Summary
Sebaceous tumours include hyperplasia, adenoma, sebaceoma and carcinoma. Importantly, the latter three are potential markers of Torre–Muir syndrome; the hereditary association of sebaceous neoplasia and internal malignancy, most commonly colorectal carcinoma. The diagnostic features, differential diagnosis, molecular diagnostics and recent advances in pathogenesis of this rare group of tumours are discussed along with Torre–Muir syndrome and recommendations for screening for this important association.
Keywords: sebaceous neoplasia, Torre–Muir syndrome, sebaceous hyperplasia, sebaceous adenoma, sebaceoma, sebaceous carcinoma, microsatellite instability
INTRODUCTION
Sebaceous tumours range from the sometimes cosmetically disturbing sebaceous hyperplasia to the potentially severe morbidity of ocular sebaceous carcinoma. These tumours show differentiation towards the sebaceous gland which secretes lipids and other substances into the hair follicle infundibulum.1,2 Perhaps the most important facet of sebaceous neoplasia is the association with Torre–Muir syndrome (TMS). In this review, the range of purely sebaceous tumours will be discussed along with associations with TMS, available diagnostic testing and recent advances in understanding of the pathogenesis of these rare tumours.
CLASSIFICATION OF SEBACEOUS LESIONS
Sebaceous hyperplasia
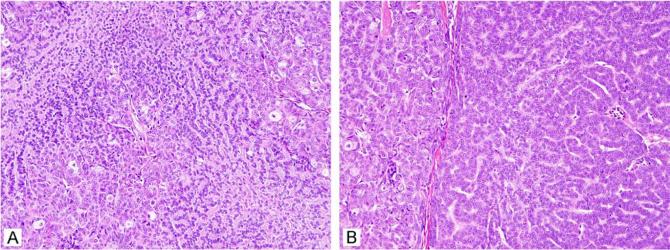
Sebaceous hyperplasia presents as a flesh-coloured papule on the face, commonly present in older men.3 It has been described in younger patients and at other sites such as the chest, penis, vulva and areola. It has sometimes been described overlying dermatofibromas, perhaps as part of the epithelial induction process described in these lesions.4,5 Sebaceous hyperplasia is small, measuring 1–3 mm, although larger forms up to 1 cm have been described. Although often suspected clinically, these lesions are sometimes mistaken for basal cell carcinoma; dermoscopy is believed to be diagnostically helpful by some.6 The lesions usually have a central dell or depression. On histology, a lobular array of well-differentiated mature sebaceous lobules is noted (Fig. 1A,B). While the lobules are greater in number and higher in the dermis than normally encountered, they are usually not much larger than the sebaceous lobules normally encountered on the face. Only one to two layers of peripheral basaloid, or germinative, cells are noted at the periphery of the lobules (Fig. 1C). The lobules are usually intimately associated with a central cystic dilated pore; the histological correlate of umbilication. Multiple tissue levels are often required to demonstrate this feature. It has been noted that the term ‘hyperplasia’ may be inappropriate as these lesions do not regress spontaneously; whether these lesions are reactive or neoplastic is not entirely clear, although most favour this to be a reactive condition. The cause of these lesions is not understood. While various genetic manipulations in transgenic mice cause hypertrophic sebaceous glands, this is not actually analogous to the human lesion, and hypertrophy appears to be a default pathway in adnexal development in the mouse. The relevance of these findings to humans is not clear. It seems likely that androgenic stimulation may play a role given its importance in normal sebaceous gland development during puberty, but this has not been established. Sebaceous hyperplasia has been reported in renal transplant patients treated with cyclosporin.7-9 Treatment has traditionally been excision and the lesions do not generally recur, although patients can develop multiple lesions.
Figure 1.
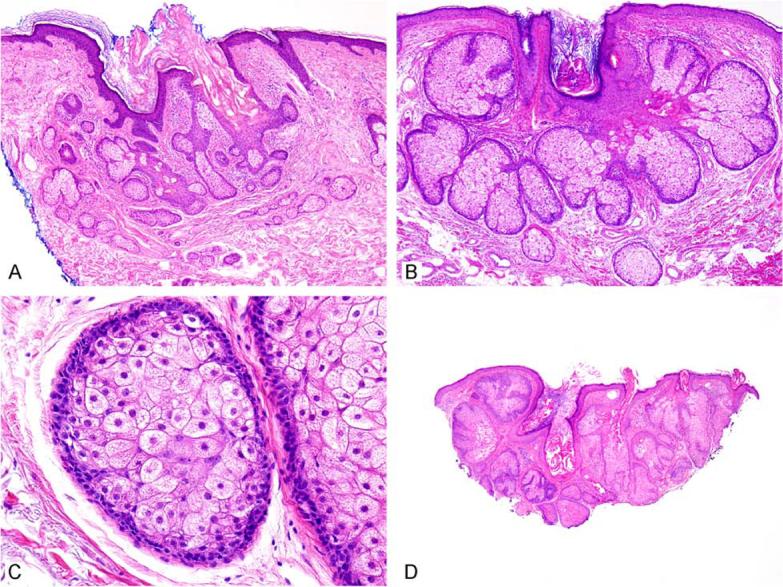
Sebaceous hyperplasia shows superficial sebaceous lobules surrounding a central pore (A, B). Higher power reveals foamy eosinophilic cytoplasm in central cells and only two basaloid or germinative cell layers at the periphery (C). (D) While most of the lesion appears to be sebaceous hyperplasia, a few of the lobules have expanded basaloid areas, indicating that this lesion is best considered as sebaceous adenoma.
Although a different entity altogether, ectopic sebaceous glands are known in the nipple area under the eponym of ‘Montgomery tubercles’ and in the lips and genital area as ‘Fordyce spots’. On the penis, these have also been referred to as ‘Tyson's glands’. Ectopic sebaceous glands are also noted in unusual places such as the tubular oesophagus (Fig. 2),10,11 but all of these ectopic glands open directly to the epithelial surface, are not associated with a follicular structure and thus are not considered to be sebaceous hyperplasia. They are common enough on the lips and genitals to be considered as a normal finding.
Figure 2.
Ectopic sebaceous gland in an oesophageal mucosa biopsy. The left and right insets magnify the sebaceous lobule and sebaceous ductal structure (courtesy of Dr. Huamin Wang, UT MD Anderson Cancer Center, Houston, Texas, USA).
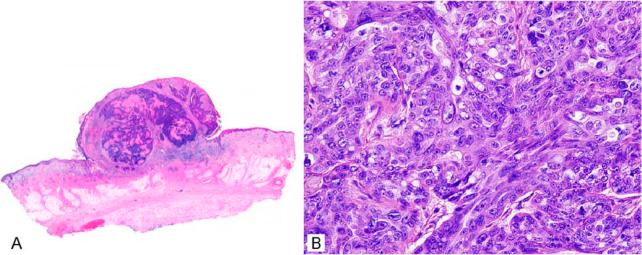
Sebaceous adenoma
Sebaceous adenomas are benign, lobular, organoid tumours showing sebaceous differentiation.12,13 The majority present in the head and neck area, but their rare presence at virtually any other cutaneous site has also been described. In addition, their presence in the oral cavity has been described.14,15 Clinically, these usually appear as non-descript flesh-coloured papules, usually 5 mm or so in greatest dimension, with a wide differential diagnosis, although basal cell carcinoma is a commonly stated clinical impression. Sometimes a more creamy white colouration can be noted with careful inspection and can provide a hint for the diagnosis. On histology, the sebaceous proliferations maintain an organoid architecture with peripheral basaloid cells that are variably expanded beyond the normal two layers, and more central mature sebocytes that often show features of holocrine secretion (Fig. 3A). The central sebocytes are larger with eosinophilic bubbly cytoplasm, although indentation of the nuclei is often less prominent than in the sebocytes of normal sebaceous glands. There can be connection with the overlying squamous epithelium (Figs 3 and 4), and sometimes these tumours appear to partially replace the surface epithelium. Mitoses are inconspicuous and cellular atypia is minimal. The overall lobular architecture of the normal sebaceous gland is maintained, but is enlarged and individual lobules show increased peripheral basaloid cells (Fig. 4C,D). This expansion of basaloid cells is a key feature for distinguishing these tumours from sebaceous hyperplasia. Sometimes sebaceous adenoma can be seen to arise in a background of surrounding prominent sebaceous glands, suggestive of sebaceous hyperplasia, but as these lesions arise on the face with a significant background of sebaceous glands, this is a difficult determination and an aetiological progression from sebaceous hyperplasia appears unlikely (Fig. 1D). By convention, the basaloid cells form no more than half of the cellularity of this tumour. Treatment is usually complete conservative surgical excision and these lesions rarely recur.
Figure 3.
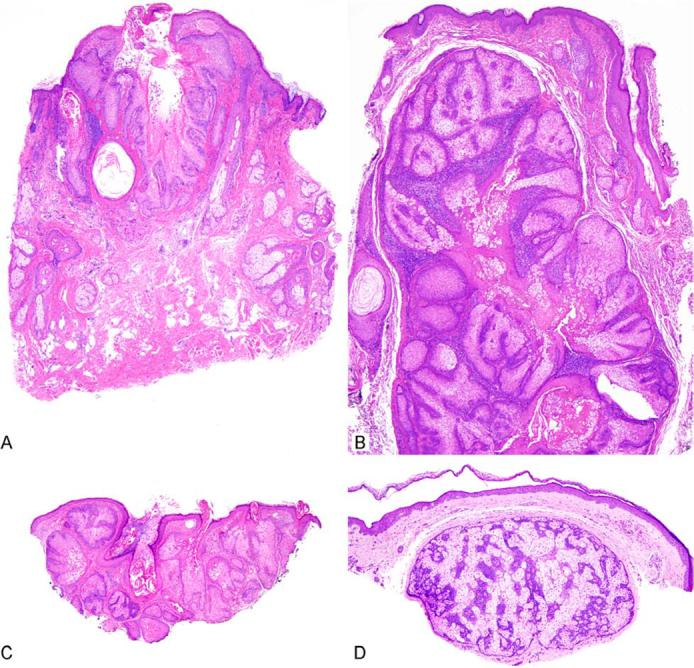
Sebaceous adenomas can be dermal or show extensive association with the overlying epidermis.
Figure 4.
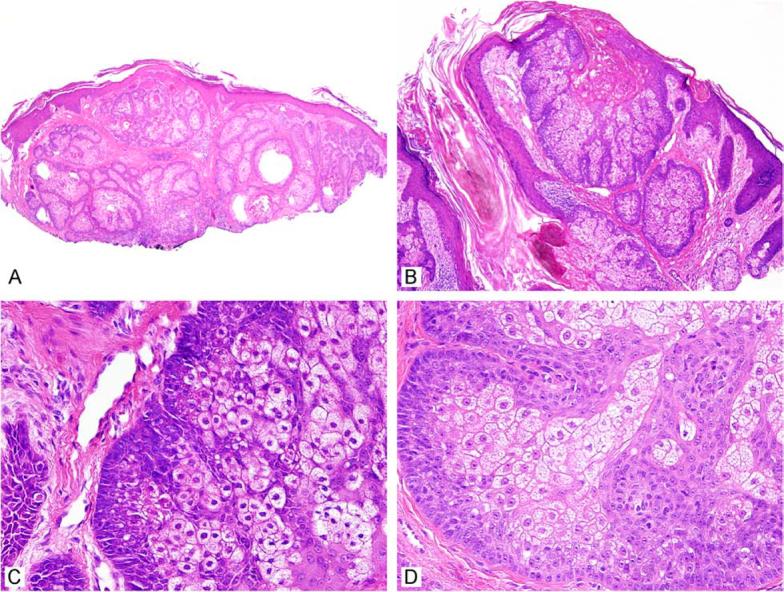
Sebaceous adenomas are well circumscribed and lobular (A, B). The higher magnification images highlight the expanded basal (germinative) cell layer (C, D).
Recently, there has been some discussion regarding whether most sebaceous adenomas are, in fact, carcinomas. While it is probably not possible to entirely exclude that any adnexal tumour, particularly one with an expanded population of basal cells, represents a low-grade malignancy, the current literature does not support sebaceous adenomas as a malignant neoplasm. Sebaceous adenomas as traditionally described are not associated with significant local recurrence or aggressive behaviour and do not appear to metastasise. Most practitioners conservatively, but completely, excise these lesions and this appears to be adequate therapy. There does not appear to be compelling justification to consider this tumour as malignant.
An extensive discussion of the differential diagnosis of clear cell lesions is presented in another article in this issue, but suffice it to say that metastatic renal cell carcinoma, clear cell melanoma, clear cell hidradenoma and various xanthomatous lesions deserve consideration. In general, the maturation of the basaloid (germinative) cells to sebocyte precursors and mature sebocytes is helpful. The central mature sebocytes will show a bubbly, multivacuolated cytoplasm with distinct indentation of the nucleus; these features are not seen in the other lesions. Sebaceous tumours lack the extensive capillary vasculature of renal cell carcinoma, and immunohistochemical studies can be helpful if confusion persists.
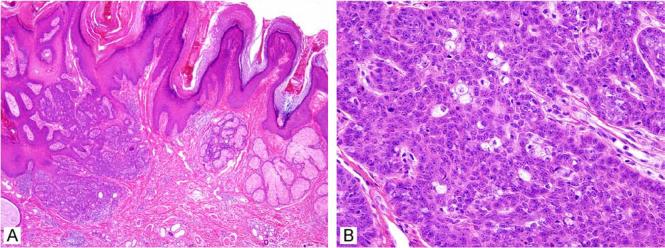
Sebaceoma
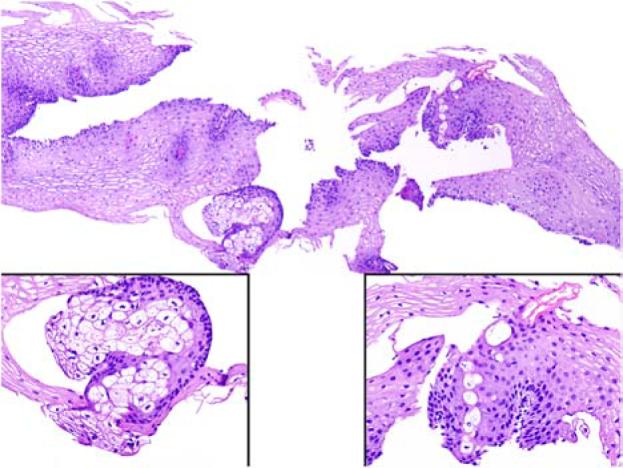
Sebaceoma was described approximately two decades ago.16 The description of this lesion includes benign sebaceous tumours with extensive (>50%) basaloid cell content.17 These lesions present similarly to sebaceous adenoma as 0.5–3-cm, flesh-coloured papules on the head and neck area in individuals in the sixth decade of life and beyond, although younger adults are sometimes encountered. This diagnosis is usually unsuspected clinically due to its rarity. There is a suggestion of female predominance, but this may decrease as additional case series are described. These lesions lose some of the organoid quality of sebaceous adenomas, and mature sebocyte differentiation can be quite focal. These lesions are usually centred in the dermis, but can involve the overlying epidermis (Fig. 5A,B). Basaloid content is prominent in these lesions, and rippled or carcinoid-like architecture in the basaloid component has been described (Fig. 6).18-20 Frequently, ductal structures are embedded that show a luminal squamous cuticle forming sebaceous ducts (Fig. 5C,D). As in most cutaneous basaloid proliferations, scattered mitoses can be seen but these lesions are well circumscribed and overt cytologic atypia is not a feature. Originally, these lesions were described as either freestanding in the dermis or attached to the overlying epidermis, although some believe that these lesions should be dermal based by definition. This diagnostic category is intended to supersede and include sebaceous epithelioma, a somewhat conceptually indistinct entity as discussed below. In the authors' opinion, sebaceoma is a benign sebaceous tumour with a clinical presentation virtually identical to that of sebaceous adenoma. The term can thus be used to describe lesions with attachment to the overlying squamous epithelium and to tumours that are based in the dermis. Perhaps the primary importance of this diagnostic category is to ensure that the sebaceous nature of these tumours is not overlooked given the sometimes basaloid predominance of the tumours.
Figure 5.
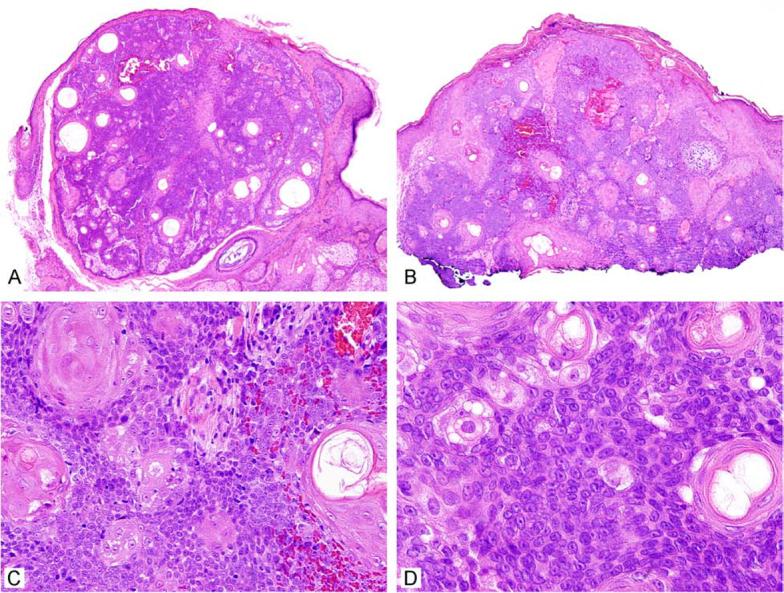
Sebaceoma can be free in the dermis or show connection to the overlying epithelium (A, B). Squamous differentiation and sebaceous ducts are sometimes present (C, D).
Figure 6.
Carcinoid-like or rippled pattern is infrequently encountered in sebaceoma and can cause diagnostic confusion.
Conceptually, adenomas and sebaceomas can be considered as two ends of a spectrum of benign sebaceous neoplasia, with the former being more organoid while the latter shows extensive basaloid differentiation. The most important point is to recognize both of these as benign lesions and potential markers of TMS as discussed below. Sebaceomas are usually treated with conservative complete excision. Sebaceomas can be differentiated from basal cell carcinoma with sebaceous differentiation as the latter shows a distinct peripheral basal cell palisading, loose fibromucinous stroma, and tumour–stroma separation artefact in formalin-fixed sections.21
Sebaceous epithelioma
The term ‘sebaceous epithelioma’ has become a difficult entity to discuss as it was initially described as a distinct entity comprised of proliferation of basaloid cells with sebaceous differentiation, but has also variably been considered as a sebaceous adenoma variant, as synonymous with basal cell carcinoma with sebaceous differentiation or something intermediate to these two. This entity has been declared as both benign and malignant by various authors. While this term does have historical merit,22 the terms ‘sebaceous adenoma’ and ‘sebaceoma’ appear to be better-defined entities and thus the authors choose not to employ the diagnostic term ‘sebaceous epithelioma’. If this term is used, it should probably best be considered as a benign sebaceous tumour, probably related to sebaceoma,16 and distinct from a basal cell carcinoma with sebaceous differentiation, although some authors argue that this lesion can be locally destructive.
Peri-ocular sebaceous carcinoma
Peri-ocular sebaceous carcinoma of the eyelid appears to arise in association with the ocular sebaceous glands such as the meibomian (tarsal) glands or glands of Zeis.23,24 Sebaceous carcinomas represent 1–2 % of malignancies of the eyelid, although estimates in series vary widely, probably due to referral bias at the institutions reporting the series. There is a slight female predominance. Only invasive melanoma at this site results in greater potential morbidity and mortality. The tumours usually arise in association with the prominent meibomian glands, and show progressive enlargement, often with erythaema, but ulceration is not usually present. Clinical presentation varies considerably leading to confusion with the more common benign conditions of chalazion or chronic blepharoconjunctivitis. Metastasis and subsequent mortality can range up to 25%, and as early recognition and aggressive treatment can reduce this considerably, an appropriate clinical index of suspicion should be maintained.25-27 Metastasis portends an aggressive clinical course. It is reasonable to biopsy presumed inflammatory lesions of the eyelid if the clinical features are suspicious, although the often small biopsies can be a challenge to interpret. Ocular sebaceous carcinoma has been reported to arise in eyelids previously exposed to radiation in treatment of another prior malignancy.
Peri-ocular sebaceous carcinomas are composed of enlarged atypical epithelioid cells usually with basaloid features and hyperchromatic nuclei (Fig. 7D). The degree of cytological atypia is significantly greater than that encountered in sebaceous adenoma or sebaceoma, but is not always prominent. The proportion of mature sebocytes present is variable, the lesion is usually more basaloid overall, and mature sebaceous differentiation can be very focal. Extensive colonization of skin/conjunctival adnexal structures is often present and pagetoid migration of malignant sebocytes can be noted (Fig. 7B). Carcinoma in the dermis or corium is infiltrative and lacks the lobular circumscription of the benign lesions described above (Fig. 7D). Mitoses, including atypical forms, can be conspicuous. From time to time, the pathologist can be asked to make a diagnosis on a small biopsy from eyelid skin or conjunctiva. The differential diagnosis will include basal cell carcinoma (the most common malignancy at this site), squamous cell carcinoma, melanoma, and rare glandular or adnexal malignancies.28
Figure 7.
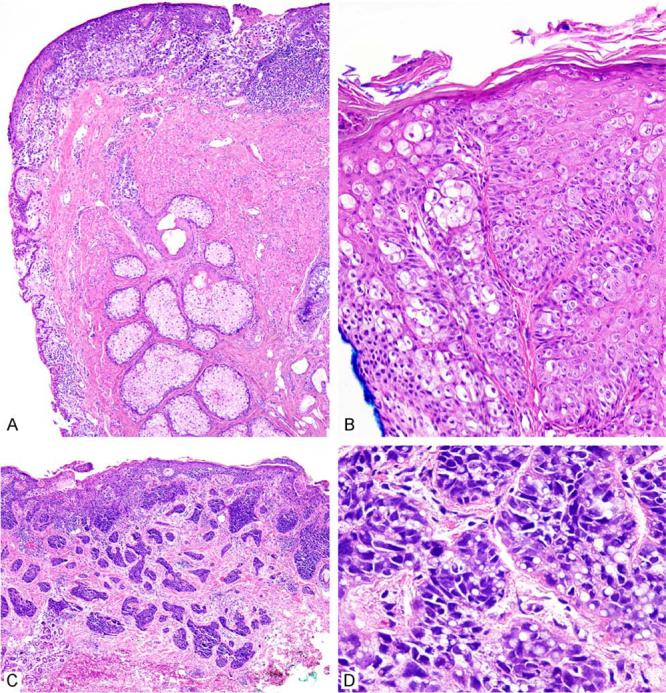
Ocular sebaceous carcinoma with adjacent prominent meibomian (sebaceous) glands and carcinoma primarily involving the cutaneous portion of the eyelid (A). Extensive involvement of the epithelial and adnexal structures can be seen with associated intra-epithelial pagetoid migration (B). Carcinomas are infiltrative and can mimic basal cell carcinoma (C), but closer inspection reveals atypical hyperchromatic nuclei with focal intracytoplasmic lipid vesicles indicating sebaceous differentiation (D).
Sometimes this difficult determination is requested on frozen section where histological artefact can obscure findings and artefactual clearing of cell cytoplasm can be a complicating factor. The ability to employ oil red-O (Fig. 10) or other adipophilic stains on fresh frozen material can compensate for this disadvantage as described below. The tumour–stroma clefting present in permanent sections is primarily an artefact of formalin fixation and is often lacking in basal cell carcinomas assessed by frozen section. Infiltrative basal cell carcinoma can be particularly vexing in this regard. The characteristic fibromucinous stroma associated with basal cell carcinoma can also be difficult to recognize on frozen sections. Noting cells with clear cytoplasm and some distortion of the nucleus can and should raise the possibility of sebaceous carcinoma. When considering a diagnosis of sebaceous adenoma or sebaceoma at this site, it is important to remember that these tumours are much less common in the eyelid than sebaceous carcinoma, and thus the histological features should be classic for these entities to make this diagnosis with confidence.29 Benign sebaceous tumours at the eyelid should be excised completely to allow full histological examination and reliable distinction from carcinoma. A melanoma at this site will often show pigmentation, but this can be absent and focal clear cell differentiation can be noted as well as pagetoid upward migration. Immunohistochemistry may be necessary to resolve these quandaries. Malignancies of the eyelid glands or adnexal structures are rare and usually have ductal differentiation, but rarely can have pagetoid features as well as clear cells. Mucinous cells within the squamous epithelium can be seen and can mimic pagetoid spread; mucicarmine or other mucin stains are helpful in this regard. In the absence of a supporting positive lipophilic stain, one sometimes cannot entirely exclude sebaceous carcinoma on a small frozen section biopsy and it is important to discuss this with the surgeon.
Figure 10.
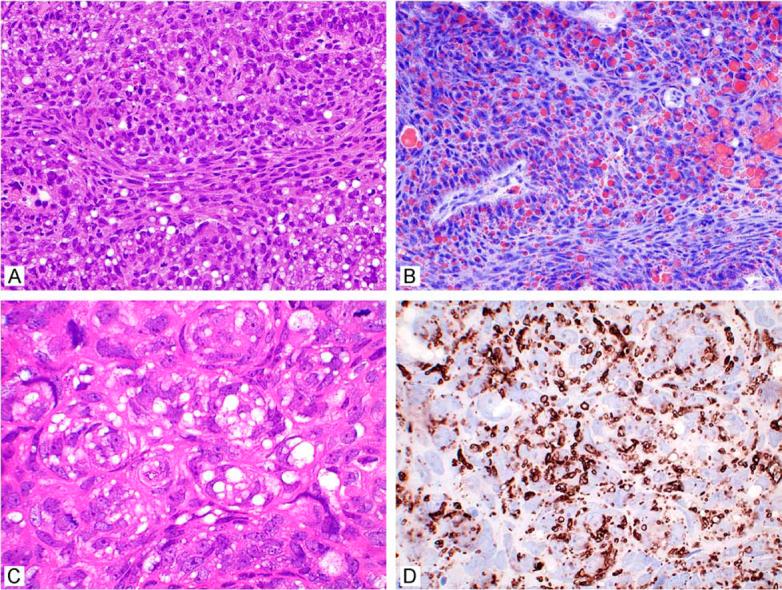
In ocular sebaceous carcinoma, when fresh tissue is available, oil red-O stain can vividly reveal intracytoplasmic lipid when findings are equivocal, particularly on frozen section (A, B). More recently, adipophilin has been demonstrated to detect sebaceous differentiation in permanent sections where lipid has been extracted during tissue processing (C, D) (courtesy of Dr. Fiona Roberts, Glasgow, UK).
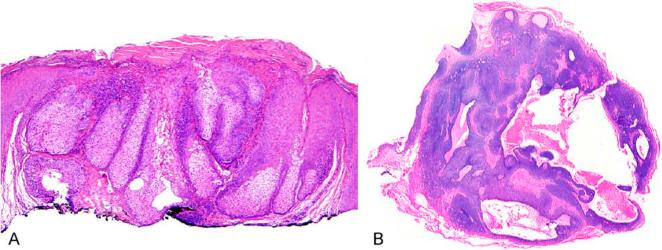
Extra-ocular sebaceous carcinoma
Despite the overwhelming relative size of the entire skin organ compared with eyelids alone, peri-ocular sebaceous carcinoma is considerably more common than non-ocular sebaceous carcinoma, which constitutes one-quarter of cases. Non-ocular sebaceous carcinoma is most frequent in the head and neck region, but up to 25% of cases can be seen elsewhere.30 Rare oral cases are noted and this tumour has been described at other unusual non-cutaneous sites, including salivary-type glands and the uterine cervix, and also rarely in association with ovarian dermoid cysts. It is primarily a disease of older adults in the seventh decade and beyond with perhaps a slight male predominance. It has been suggested that this lesion may have a better prognosis than peri-ocular sebaceous carcinoma, but other studies have indicated that the pattern of metastasis and mortality are similar.30-32 Additional well-defined series of extra-ocular cases are needed to clarify this issue further.
Extra-ocular sebaceous carcinoma presents as an erythaematous papule or nodule that can sometimes ulcerate. Histological examination reveals a proliferation of atypical enlarged basaloid cells, often with minimal or focal mature sebaceous differentiation (Fig. 8). In contrast to adenoma and sebaceoma, sebaceous carcinoma most commonly has an infiltrative border. However, it is important to note that cases can be rather well circumscribed but have extreme cytologic atypia unacceptable in a benign lesion, or have relatively low-grade nuclei with a distinctively infiltrative architecture. Both of these scenarios are best interpreted as carcinoma. In contrast to peri-ocular carcinoma, pagetoid intra-epithelial migration is uncommon. Squamous differentiation can be present. Mitoses may be frequent and include atypical forms; foci of necrosis can be noted. Cases must be distinguished from basal cell carcinoma and perhaps amelanotic melanoma according to the principles noted above for peri-ocular carcinomas. Immunophenotypic characterization of sebaceous carcinoma is discussed in greater detail below.
Figure 8.
Non-ocular sebaceous carcinoma with polypoid appearance, an infiltrative architecture and areas of necrosis (A). Mature sebaceous differentiation can be focal in sebaceous carcinoma (B).
Other sebaceous tumours and conditions
A wide variety of other sebaceous tumours are noted, but these are not known to be associated with TMS and will be only be mentioned briefly. Naevus sebaceus is a congenital lesion that shows a sebaceous component that varies as the individual ages. These lesions are well described to be associated with both benign and malignant tumours arising in their midst, including sebaceous adenomas, sebaceomas and carcinomas (Fig. 9).33,34 Often the lesions arising in the context of naevus sebaceus are difficult to classify precisely.35 Steatocystomas and cutaneous dermoid cysts characteristically show sebaceous differentiation. A lesion termed ‘superficial epithelioma with sebaceous differentiation’ has rarely been described.36,37 Too few cases are described to know whether this lesion is associated with TMS. Sebaceous differentiation has also been described in various follicular tumours, perhaps most prominently sebaceous trichofolliculoma, microcystic adnexal carcinomas, cutaneous mixed tumours and pilar sheath acanthomas. Folliculosebaceous cystic hamartomas show a variable sebaceous component. Basal cell carcinoma with sebaceous differentiation has been discussed above. A rarely described tumour of the hair mantle termed ‘mantleoma’ can show focal sebaceous differentiation.38 Furthermore, it is not unexpected that any follicular tumour, particularly those showing differentiation towards the upper or infundibular portion of the follicle, could show sebaceous differentiation from time to time. None of this group of lesions is known to be associated with TMS and these lesions will not be discussed further.
Figure 9.
Naevus sebaceus (A) with a sebaceous tumour arising within it (B) that shows unusual architecture but is likely to be benign sebaceoma.
Practice Points: Criteria for Malignancy in Sebaceous Neoplasia
Sebaceous tumors showing an infiltrative border or cytologic atypia unacceptable in a benign lesion should be regarded as malignant.
Tumor necrosis in basaloid areas is a strong indicator of malignancy, but should not be confused with areas of holosection in sebaceous adenomas.
Mitoses are not a strict criteria for malignancy as sebaceomas can sometimes show conspicuous mitotic activity. An unusual number of mitoses or specifically atypical mitotic forms are concerning features that must be interpreted within the overall appearance of the tumor.
Pagetoid intraepithelial migration indicates malignancy, but is not commonly encountered in non-ocular cases.
All sebaceous tumors of the eyelid should be regarded as malignant unless they clearly meet criteria for a benign category given that periocular sebaceous carcinoma is much more common than benign sebaceous lesions at this site.
Sebaceous tumors associated with the Torre-Muir Syndrome (TMS) can sometimes show usual cytologic and architectural features that complicate definitive assessment of the tumor as benign or malignant. As sebaceous tumors, benign or malignant, are usually completely excised, this determination is often not critical. However, it is important to remark on the atypical features and inability to strictly classify a lesion as benign so that appropriate clinical follow-up can be initiated.
Special stains and immunohistochemistry
While sebaceous differentiation can usually be assessed histologically by identifying mature sebocytes with eosinophilic bubbly cytoplasm and indented or crenylated nuclei, sometimes these features are focal and not entirely convincing, and additional studies can be useful. In particular, cases of sebaceous carcinoma can show a very limited mature sebaceous component, and while clear to bubbly lightly eosinophillic cytoplasm is noted, the enlarged nuclei are often not indented. This is also sometimes noted in the lower-grade nuclei of sebaceoma. When frozen material is present, an oil red-O stain can vividly demonstrate intracytoplasmic lipid (Fig. 10 A,B). It is important to ensure that the tiny fat globules are present in the cytoplasm as there are often significant lipid collections in the dermis or submucosa that can be misinterpreted. Unfortunately, lipid is extracted during the organic phase of tissue processing, and thus this stain cannot be performed in formalin-fixed paraffin-embedded material. A family of proteins present in the lipid globules and the membranes surrounding them has been described and includes adipophilin, perilipin and TIP 47.39-41 These proteins are crosslinked by formalin and retained in permanent sections. A recent study has elegantly demonstrated the usefulness of adipophilin in detecting sebaceous differentiation (Fig. 10C,D).41 Additional studies are needed to further investigate the sensitivity and specificity of this antibody, but the initial results appear promising. Immunohistochemistry for the androgen receptor can provide evidence of sebaceous differentiation, but the specificity of this finding requires further investigation.42 Sebaceous cells are reactive for epithelial membrane antigen (EMA), but negative for carcinoembryonic antigen (CEA).43,44 EMA can be useful, but it is important to note that squamous or basaloid cells of non-sebaceous lineage express EMA focally and this can cause confusion. The presence of cytokeratin 7 and absence of BerEP4 in the basaloid cells comprising sebaceous lesions is believed to be useful to distinguish sebaceous tumours from basal cell carcinoma (where the opposite profile is usually noted). A panel of BerEP4 and EMA, where sebaceomas are virtually all negative for BerEP4 with strong focal reactivity for EMA while basal cell carcinomas are almost all reactive for BerEP4 and usually negative for EMA, can be of use in this differential.45 However, given the rarity of basal cell carcinoma with sebaceous differentiation, these particular lesions have not been surveyed adequately. Indeed, immunohistochemochemical results themselves can be disappointing and often confusing when applied to cases that are difficult and challenging on histology.
Practice Points: Additional Reagents Useful in the Differential Diagnosis of Sebaceous Tumors
Oil Red-O: Adipophilic special stain; must have fresh frozen material.
Adipophilin: Newly characterized antibody against a protein associated with intracytoplasmic lipid vesicles; works on formalin-fixed, paraffin-embedded tumors; requires additional study of sensitivity and specificity.
Epithelial Membrane Antigen (EMA): Highlights mature sebocytes, but focal expression can be seen in other cutaneous epithelial tumors.
BerEP4: Seen in most basal cell carcinomas, but is not usually present in sebaceomas; more study is needed.
Cytokeratin 7 (CK7): Present in most sebaceous neoplasia, usually absent in basal cell carcinoma.
Use of these antibodies in panels, perhaps including other markers for lesions entering the differential diagnosis in a particular case, is usually more helpful than application of a single immunohistochemical stain.
Torre–Muir syndrome
Torre and Muir independently described cases of what has become known as ‘TMS’ in 1967 and 1968.46,47 TMS is the usually hereditary association of sebaceous neoplasia and internal malignancy.48 While the initial cases involved colorectal carcinoma, multiple malignancies including uterine, renal pelvis, breast and others are now described as part of the spectrum. A range of sebaceous neoplasia is also encountered including adenoma, sebaceoma, what has been referred to as ‘epithelioma’ and carcinoma.49-54 Some of the sebaceous tumours are difficult to classify and a minority of cases may show keratoacanthoma-like architecture or cystic change (Fig. 11).51,52,55,56 Some authorities have found it difficult to be certain whether the sebaceous neoplasms associated with TMS are benign or malignant given their unusual features, but reports of these tumours behaving aggressively in this syndrome are rare. Ocular sebaceous carcinoma can also be associated, but the link is not nearly as strong as with sebaceous tumours at other sites.54,57 A single sebaceous tumour can be a marker of TMS.58,59
Figure 11.
Keratoacanthoma-like (A) or cystic (B) architecture can be seen in Torre–Muir syndrome, but is not an entirely specific or sensitive marker.
Practice Points: Association of Torre-Muir Syndrome (TMS) and Sebaceous Neoplasia
Torre-Muir Syndrome (TMS) is the usually hereditary association of cutaneous sebaceous neoplasia and internal malignancy. Sebaceous neoplasia can be the presenting sign.
Torre-Muir syndrome is a rare subset of the hereditary non-polyposis colorectal carcinoma (HNPCC) syndrome.
Sebaceous tumors in TMS include sebaceous adenoma, sebaceoma, and sebaceous carcinoma. Sebaceous hyperplasia and ectopic sebaceous glands do not appear to be significant markers of the syndrome.
Sebaceous adenoma is believed to be the most specific marker of TMS and these can sometimes show cystic change or keratoacanthoma-like architecture.
Sebaceous carcinoma, particularly periocular, is less frequently associated with TMS unless present outside of the head and neck region.
Multiple sebaceous tumors at any location or the presence of even a single tumor outside of the head and neck region are strong indicators of TMS.
Regardless of age, all patients with sebaceous neoplasia, perhaps excepting periocular sebaceous carcinoma, deserve at least clinical screening for TMS.
More recently, it has become clear that TMS is a subset of the hereditary non-polyposis colorectal carcinoma (HNPCC) syndrome, representing about 1–2% at most of these cases.60,61 The majority of these syndromes are associated with microsatellite instability (MSI) associated with loss of DNA mismatch repair proteins, most commonly MLH1 and MSH2.60,62,63 MSI results when mistakes made by the DNA replicative machinery, primarily in areas of stretches of DNA composed of mono- or dinucleatide repeats (microsatellites), are not detected and repaired, and thus result in accumulation of deletions or insertions.64 These changes can be detected using polymerase chain reaction (PCR)-based assays using a panel recommended by an international expert panel sponsored by the National Cancer Institute.65-67 The most common format is PCR amplification of multiple established microsatellite regions from both tumour and normal tissue with size fractionation of the amplicons. MSI shows increased variation in microsatellite length over that seen in normal tissue (Fig. 13). This test is currently the gold standard for demonstration of MSI. In general, MSI demonstrated at greater than or equal to two of five or six loci, or more than three of 10 loci, defines a tumour as being MSI. MSI can be seen in fewer loci and this has provisionally been termed ‘low MSI’, but this has not turned out to be a good marker for loss of DNA mismatch repair proteins and is of uncertain biological and clinical relevance. While this test has not been validated thoroughly for sebaceous neoplasia, in the limited published reports and the authors' personal experience, it seems to have excellent sensitivity and specificity, with sebaceous neoplasia usually showing instability in at least four of the six loci that are tested routinely.68
Figure 13.
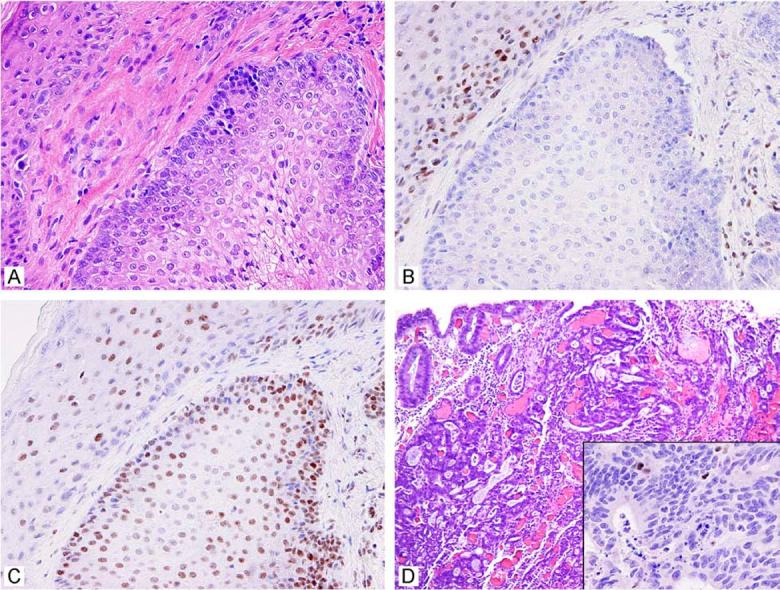
Portion of a sebaceous adenoma (A). Immunohistochemistry for DNA mismatch repair proteins revealed that the cells were deficient for MSH2 (B) while MLH1 (C) was intact. The overlying squamous epithelium provides a positive control. This patient also had a caecal colonic carcinoma deficient for MSH2 (D, inset).
HNPCC and TMS usually result from an inherited defect in one allele of either MLH1 or MSH2, with a second hit to the remaining wild-type allele completing the Knudsen two-hit model for loss-of-function mutations and resulting in MSI.69 A variety of mechanisms from various types of mutation to methylation suppression can provide this second hit. Loss of MSH2 or MLH1 can be detected reliably by demonstrating loss of nuclear reactivity in tumour cells on immunohistochemistry with specificity approaching 100% and sensitivity of >90% in colonic carcinoma.70-73 It should be noted that both colonic carcinomas and endometrial carcinomas also show somatic loss of MLH1 by methylation suppression in 10–15% of sporadic carcinomas. Thus this somatic event occurs at up to 20 times the rate of HNPCC in the population. HNPCC itself shows a roughly equal distribution between loss of MLH1 and MSH2, while TMS shows an excess of MSH2 loss of approximately 10 to one over MLH1 loss.60 Thus sebaceous neoplasia is unique in that mismatch repair deficiency is primarily accounted for by MSH2 loss rather than MLH1 loss in most cases.60,74-76 This also implies that sporadic MSI is uncommon in sebaceous neoplasia. In the authors' series of approximately 100 unselected sebaceous neoplasms, roughly 40% (all adenomas) were mismatch repair protein deficient, while only about 20% of carcinomas were deficient. Carcinomas in the head and neck region showed a rate of deficiency of less than 5%. However, for the relatively uncommon tumours, both benign and malignant, occurring outside of the head and neck region (most common site by far, accounting for 80% of cases), virtually all tumours showed mismatch repair deficiency, making non-head and neck sites especially strong indicators of TMS.77 Since there are no well-described mechanisms for somatic loss of MSH2 and MLH1 loss is so uncommon in sebaceous neoplasia, demonstration of the loss of either of these proteins is a definite indication for clinical screening, and potentially for genetic counselling for the individual and other relatives possibly affected. Guidelines for the clinical evaluation of patients with sebaceous tumours have been reviewed recently.49,62,78
Multiple sebaceous tumours and sebaceous tumours occurring before the age of 50 years are also strong indicators of TMS. Loss of mismatch repair genes in both a sebaceous tumour and an internal malignancy is virtually diagnostic (Fig. 13). In practice, the authors offer screening for all patients with sebaceous neoplasms (except ocular sebaceous carcinoma) by immunohistochemistry for MLH1 and MSH2 deficiency, regardless of patient age, as sebaceous tumours can be late onset and sometimes only a single cutaneous tumour is present in this syndrome.58 The importance of this recognition is that sebaceous lesions can precede or be concurrent with internal malignancy.48 While there are other mismatch repair proteins that can be involved such as MLH6, PMS1, PMS2 and others, the prevalence of these mutations causing HNPCC or TMS is so low that the authors do not use these in routine screening. If MSH2 or MLH1 is lost, it is generally recommended that a patient receive further work-up for TMS. If they are both intact, it is noted that while TMS is less likely, it is not excluded as up to 20% of cases can be intact. If there is still clinical suspicion for TMS at this point, the PCR-based test for MSI can be employed as this will detect MSI caused by loss of proteins other than MSH2 and MLH1. Finally, there are a minority of cases where this syndrome does not appear to be associated with MSI at all, and these cases probably arise from an entirely different genetic mechanism which is currently undescribed. This form of TMS must be defined at this point by strict clinical and geneological criteria, and represents a small minority of cases. These cases with intact mismatch repair proteins that lack MSI warrant further study. The actual prevalence of such cases is not really known as the syndrome itself is rare, definitive genetic testing is not always employed, and the syndrome appears to go unrecognized in some instances.
Immunohistochemistry for MSH2 and MLH1 is also useful in that it indicates the specific gene that is involved and allows targeted mutational screening of that gene. Genetic testing for mutations in both MSH2 and MLH1 is arduous as the genes are relatively large and the mutations include deletions, insertions, point mutations and other aberrations leading to loss of function in virtually all exons of the gene.79 Genetic testing can be worthwhile if individuals desire this information, as it may confirm whether an individual in an affected family cohort is at risk at an early stage so that appropriate screening can be initiated.
Practice Points: Testing for Torre-Muir Syndrome (TMS) / Hereditary Non-Polyposis Colorectal Carcinoma (HNPCC) Syndrome
The initial assessment for TMS is the recognition of the association of sebaceous neoplasia and internal malignancy.
Additional testing can include personal and family medical history, immunohistochemistry for mismatch repair proteins such as MSH2 or MLH1, PCR-based testing for microsatellite instability (MSI), or mutational screening for the MSH2 or MLH1 genes depending on the level of clinical suspicion and wishes of the patient and their family.
TMS syndrome much more commonly involves mutation of MSH2 while HNPCC shows roughly equal prevalence of MLH1 and MSH2.
As both HNPCC syndrome and TMS show identical genetic defects in many cases, affected members in a single family tree can show clinical signs of either syndrome.
Timely diagnosis of TMS provides important information for the individual patient and for potentially affected family members.
As this syndrome is hereditary, early involvement of an experienced clinical genetics counseling group is critical for successful management of patients and their families.
Sebaceous tumour pathogenesis: recent advances
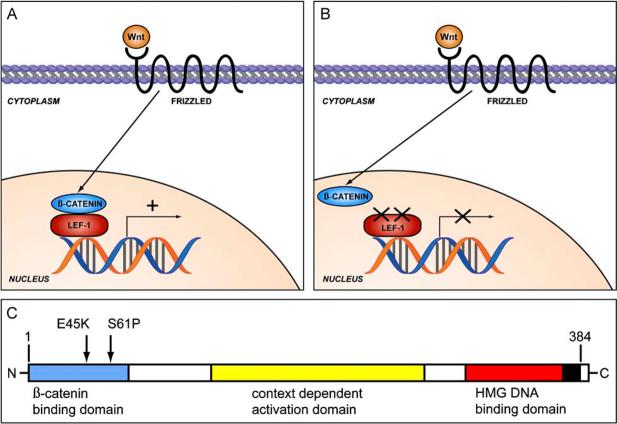
At least three strains of transgenic mice have been described to develop sebaceous tumours: mice deficient in MSH2; mice expressing mutant cutaneous LEF-1 that cannot interact with β-catenin; and mice overexpressing hedgehog protein in skin.80-82 The first group of mice basically replicates the human TMS syndrome. The presence of sebaceous tumours in the latter two groups of mice was a surprising finding. Lymphocyte enhancing factor 1 (Lef-1) is a member of the T-cell factor family of transcription factors, and in its basal state it is bound to DNA and probably acts as a gene suppressor.83,84 When β-catenin translocates to the nucleus under the control of the Wnt pathway, it binds to Lef-1 and allows gene transcription to occur (Fig. 14A,B).85 Lef-1 protein that is mutated or deleted at its N-terminus cannot interact with β-catenin and thus these genes are not activated, although β-catenin may still function in other ways. Based on the findings in mice, the authors have recently demonstrated that a subset of sebaceous adenomas and sebaceomas have dual inactivating mutations in the N-terminal β-catenin binding domain of Lef-1 (Fig. 14C).86 Interestingly, activating mutations in β-catenin are found in both benign and malignant hair tumours, most notably pilomatrical tumours.87,88 The hedgehog pathway is best known for its involvement in Gorlin syndrome and sporadic basal cell carcinomas, but also appears to be involved in the development of sebaceous tumours.89 Interestingly, these same pathways also seem to contribute to normal development of adnexal structures and skin surface epithelium.
Figure 14.
The Wnt pathway leading to nuclear translocation of β-catenin is complex, but this greatly simplified figure shows that nuclear β-catenin is normally able to bind to Lef-1 and allow gene transcription (A), but cannot perform this function when Lef-1 is mutated (B). The dual mutations (E45K and S61P) in the N-terminal β-catenin interaction domain of Lef-1 demonstrated in a subset of sebaceous adenomas and sebaceomas blocks the interaction between these proteins and thus downstream gene transcription (C).
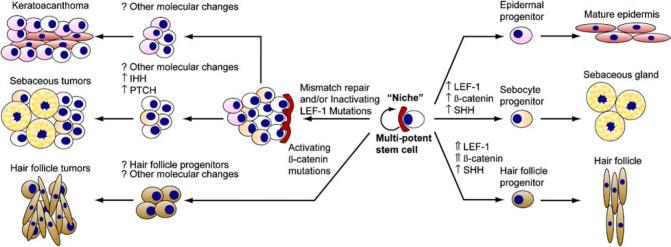
Multipotent stem cells in the skin are well characterized and present in the bulge region of the follicle (near the insertion of the erector pili muscle) and also in the follicular epithelium.84,90,91 As these stem cells persist for the life of the organism, they represent an ideal compartment for the accumulation of the multiple mutations likely required to produce tumours, particularly malignant tumours. The Wnt, β-catenin, Lef-1 and hedgehog proteins and the signalling pathways in which they reside appear to function in normal development of adnexal structure, but when dysregulated may lead to tumourigenesis. There is much current discussion and some preliminary data that solid tumours may contain a subset of stem cells, so-called ‘cancer stem cells’, that comprise no more than a few percent of all tumour cells.92-94 These cells may function similarly to their role in normal tissues in that their asymmetric division replenishes the stem cell and also gives rise to a more committed cell that proceeds down a particular cell lineage pathway. This model provides an attractive explanation for the heterogeneity encountered in many tumours, and prompts an intriguing hypothesis regarding the ability of most cancers to recur despite extensive cytoreduction with chemotherapy. Cancer stem cells may evade the toxicity of traditional chemotherapeutic agents due to their relatively quiescent replicative rate. Cells with some immunohistochemical properties of multipotent cutaneous stem cells, such as keratin 15 reactivity, are present in sebaceous tumours and it is tempting to postulate that stem cells may the source of sebaceous and other cutaneous neoplasia (Fig. 15).95,96 Better understanding of this process may provide exciting insights into tumour biology and perhaps better treatments for our patients.
Figure 15.
Stem cell model for sebaceous tumour development. Cutaneous multipotent stem cells can develop into follicular, interfollicular epidermal or sebaceous structures under a variety of signals (right side). Intense signalling through the Wnt pathway resulting in increased β-catenin and Lef-1 activation leads to a follicular fate, while less intense signalling allows a sebaceous fate and absence of signalling to an interfollicular epidermal fate. Regarding tumourigenesis (left side), activating mutations in β-catenin, presumably acting with Lef-1, leads to hair follicle tumours such as pilomatrixomas. Abrogation of the Lef-1 pathway, likely in combination with other events, leads to sebaceous tumourigenesis and perhaps keratoacanthoma-like lesions as well. The role of stem cells in this process is not well characterized, but this is an attractive model since stem cells persist for the life of the organisms and thus can more readily be the target of the multiple mutations likely required for tumourigenesis. SHH, sonic hedgehog; IHH, indian hedgehog; PTCH, patched.
Figure 12.
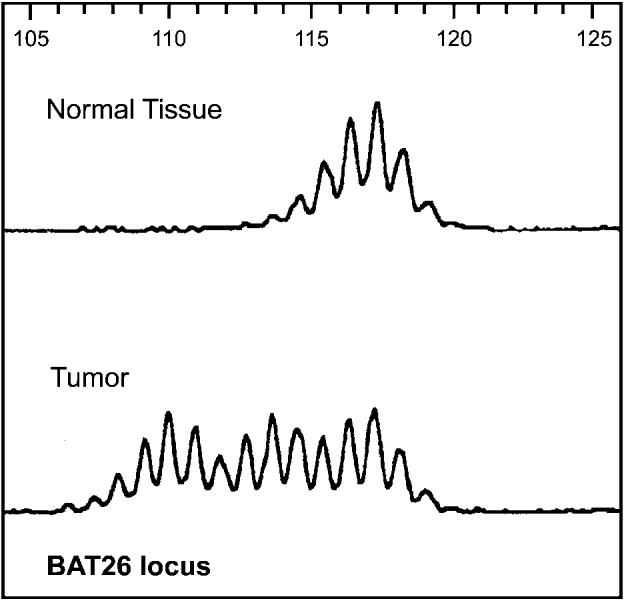
Microsatellite instability in sebaceous tumours can also be demonstrated by a polymerase-chain-reaction-based assay as seen here at the BAT-26 locus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Porter AM. Why do we have apocrine and sebaceous glands? J R Soc Med. 2001;94:236–237. doi: 10.1177/014107680109400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danby FW. Why we have sebaceous glands. J Am Acad Dermatol. 2005;52:1071–1072. doi: 10.1016/j.jaad.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Marks R. Sebaceous gland hyperplasia and senile comedones: a prevalence study in elderly hospitalized patients. Br J Dermatol. 1987;117:231–236. doi: 10.1111/j.1365-2133.1987.tb04121.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis TT, Calilao G, Fretzin D. Sebaceous hyperplasia overlying a dermatofibroma. Am J Dermatopathol. 2006;28:155–157. doi: 10.1097/01.dad.0000175527.96873.fa. [DOI] [PubMed] [Google Scholar]
- 5.Requena L, Roo E, Sanchez Yus E. Plate-like sebaceous hyperplasia overlying dermatofibroma. J Cutan Pathol. 1992;19:253–255. doi: 10.1111/j.1600-0560.1992.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 6.Zaballos P, Ara M, Puig S, Malvehy J. Dermoscopy of sebaceous hyperplasia. Arch Dermatol. 2005;141:808. doi: 10.1001/archderm.141.6.808. [DOI] [PubMed] [Google Scholar]
- 7.Salim A, Reece SM, Smith AG, et al. Sebaceous hyperplasia and skin cancer in patients undergoing renal transplant. J Am Acad Dermatol. 2006;55:878–881. doi: 10.1016/j.jaad.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Pang SM, Chau YP. Cyclosporin-induced sebaceous hyperplasia in renal transplant patients. Ann Acad Med Singapore. 2005;34:391–393. [PubMed] [Google Scholar]
- 9.Bencini PL, Montagnino G, Sala F, De Vecchi A, Crosti C, Tarantino A. Cutaneous lesions in 67 cyclosporin-treated renal transplant recipients. Dermatologica. 1986;172:24–30. doi: 10.1159/000249288. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Jin SY, Shim CS. Esophageal ectopic sebaceous glands. Clin Gastroenterol Hepatol. 2006 doi: 10.1016/j.cgh.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 11.De La Pava S, Pickren JW. Ectopic sebaceous glands in the esophagus. Arch Pathol. 1962;73:397–399. [PubMed] [Google Scholar]
- 12.Rulon DB, Helwig EB. Cutaneous sebaceous neoplasms. Cancer. 1974;33:82–102. doi: 10.1002/1097-0142(197401)33:1<82::aid-cncr2820330115>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Prioleau PG, Santa Cruz DJ. Sebaceous gland neoplasia. J Cutan Pathol. 1984;11:396–414. doi: 10.1111/j.1600-0560.1984.tb00397.x. [DOI] [PubMed] [Google Scholar]
- 14.Epker BN, Henny FA. Intra-oral sebaceous gland adenoma. Cancer. 1971;27:987–1000. doi: 10.1002/1097-0142(197104)27:4<987::aid-cncr2820270434>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Orlian AI, Salman L, Reddi T, Yamane GM, Chaudhry AP. Sebaceous adenoma of the oral mucosa. J Oral Med. 1987;42:38–39. [PubMed] [Google Scholar]
- 16.Troy JL, Ackerman AB. Sebaceoma. A distinctive benign neoplasm of adnexal epithelium differentiating toward sebaceous cells. Am J Dermatopathol. 1984;6:7–13. [PubMed] [Google Scholar]
- 17.Misago N, Mihara I, Ansai S, Narisawa Y. Sebaceoma and related neoplasms with sebaceous differentiation: a clinicopathologic study of 30 cases. Am J Dermatopathol. 2002;24:294–304. doi: 10.1097/00000372-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kazakov DV, Kutzner H, Rutten A, Mukensnabl P, Michal M. Carcinoid-like pattern in sebaceous neoplasms: another distinctive, previously unrecognized pattern in extra-ocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195–203. doi: 10.1097/01.dad.0000157464.95204.fc. [DOI] [PubMed] [Google Scholar]
- 19.Misago N, Narisawa Y. Rippled-pattern sebaceoma. Am J Dermatopathol. 2001;23:437–443. doi: 10.1097/00000372-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kiyohara T, Kumakiri M, Kuwahara H, Saitoh A, Ansai S. Rippled-pattern sebaceoma: a report of a lesion on the back with a review of the literature. Am J Dermatopathol. 2006;28:446–448. doi: 10.1097/01.dad.0000211504.14371.b2. [DOI] [PubMed] [Google Scholar]
- 21.Misago N, Suse T, Uemura T, Narisawa Y. Basal cell carcinoma with sebaceous differentiation. Am J Dermatopathol. 2004;26:298–303. doi: 10.1097/00000372-200408000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Dinneen AM, Mehregan DR. Sebaceous epithelioma: a review of twenty-one cases. J Am Acad Dermatol. 1996;34:47–50. doi: 10.1016/s0190-9622(96)90833-6. [DOI] [PubMed] [Google Scholar]
- 23.Shields JA, Demirci H, Marr BP, Eagle RC, Jr, Shields CL. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004;111:2151–2157. doi: 10.1016/j.ophtha.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Callahan EF, Appert DL, Roenigk RK, Bartley GB. Sebaceous carcinoma of the eyelid: a review of 14 cases. Dermatol Surg. 2004;30:1164–1168. doi: 10.1111/j.1524-4725.2004.30348.x. [DOI] [PubMed] [Google Scholar]
- 25.Rao NA, Hidayat AA, McLean IW, Zimmerman LE. Sebaceous carcinomas of the ocular adnexa: a clinicopathologic study of 104 cases, with five-year follow-up data. Hum Pathol. 1982;13:113–122. doi: 10.1016/s0046-8177(82)80115-9. [DOI] [PubMed] [Google Scholar]
- 26.Doxanas MT, Green WR. Sebaceous gland carcinoma. Review of 40 cases. Arch Ophthalmol. 1984;102:245–249. doi: 10.1001/archopht.1984.01040030195025. [DOI] [PubMed] [Google Scholar]
- 27.Muqit MM, Roberts F, Lee WR, Kemp E. Improved survival rates in sebaceous carcinoma of the eyelid. Eye. 2004;18:49–53. doi: 10.1038/sj.eye.6700523. [DOI] [PubMed] [Google Scholar]
- 28.Marback EF, Costa AL, Nossa LM, Marback RL, Rao NA. Eyelid skin adenoid cystic carcinoma: a clinicopathological study of one case simulating sebaceous gland carcinoma. Br J Ophthalmol. 2003;87:118–119. doi: 10.1136/bjo.87.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AD, Mudhar HS, Bhola R, Rundle PA, Rennie IG. Sebaceous adenoma of the eyelid in Muir–Torre syndrome. Arch Ophthalmol. 2005;123:562–565. doi: 10.1001/archopht.123.4.562. [DOI] [PubMed] [Google Scholar]
- 30.Wick MR, Goellner JR, Wolfe JT, 3rd, Su WP. Adnexal carcinomas of the skin. II. Extraocular sebaceous carcinomas. Cancer. 1985;56:1163–1172. doi: 10.1002/1097-0142(19850901)56:5<1163::aid-cncr2820560533>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Moreno C, Jacyk WK, Judd MJ, Requena L. Highly aggressive extraocular sebaceous carcinoma. Am J Dermatopathol. 2001;23:450–455. doi: 10.1097/00000372-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Reina RS, Parry E. Aggressive extraocular sebaceous carcinoma in a 52-year-old man. Dermatol Surg. 2006;32:1283–1286. doi: 10.1111/j.1524-4725.2006.32292.x. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda K, Doi T, Kosaka H, Tasaki N, Yoshioka H, Kakibuchi M. Sebaceous carcinoma arising in naevus sebaceus. J Dermatol. 2005;32:641–644. doi: 10.1111/j.1346-8138.2005.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 34.Miller CJ, Ioffreda MD, Billingsley EM. Sebaceous carcinoma, basal cell carcinoma, trichoadenoma, trichoblastoma, and syringocystadenoma papilliferum arising within a naevus sebaceus. Dermatol Surg. 2004;30:1546–1549. doi: 10.1111/j.1524-4725.2004.30552.x. [DOI] [PubMed] [Google Scholar]
- 35.Alessi E, Wong SN, Advani HH, Ackerman AB. Naevus sebaceus is associated with unusual neoplasms. An atlas. Am J Dermatopathol. 1988;10:116–127. doi: 10.1097/00000372-198804000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Friedman KJ, Boudreau S, Farmer ER. Superficial epithelioma with sebaceous differentiation. J Cutan Pathol. 1987;14:193–197. doi: 10.1111/j.1600-0560.1987.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 37.Rothko K, Farmer ER, Zeligman I. Superficial epithelioma with sebaceous differentiation. Arch Dermatol. 1980;116:329–331. [PubMed] [Google Scholar]
- 38.Steffen C. Mantleoma. A benign neoplasm with mantle differentiation. Am J Dermatopathol. 1993;15:306–310. [PubMed] [Google Scholar]
- 39.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–19155. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 40.Robenek H, Robenek MJ, Troyer D. PAT family proteins pervade lipid droplet cores. J Lipid Res. 2005;46:1331–1338. doi: 10.1194/jlr.M400323-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Muthusamy K, Halbert G, Roberts F. Immunohistochemical staining for adipophilin, perilipin and TIP47. J Clin Pathol. 2006;59:1166–1170. doi: 10.1136/jcp.2005.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426–431. doi: 10.1097/00000372-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Johnson JS, Lee JA, Cotton DW, Lee WR, Parsons MA. Dimorphic immunohistochemical staining in ocular sebaceous neoplasms: a useful diagnostic aid. Eye. 1999;13:104–108. doi: 10.1038/eye.1999.19. [DOI] [PubMed] [Google Scholar]
- 44.Ansai S, Hashimoto H, Aoki T, Hozumi Y, Aso K. A histochemical and immunohistochemical study of extra-ocular sebaceous carcinoma. Histopathology. 1993;22:127–133. doi: 10.1111/j.1365-2559.1993.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 45.Fan, Carr, Lazar, Calonje, et al. in press.
- 46.Torre D. Multiple sebaceous tumours. Arch Dermatol. 1968;98:549–551. doi: 10.1001/archderm.98.5.549. [DOI] [PubMed] [Google Scholar]
- 47.Muir EG, Bell AJ, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54:191–195. doi: 10.1002/bjs.1800540309. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz RA, Torre DP. The Muir–Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90–104. doi: 10.1016/0190-9622(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 49.Lynch HT, Fusaro RM, Lynch PM. Sebaceous skin lesions as clues to hereditary non-polyposis colorectal cancer. J Invest Dermatol. 2006;126:2158–2159. doi: 10.1038/sj.jid.5700534. [DOI] [PubMed] [Google Scholar]
- 50.Tsalis K, Blouhos K, Vasiliadis K, Tsachalis T, Angelopoulos S, Betsis D. Sebaceous gland tumours and internal malignancy in the context of Muir–Torre syndrome. A case report and review of the literature. World J Surg Oncol. 2006;4:8. doi: 10.1186/1477-7819-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbott JJ, Hernandez-Rios P, Amirkhan RH, Hoang MP. Cystic sebaceous neoplasms in Muir–Torre syndrome. Arch Pathol Lab Med. 2003;127:614–617. doi: 10.5858/2003-127-0614-CSNIMS. [DOI] [PubMed] [Google Scholar]
- 52.Misago N, Narisawa Y. Sebaceous neoplasms in Muir–Torre syndrome. Am J Dermatopathol. 2000;22:155–161. doi: 10.1097/00000372-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Burgdorf WH, Pitha J, Fahmy A. Muir–Torre syndrome. Histologic spectrum of sebaceous proliferations. Am J Dermatopathol. 1986;8:202–208. doi: 10.1097/00000372-198606000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Cohen PR. Sebaceous carcinomas of the ocular adnexa and the Muir–Torre syndrome. J Am Acad Dermatol. 1992;27:279–280. doi: 10.1016/s0190-9622(08)80752-9. [DOI] [PubMed] [Google Scholar]
- 55.Rutten A, Burgdorf W, Hugel H, et al. Cystic sebaceous tumours as marker lesions for the Muir–Torre syndrome: a histopathologic and molecular genetic study. Am J Dermatopathol. 1999;21:405–413. doi: 10.1097/00000372-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Halling KC, Honchel R, Pittelkow MR, Thibodeau SN. Microsatellite instability in keratoacanthoma. Cancer. 1995;76:1765–1771. doi: 10.1002/1097-0142(19951115)76:10<1765::aid-cncr2820761013>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Ponti G, Ponz de Leon M, Losi L, et al. Different phenotypes in Muir–Torre syndrome: clinical and biomolecular characterization in two Italian families. Br J Dermatol. 2005;152:1335–1338. doi: 10.1111/j.1365-2133.2005.06506.x. [DOI] [PubMed] [Google Scholar]
- 58.Rothenberg J, Lambert WC, Vail JT, Jr., Nemlick AS, Schwartz RA. The Muir–Torre (Torre's) syndrome: the significance of a solitary sebaceous tumour. J Am Acad Dermatol. 1990;23:638–640. doi: 10.1016/s0190-9622(08)81072-9. [DOI] [PubMed] [Google Scholar]
- 59.Donati P. Solitary sebaceoma in Muir–Torre syndrome. Int J Dermatol. 1996;35:601–602. doi: 10.1111/j.1365-4362.1996.tb03671.x. [DOI] [PubMed] [Google Scholar]
- 60.Mangold E, Pagenstecher C, Leister M, et al. A genotype-phenotype correlation in HNPCC: strong predominance of msh2 mutations in 41 patients with Muir–Torre syndrome. J Med Genet. 2004;41:567–572. doi: 10.1136/jmg.2003.012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barana D, van der Klift H, Wijnen J, et al. Spectrum of genetic alterations in Muir–Torre syndrome is the same as in HNPCC. Am J Med Genet A. 2004;125:318–319. doi: 10.1002/ajmg.a.20523. [DOI] [PubMed] [Google Scholar]
- 62.Ponti G, Losi L, Pedroni M, et al. Value of MLH1 and MSH2 mutations in the appearance of Muir–Torre syndrome phenotype in HNPCC patients presenting sebaceous gland tumours or keratoacanthomas. J Invest Dermatol. 2006;126:2302–2307. doi: 10.1038/sj.jid.5700475. [DOI] [PubMed] [Google Scholar]
- 63.Ollila S, Fitzpatrick R, Sarantaus L, et al. The importance of functional testing in the genetic assessment of Muir–Torre syndrome, a clinical subphenotype of HNPCC. Int J Oncol. 2006;28:149–153. [PubMed] [Google Scholar]
- 64.Abdel-Rahman WM, Peltomaki P. Molecular basis and diagnostics of hereditary colorectal cancers. Ann Med. 2004;36:379–388. doi: 10.1080/07853890410018222. [DOI] [PubMed] [Google Scholar]
- 65.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 67.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 68.Peris K, Onorati MT, Keller G, et al. Widespread microsatellite instability in sebaceous tumours of patients with the Muir–Torre syndrome. Br J Dermatol. 1997;137:356–360. [PubMed] [Google Scholar]
- 69.Kruse R, Rutten A, Hosseiny-Malayeri HR, et al. ‘Second hit’ in sebaceous tumours from Muir–Torre patients with germline mutations in MSH2: allele loss is not the preferred mode of inactivation. J Invest Dermatol. 2001;116:463–465. doi: 10.1046/j.1523-1747.2001.01265.x. [DOI] [PubMed] [Google Scholar]
- 70.Marcus VA, Madlensky L, Gryfe R, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumours. Am J Surg Pathol. 1999;23:1248–1255. doi: 10.1097/00000478-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumours. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 72.Casey G, Lindor NM, Papadopoulos N, et al. Conversion analysis for mutation detection in MLH1 and MSH2 in patients with colorectal cancer. JAMA. 2005;293:799–809. doi: 10.1001/jama.293.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazar AJ, Ribic C, Zubovits J, Moore M, Gallinger S, Redston R. Immunohistochemistry for deficiency of mismatch repair proteins MLH1, MSH2 and MSH6 identifies colorectal carcinoma with high-frequency microsatellite instability (USCAP Abstract) Mod Pathol. 2002;15(Suppl 1):135A. [Google Scholar]
- 74.Mathiak M, Rutten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumours from patients with Muir–Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338–343. doi: 10.1097/00000478-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Liang SB, Furihata M, Takeuchi T, Sonobe H, Ohtsuki Y. Reduced human mismatch repair protein expression in the development of precancerous skin lesions to squamous cell carcinoma. Virchows Arch. 2001;439:622–627. doi: 10.1007/s004280100445. [DOI] [PubMed] [Google Scholar]
- 76.Popnikolov NK, Gatalica Z, Colome-Grimmer MI, Sanchez RL. Loss of mismatch repair proteins in sebaceous gland tumours. J Cutan Pathol. 2003;30:178–184. doi: 10.1034/j.1600-0560.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 77.Lazar AJ, Redston M, Grayson W, Calonje E. Analysis of sebaceous hyperplasia, adenoma and carcinoma by immunohistochemistry for deficiency of DNA mismatch repair proteins MLH1, MSH2 and MSH6 (USCAP Abstract) Mod Pathol. 2004;17(Suppl 1):94A. [Google Scholar]
- 78.Ponti G, Losi L, Di Gregorio C, et al. Identification of Muir–Torre syndrome among patients with sebaceous tumours and keratoacanthomas: role of clinical features, microsatellite instability, and immunohistochemistry. Cancer. 2005;103:1018–1025. doi: 10.1002/cncr.20873. [DOI] [PubMed] [Google Scholar]
- 79.Papadopoulos N, Lindblom A. Molecular basis of HNPCC: mutations of MMR genes. Hum Mutat. 1997;10:89–99. doi: 10.1002/(SICI)1098-1004(1997)10:2<89::AID-HUMU1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 80.Reitmair AH, Redston M, Cai JC, et al. Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice. Cancer Res. 1996;56:3842–3849. [PubMed] [Google Scholar]
- 81.Reitmair AH, Schmits R, Ewel A, et al. MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet. 1995;11:64–70. doi: 10.1038/ng0995-64. [DOI] [PubMed] [Google Scholar]
- 82.Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 84.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeda H, Lyle S, Lazar AJ, Zouboulis CC, Smyth I, Watt FM. Human sebaceous tumours harbor inactivating mutations in LEF1. Nat Med. 2006;12:395–397. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- 87.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 88.Lazar AJ, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148–157. doi: 10.1111/j.0303-6987.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 89.Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J Investig Dermatol Symp Proc. 1999;4:296–301. doi: 10.1038/sj.jidsp.5640233. [DOI] [PubMed] [Google Scholar]
- 92.Burkert J, Wright NA, Alison MR. Stem cells and cancer: an intimate relationship. J Pathol. 2006;209:287–297. doi: 10.1002/path.2016. [DOI] [PubMed] [Google Scholar]
- 93.Finlan LE, Hupp TR. Epidermal stem cells and cancer stem cells: insights into cancer and potential therapeutic strategies. Eur J Cancer. 2006;42:1283–1292. doi: 10.1016/j.ejca.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 94.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–457. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 95.Misago N, Narisawa Y. Cytokeratin 15 expression in neoplasms with sebaceous differentiation. J Cutan Pathol. 2006;33:634–641. doi: 10.1111/j.1600-0560.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 96.Bieniek R, Lazar AJ, Photopoulos C, Lyle S. Sebaceous tumours contain a subpopulation of cells expressing the keratin 15 stem cell marker. Br J Dermatol. 2007 doi: 10.1111/j.1365-2133.2006.07623.x. in press. [DOI] [PubMed] [Google Scholar]