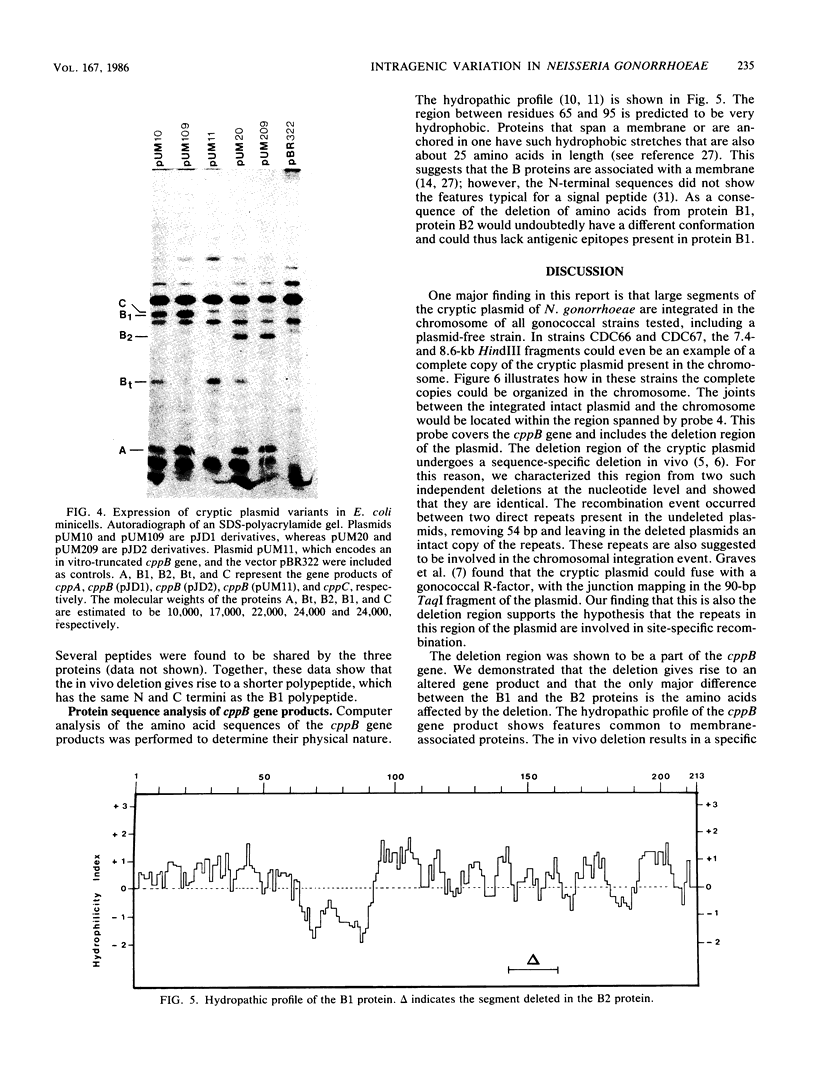
Cryptic plasmid DNA of Neisseria gonorrhoeae was found integrated into the gonococcal chromosome in both plasmid-bearing strains and plasmid-free strains. At several chromosomal locations only segments of the plasmid were found. However, in at least two strains an intact copy of the plasmid seemed to be present with the joints between the plasmid and the chromosomal DNA being located within the cppB gene of the cryptic plasmid. The cppB gene was shown to undergo a sequence-specific intragenic deletion. The deletion removed 54 base pairs, representing 18 amino acids, and did not affect the reading frame. It is proposed that the cryptic plasmid integrates into the chromosome and other gonococcal plasmids within this site-specific deletion region. Models for the site-specific recombination are presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström S., Norlander L., Norqvist A., Normark S. Contribution of a TEM-1-like beta-lactamase to penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Apr;13(4):618–623. doi: 10.1128/aac.13.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Olsson O., Normark S. Common evolutionary origin of chromosomal beta-lactamase genes in enterobacteria. J Bacteriol. 1982 May;150(2):528–534. doi: 10.1128/jb.150.2.528-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. The mechanism of phage lambda site-specific recombination: site-specific breakage of DNA by Int topoisomerase. Cell. 1983 Dec;35(3 Pt 2):795–803. doi: 10.1016/0092-8674(83)90112-5. [DOI] [PubMed] [Google Scholar]
- Davies J. K., Normark S. A relationship between plasmid structure, structural lability, and sensitivity to site-specific endonucleases in Neisseria gonorrhoeae. Mol Gen Genet. 1980 Jan;177(2):251–260. doi: 10.1007/BF00267436. [DOI] [PubMed] [Google Scholar]
- Foster R. S., Foster G. C. Electrophoretic comparison of endonuclease-digested plasmids from Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1297–1304. doi: 10.1128/jb.126.3.1297-1304.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. F., Biswas G. D., Sparling P. F. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol. 1982 Dec;152(3):1071–1077. doi: 10.1128/jb.152.3.1071-1077.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundström T., Jaurin B., Edlund T., Normark S. Physical mapping and expression of hybrid plasmids carrying chromosomal beta-lactamase genes of Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1127–1134. doi: 10.1128/jb.143.3.1127-1134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Normark S. Sequence-specific DNA modification in Neisseria gonorrhoeae. J Bacteriol. 1983 Sep;155(3):1324–1332. doi: 10.1128/jb.155.3.1324-1332.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Morisato D., Kleckner N. Transposase promotes double strand breaks and single strand joints at Tn10 termini in vivo. Cell. 1984 Nov;39(1):181–190. doi: 10.1016/0092-8674(84)90204-6. [DOI] [PubMed] [Google Scholar]
- Norlander L., Davies J. K., Hagblom P., Normark S. Deoxyribonucleic acid modifications and restriction endonuclease production in Neisseria gonorrhoeae. J Bacteriol. 1981 Feb;145(2):788–795. doi: 10.1128/jb.145.2.788-795.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlander L., Davies J., Norqvist A., Normark S. Genetic basis for colonial variation in Neisseria gonorrhoeae. J Bacteriol. 1979 Jun;138(3):762–769. doi: 10.1128/jb.138.3.762-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts M., Piot P., Falkow S. The ecology of gonococcal plasmids. J Gen Microbiol. 1979 Oct;114(2):491–494. doi: 10.1099/00221287-114-2-491. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Blake M., Gotschlich E. C. Intra-strain heterogeneity of gonococcal pili is related to opacity colony variance. J Exp Med. 1980 Mar 1;151(3):716–725. doi: 10.1084/jem.151.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Billyard E., So M., Storzbach S., Meyer T. F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985 Feb;40(2):293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Senior A. E. Secondary and tertiary structure of membrane proteins involved in proton translocation. Biochim Biophys Acta. 1983 Jul 15;726(2):81–95. doi: 10.1016/0304-4173(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences are not uniformly hydrophobic. J Mol Biol. 1982 Aug 15;159(3):537–541. doi: 10.1016/0022-2836(82)90300-x. [DOI] [PubMed] [Google Scholar]