Abstract
Clinicians treating human immunodeficiency virus (HIV)-infected patients with substance use disorders often face the challenge of managing patients' acute or chronic pain conditions while keeping in mind the potential dangers of prescription opiate dependence. In this clinical review, we critically appraise the existing data concerning barriers to appropriate treatment of pain among HIV-infected patients with substance use disorders. We then analyze published studies concerning the choice of pharmacological pain control regimens for acute and chronic pain conditions in HIV-infected patients, keeping in mind HIV-specific issues related to drug interactions and substance use disorders. We summarize this information in the form of flowcharts for physicians approaching HIV-infected patients who present with complaints of pain, providing evidence-based guidance for the structuring of pain management services and for addressing aberrant drug-taking behaviors.
Keywords: HIV/AIDS, Injection drug users, Substance use, Chronic pain, Opiates, Clinical care
1. Introduction
The twin problems of pain and drug dependence affect overlapping populations; as many as 5 to 7 million Americans are believed to experience acute or chronic pain from a variety of causes while having a history of drug dependence (Rosenblum et al., 2003). Because illicit drug use is a risk factor for the acquisition of human immunodeficiency virus (HIV), a significant proportion of HIV-infected patients with pain syndromes are likely to have a comorbid substance use disorder. This article reviews the current evidence concerning the clinical management of pain for HIV-infected patients with substance use disorders. Our definition of pain includes nociceptive and neuropathic pain as well as acute and chronic pain. Our use of the term “drug dependence” refers both to histories confirmed by patients and dependence observed by clinicians but denied by patients. We have narrowed our review to literature related to pain management in an ambulatory HIV care setting, as inpatient pain control and end-of-life pain control among HIV-infected patients have been discussed extensively elsewhere (Frich & Borgbjerg, 2000).
2. Methodology
A MEDLINE database search was conducted using the keywords “HIV,” “pain,” “acute pain,” “chronic pain,” “opiates,” “dependence,” and “substance abuse” to generate the set of data to be evaluated in this article. A list of 2,083 articles was constructed using all combinations of these keywords; of these articles, 1,941 were immediately excluded by two of the authors (S.B. and R.D.B.) for having no relevance to the subject at hand. Of the 142 remaining references, 54 failed to comment on aspects of pain management for HIV-infected patients, mentioning HIV only in passing and without accompanying data or other potentially useful clinical information. The remaining 88 references formed the basis for this review. Of these, 22 described prospective randomized controlled trials, 32 described retrospective case–control studies, 13 presented case reports, and the remaining involved guidelines from authoritative bodies (n = 5) or prior reviews of relevant literature (n = 16).
Using the experimental studies as a backbone for our review, we organized our article into six major sections for which data were available: barriers to treating pain, initial regimens for treatment, acute pain management, chronic pain management, structuring the delivery of pain management services, and addressing aberrant patient behavior.
3. Barriers to treatment
Self-reports of pain among HIV-infected patients in clinical care settings range from 28% to 97%, varying with the method of assessing pain (verbal report vs. pain scales) and with the setting (clinics vs. hospice facilities; Frich & Borgbjerg, 2000; Gorman & Newshan, 1998; Kimball & McCormick, 1996; Vogl et al., 1999). Two studies have compared self-reports of untreated or undertreated pain in the HIV-infected population with the conditions and circumstances surrounding the pain (Kirsh, Whitcomb, Donaghy, & Passik, 2002; Larue, Fontaine, & Colleau, 1997); these studies observed greater rates of self-reported untreated or undertreated pain among HIV-infected patients (80%) than among patients with cancer (42%) who experience conditions that were judged by the study authors to be equally painful (Cleeland et al., 1994). Among HIV-infected patients, women, less-educated patients, and patients with histories of injection drug use (IDU) have been reported to be more likely than others to receive less pain treatment services than other patients who present with equivalent conditions (Kirsh et al., 2002; Larue et al., 1997).
A few studies have focused exclusively on pain management among patients with a history of IDU. In one study that involved 516 ambulatory HIV-infected patients, those with IDU histories were more likely to receive less analgesic medications from their physicians than other patients with the equivalent conditions. These patients reported less symptomatic relief and more psychiatric distress than did other patients. Reports of pretreatment pain intensity and functional impairment were equivalent between those confirmed to be misusing or abusing drugs and those who were not, indicating that patients with histories of drug use may have been undertreated. Patients' pain reports were compared with their disease status to further evaluate this possibility, and this analysis revealed that patients with an abuse history were, according to the authors of the study, unlikely to be exaggerating their reports of pain in comparison to other patients (Breitbart et al., 1997). This conclusion has been made from other comparisons of HIV-infected patients with and without IDU histories (Gorman & Newshan, 1998). It has subsequently been argued that an IDU history is a complication of, not a contraindication to, comprehensive pain management services that include pharmacological therapies (Gourlay, Heit, & Almahrezi, 2005).
Regardless of the literature concerning undertreatment, there are numerous challenges to the provider when encountering patients with a history of either active or former substance misuse (see Table 1). Because pain is inherently subjective, clinicians are rarely assured of its existence until its intensity is so severe as to produce physical signs (Gorman & Newshan, 1998). As a result, clinicians may believe that a patient is drug seeking or that pain management with a narcotic may precipitate a relapse into drug abuse. Current evidence suggests that physicians are, however, poor at predicting pain medication misuse and abuse among patients (Gourlay et al., 2005).
Table 1.
Common reasons cited for inadequate treatment of pain among patients with recognized drug dependence
| Physician concerns |
| Drug seeking by patients |
| Diversion |
| Relapse to substance abuse |
| Legal repercussions |
| Inadequate skills |
| Unavailability of specialists |
| Patient concerns |
| Relapse to substance abuse |
| Anticipated physical discomforts (thinking that medicines will be injected; fearing side effects) |
| Concerns about insurance coverage/cost |
| Fearing accusations of malingering |
| Perceived “weakness” in taking medications for pain |
Note. Based on information from Walker et al. (1992) and Wood et al. (1990).
Patient perspectives have also been reviewed in the literature. HIV-infected patients with and without a history of substance abuse have been reported to experience worries when the topic of pain medication is raised. Surveys of patients who receive care in the ambulatory setting reveal that their most frequently endorsed concerns relate to the addictive potential of medications and perceived physical discomforts associated with taking them (Breitbart et al., 1998; Frich & Borgbjerg, 2000; Passik et al., 2000).
In survey-based studies, many providers report sharing these concerns and express the belief that they are inadequately trained in pain management (Breitbart, Kaim, & Rosenfeld, 1999; Miller, Sheppard, Colenda, & Magen, 2001). In a survey of 492 HIV care providers attending continuing education symposia around the United States, the most endorsed barriers to pain management were those related to lack of knowledge or lack of access to pain management specialists, followed by concerns of abuse and addiction among patients. Interestingly, the most experienced group of providers were the least likely to cite abuse as a barrier, whereas those with the least experience were most likely to cite substance abuse, diversion, or state regulatory concerns as barriers to pain management (Prater, Zylstra, & Miller, 2002).
There has been recent clarification about the regulatory barriers concerning the prescription of pain medication. As reviewed recently, state regulations concerning the prescription of opiates do not apply to prescriptions for pain relief, as per the Psychotropic Substance Act of 1978. The more stringent Uniform Controlled Substances Act of 1970 also does not regulate the use of narcotics for pain relief (Dews & Mekhail, 2004; Jaffe & O'Keeffe, 2003). Based upon our review, it appears that no current legal barriers prevent the prescription of Schedule II, III, or IV drugs for chronic pain related to HIV/AIDS (Dallocchio, Buffa, Mazzarello, & Chiroli, 2000; Morello, Leckband, Stoner, Moorhouse, & Sahagian, 1999; Saarto & Wiffen, 2005).
4. Choosing an initial regimen
Some authors have suggested that clinicians should begin encounters with a patient in pain with a verbal acknowledgement of the patient's report of distress (Koo, 2003; Prater et al., 2002). These authors argue that the acknowledgement of pain may be a starting point to discuss its management, particularly among patients with a history of substance abuse whose pain may have not been acknowledged by others. Case reports and surveys have also suggested a format for history taking among patients with a history of substance abuse who also have a history of HIV or HIV-related risk factors (see Table 2; Koo, 2003; Prater et al., 2002; Streltzer, 2001). Although supported through clinical experience rather than empirical evidence, the approaches outlined are intended to place the patient at ease while gleaning important information about past drug use behaviors that the clinician may wish to take into account. The related examination of the painful site may or may not lead the clinician to an initial diagnosis, but it has been suggested that distinguishing neuropathic and non-neuropathic pain even in the absence of specific diagnoses may provide an initial direction for therapy (Passik & Kirsh, 2004a). For example, amitriptyline and gabapentin have been demonstrated to provide some relief for patients with neuropathic pain but have not been reported to relieve nonneuropathic pain syndromes among patients with a history of substance abuse (Passik & Kirsh, 2004a).
Table 2.
Eliciting a pain history
| Recommendations to consider: |
| Inquire openly about a patient's past personal and family history of substance use |
| Include use of alcohol and over-the-counter preparations |
| Solicit every history to eliminate profiling any patient or missing patients who do not fit a “drug-abusing profile” |
| Complaints of chronic pelvic and rectal pain that may disguise a patient's history of sexual assault (Streltzer, 2001) |
| Suggested questions to put the patient at ease, based on Koo (2003): |
| Instead of asking “how many pain pills do you use?”, ask “how many pills does it take to relieve your pain?” |
| Instead of asking “how often do you take them?”, ask “how long do they really work for your pain?” |
| Instead of asking “do you use other drugs?”, ask “do you wake with pain; do you need more medication to get back to sleep?” |
| Examination, based on Prater et al. (2002): |
| Direct observation of the painful site |
| Assessment of the patient's range of motion at the site if relevant |
| During the examination, ask patients about their goals for therapy (providing a realistic view of the extent of pain and impairment) |
Existing data suggest that nonopioid and behavioral interventions ranging from relaxation therapy to antidepressant medications may be effective for mild pain complaints among HIV-infected patients (Lebovits et al., 1989; World Health Organization [WHO], 1986). For pain management of these mild pain complaints, the WHO stepladder of pain medications (see Table 3) is also one currently recommended approach adopted by many expert bodies (WHO, 1986). This WHO stepladder involves the introduction of opiates after an initial attempt at nonopiate therapy, such as nonsteroidal drugs or acetaminophen (see Figs. 1 and 2). The stepladder also includes adjuvant therapies (Table 3), which, the WHO argues, enhance the pain-relieving effects of opiates, possibly allowing clinicians to use lower opiate doses.
Table 3.
Stepwise approach to common pain medications
| Pain level | Commonly used medications | Typical oral dosagesa | Equivalent dosages for oral opiates |
|---|---|---|---|
| Step 1: Mild pain | Acetaminophen | 325–1,000 mg every 4–6 h | |
| (nonnarcotic medications) | NSAIDS | ||
| Cox-2 inhibitors | Varies by drug | ||
| Amitriptylineb | 75–150 mg/day | ||
| Gabapentinb | 3,600 mg/day | ||
| Step 2: Moderate pain | Codeine | 15–60 mg every 4–6 h | 200 mg every 4 h |
| (weak opioids used in addition to those in Step 1) | Hydrocodone | 7.5 mg every 4–6 h | |
| Step 3: Severe pain | Morphine | 10–30 mg every 3–4 h | 30 mg every 4 h |
| (strong opioids used in addition to those in Step 1) | Oxycodone | 5–30 mg every 4 h | 15–20 mg every 4 h |
| Hydromorphone | 2–8 mg every 3–4 h | 8 mg every 4 h | |
| (start 2–4 mg every 3–4 h for opiate-naïve patients) | |||
| Methadone | 2.5–10 mg every 3–6 h; 80–160 mg for tolerant patients | 20 mg every 6–12 hc | |
| Extended-release oxycodone | 15–30 mg every 8–12 h | 45–60 mg every 12 h | |
| Extended-release morphine | 15–30 mg every 8–12 h; 200 mg for opiate-tolerant patients | 90 mg every 12 h | |
| Adjuvant medications | Corticosteroids: | ||
| (can be added to any step of the ladder)d | Dexamethasone | 16–96 mg qd | |
| Prednisone | 40–100 mg qd | ||
| Anticonvulsants: | |||
| Carbamazepine | 200–1,600 mg qd | ||
| Phenytoin | 300–500 mg qd | ||
| Antidepressants: | |||
| Amitriptyline | 25–150 mg qd | ||
| Doxepin | 25–150 mg qd | ||
| Imipramine | 20–150 mg qd | ||
| Trazodone | 75–225 mg qd | ||
| Neuroleptics: | |||
| Methotrimeprazine | 40–80 mg qd | ||
| Antihistamines: | |||
| Hydroxyzine | 300–450 mg qd | ||
| Local anesthetics/antiarrhythmics: | |||
| Lidocaine | 5 mg/kg | ||
| Mexiletine | 450–600 mg qd | ||
| Tocainide | 20 mg /kg | ||
| Psychostimulants: | |||
| Dextroamphetamine | 5–10 mg qd | ||
| Methylphenidate | 10–15 mg qd |
Note. Based on information from Ballantyne (2002), Ballantyne and Mao (2003), HIVpositive.com (2005), Prater et al. (2002), Streltzer (2001), and WHO (1986). Note that all listed medications are metabolized by the cytochrome P450 system, thus presenting potential drug interactions with antiretroviral medications.
Typical dosages are achieved after slow titration as discussed in the text.
Both amitriptyline and gabapentin should be used for neuropathic pain and should be slowly titrated to a therapeutic dose to allow patients to slowly grow accustomed to the side effects of these medications.
Use a ratio of 1 mg methadone: 4 mg morphine up to 90 mg methadone, then use a 1:8 ratio for 90–300 mg methadone and a 1:12 ratio for >300 mg methadone.
See text concerning drug interactions with HIV-related medications.
Fig. 1.
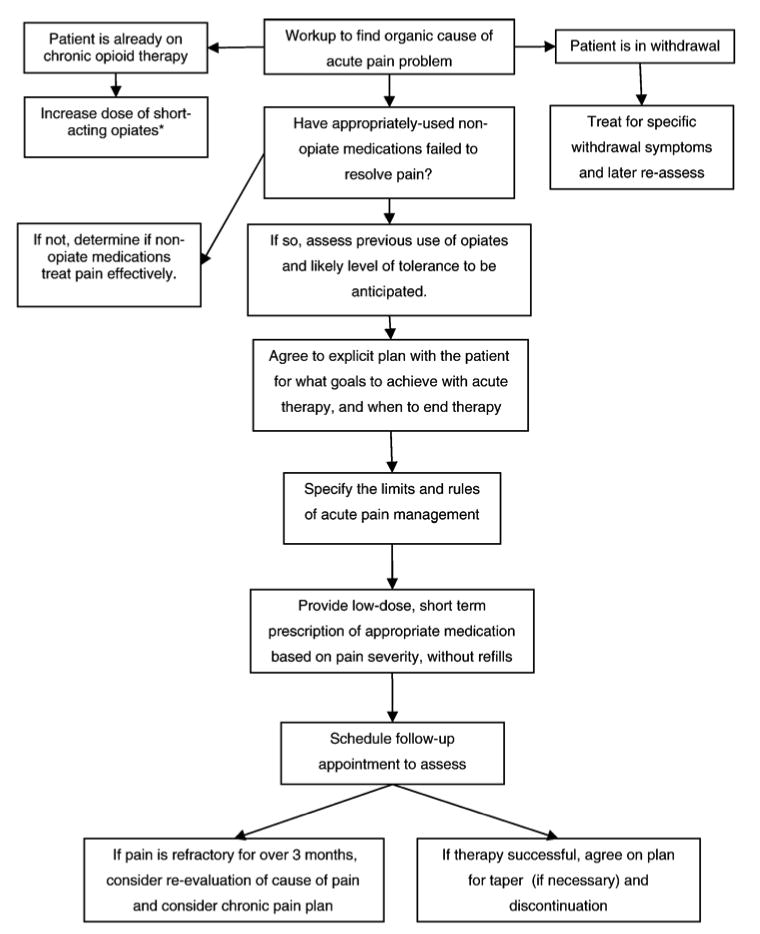
Stepwise approach for acute pain. Based on information from Ballantyne (2002), Ballantyne and Mao (2003), and Gourlay et al. (2005).
*See text; patients on chronic opiate substitution therapy using buprenorphine should either change the frequency of dosing or change to a full-opiate agonist during acute pain treatment; then, patients are reinduced onto buprenorphine after adequate acute pain treatment.
Fig. 2.
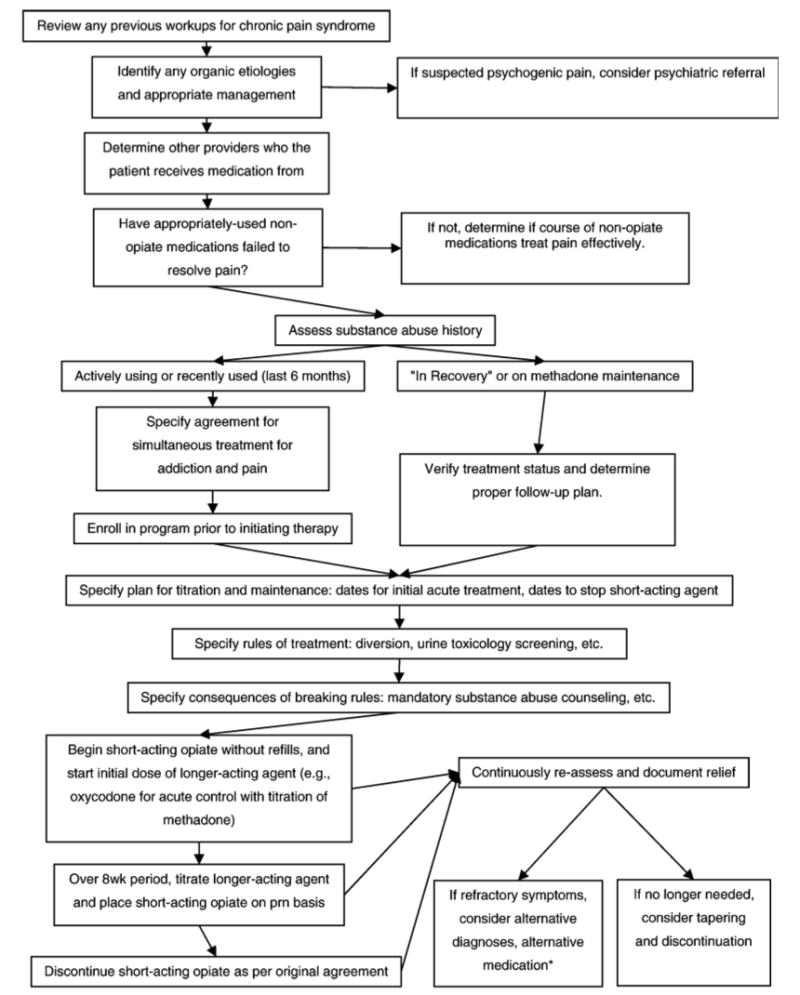
Stepwise approach for chronic pain. Based on information from Ballantyne and Mao (2003).
*Equivalent dosages specified in Table 3.
Clinicians should be aware that some pharmacological therapies for pain may interact with antiretroviral therapy (Gross, Bilker, Friedman, Coyne, & Strom, 2002). A detailed review of the known interactions between methadone, buprenorphine, and antiretroviral medications is beyond the scope of this review but is the subject of recent literature (Basu, Chwastiak, & Bruce, 2005; Bruce, Altice, Gourevitch, & Friedland, 2006). Most notably, efavirenz and nevirapine have been found to induce the metabolism of methadone and may precipitate opioid withdrawal in individuals chronically maintained on methadone (Basu et al., 2005; Bruce et al., 2006). It is still possible to use methadone while using medications that may induce liver enzymes. This is because the liver enzymes of HIV-infected patients who have taken efavirenz and nevirapine for several weeks will have reached a new equilibrium rate of metabolism above the original baseline. Therefore, the methadone titration can be started after this new equilibrium has been reached, and the antiretrovirals will not precipitate withdrawal (Basu et al., 2005; Bruce et al., 2006).
Other concerns about interactions between antiretrovirals and medications listed on the WHO stepladder include the fact that ritonavir increases the glucuronidation and decreases the plasma circulation of morphine; this may precipitate withdrawal in chronic morphine users (Schweiger et al., 1988). The adjuvant medication carbamazepine can lead to bone marrow dyscrasias and is hepatotoxic (worrisome for patients infected with hepatitis C; Penttila, Syvalahti, Hinkka, Kuusela, & Scheinin, 2001). Another adjuvant, amitriptyline, may cause dry mouth and dry skin, which have been reported to be a problem for many HIV-infected patients (Bloodworth, 2005).
Opiates, of course, present their own unique side effects and concerns. It has been reported that, among patients with a history of substance abuse, those receiving opiates appear to reduce their pain scale ratings by 27.8% over the course of treatment, as against 6.8% for patients receiving placebo (Prater et al., 2002). According to a meta-analysis of cohort studies, about two thirds of the opiate-receiving patients experience nausea, vomiting, sedation, or minor side effects (Prater et al., 2002). Serious adverse events such as hospitalization, respiratory depression, or death have been rarely reported among outpatients, occurring in 1.3% of patients based on eight studies of 1,168 subjects who were followed up for at least 26 weeks (Prater et al., 2002). Major side effects such as respiratory depression or decreased life expectancy among outpatients have not been shown to result from large doses when patients are still complaining of pain (Galer, Coyle, Pasternak, & Portenoy, 1992). That said, daily doses above 180 mg for morphine or morphine equivalents have not been validated in clinical trials of chronic pain patients; we argue that ceiling doses are ultimately a function of an individual patient's unique response to opiates. Physicians should titrate the medication dose until pain relief is achieved at the lowest possible dose in accordance with expert guidelines (Prater et al., 2002).
5. Managing acute pain
It has been argued that patients should be treated equally regardless of their substance use profile, although those with a history of opiate abuse may require closer follow-up and supervision (Gorman & Newshan, 1998). When faced with acute pain, the patient with a history of opiate abuse may need to be treated with short-acting opiates that can be rapidly titrated to effect and then decreased as pain decreases. According to a review of data on short-acting analgesics, clinical data suggest that short-acting agents do not appear to prolong or enhance dependence in the acute pain setting among HIV-infected patients with a history of substance abuse (including opiate abuse; Gorman & Newshan, 1998).
Patients with a history of opiate dependence may require higher-than-usual doses of opiates during acute treatment, given their tolerance, according to two studies on analgesia among such patients (Hughes, Bickel, & Higgins, 1991; Umbricht et al., 2003). Opiate doses can still be rapidly lowered in these patients as the pain resolves (Basu et al., 2005).
For patients with acute pain who are actively abusing opiates, methadone is effective both in providing analgesia and in preventing opiate withdrawal symptoms that might promote the misuse or abuse of prescribed short-acting opiates. In the acute pain setting, methadone typically starts at 30 mg for the first day and is tapered in 5- to 10-mg increments per day until discontinuation (Passik & Kirsh, 2004a). Methadone has an analgesic effect of 6 to 8 hours and produces tolerance more slowly than more euphoria-producing opiates (Fishman, Wilsey, Mahajan, & Molina, 2002). Methadone may, however, have a prolonged and unpredictable half-life beyond an average of 12 to 16 hours, which may cause it to accumulate when used for any length of time (Compton, 1994). Because methadone has a limited analgesic effect after 8 hours, it may not provide adequate analgesia for some opioid-tolerant individuals for the entire day. Practitioners should be aware of the possible need to provide additional short-acting opioids for opioid-tolerant individuals to achieve adequate pain relief. However, clinicians should always begin with methadone and proceed cautiously with the introduction of other opioids. Because it is prohibited by federal law to prescribe methadone for the treatment of opioid dependence, except through federally licensed methadone maintenance treatment programs (MMTPs), the dose of methadone that was started on patients as suggested above must be tapered off as the acute pain resolves. Practitioners could use this time as an opportunity to discuss formal drug treatment and enrollment in an MMTP, which has been found to provide effective structured support to HIV-infected persons recovering from opiate addictions (Prater et al., 2002).
Patients on buprenorphine maintenance may also require special consideration. Buprenorphine is a partial opiate agonist with unique properties; as a partial agonist, it is not reported to contribute to a feeling of euphoria (unlike methadone). In addition, buprenorphine has a “ceiling” effect, limiting its opiate effects and, therefore, enhancing its safety profile due to its low potential to induce respiratory depression (Basu, Smith-Rohrberg, Bruce, & Altice, 2006). This safety profile has led regulators to be less stringent with buprenorphine than with methadone; unlike methadone, buprenorphine does not require enrollment in a specialized clinic; rather, it can be prescribed by community practitioners after an 8-hour training course (Jaffe & O'Keeffe, 2003). This has made it attractive to clinicians treating HIV-infected patients (Basu et al., 2006).
In the context of pain management, it is notable that buprenorphine has a high binding affinity, and therefore, short-acting analgesics may or may not provide adequate pain control because they cannot displace buprenorphine from opiate receptors. There are three approaches that have been identified for the treatment of acute pain in individuals on buprenorphine maintenance (Alford, Compton, & Samet, 2006). An initial trial of changing the dosing frequency, the amount of buprenorphine, or both can be considered (e.g., from 16 mg once daily to 8 mg three times daily). However, due to the ceiling effect of buprenorphine, it is unlikely that any additional analgesia will be experienced beyond 32 mg in a 24-hour period (Basu et al., 2006). Secondly, because buprenorphine does not occupy the opiate receptor indefinitely, some individuals will find that the addition of a short-acting full opiate agonist is helpful in providing some additional pain relief. Although there is limited evidence in this regard, we would argue that a trial of a short-acting agent (e.g., oxycodone) could be considered. If the first two approaches are insufficient, we would argue that buprenorphine could be substituted with a full opiate agonist such as methadone to provide effective analgesia.
A summary approach to acute pain control in HIV-infected patients is summarized in Fig. 1, which is based upon two general reviews on the use of opiate therapy, combined with a recent review of clinical trials related to opiate use among HIV-infected patients (Ballantyne & Mao 2003; Breitbart & McDonald, 1996; Gourlay et al., 2005). The flowchart begins with a workup of the etiology of the acute pain problem, treatment for withdrawal symptoms, and management with short-acting therapy for patients on chronic opiates. For those not on chronic opiates, it describes the WHO stepladder approach, beginning with nonopiate medications and advancing to short-term opiate therapies with schedules for follow-up and to either taper-down therapy or continuation on a chronic therapy plan.
6. Managing chronic pain
Based upon three current assessments and sets of recommendations from researchers on chronic pain management, we have constructed a rubric for practitioners who encounter HIV-infected individuals with chronic pain (see Fig. 2; Ballantyne, 2002; Bloodworth, 2005; Kirsh et al., 2002). Below, we explain the empirical basis for the nonintuitive parts of this figure. The flowchart begins with a workup of organic etiologies for the pain, a trial of nonopiate medications, and an assessment of substance use history. We then describe the explicit planning of a therapeutic pain medication course and methods of creating rules and agreements with patients to avoid conflict.
Recommendations of the assessment and structure of chronic pain management appear to be based more on clinical experience than on empirical studies. During the assessment of patients with a recent history of opiate use, we suggest—based upon our review of current literature and clinical experience—that practitioners be particularly vigilant about patients with a recent history of use (last use <6 months prior to assessment). Patients with a recent history of opiate abuse appear to be at greatest risk of withdrawal; current evidence suggests that their symptoms may be managed with 15 to 20 mg/day of methadone, tapering down until discontinuation (Gorman & Newshan, 1998). Methadone can also be used as a long-acting analgesic, but current data suggest that the total dose should be split into segments to allow methadone to be given several times per day, as once-daily dosing does not appear to be effective for providing analgesic therapy (Ballantyne & Mao, 2003).
Regardless of the length of time since last use of opiates (in the context of either therapy or abuse), other authors have recommended that a treatment course involving opiate therapy should begin with a trial period of dose initiation and adjustment (Antoin & Beasley, 2004). During this trial period, the practitioner can individualize the structure under which opiate medications will be taken. Some practitioners find this period particularly challenging, as the upward titration of long-acting opiate medications can be a slow process and may be complicated when a patient seeks additional short-acting agents either due to an exacerbation of the underlying painful condition or for relief as the dose of chronic opiate medications is titrated upward (Ballantyne & Mao, 2003). In the setting of an exacerbation of an underlying painful condition, it has been argued that the active user should present for an evaluation to obtain a short-acting agent for the pain; this can allow the physician to ascertain if the patient is diverting or abusing the prescribed medications (Kirsh et al., 2002). Short-acting agents can also be used to help adjust the initial dose of long-acting opiates but should not typically be part of the long-term plan unless the patient requires three or four doses per week for breakthrough pain. Generally, a short-acting opiate can be initiated and tapered off as a long-acting agent is added to provide stabilization and a basal level of pain control (Ballantyne, 2002). Patients can have exacerbations in pain and will, on occasion, need short-acting agents to provide relief during those exacerbations (Prater et al., 2002).
In our clinical opinion, some medicines should probably be avoided by clinicians who treat patients with a substance use history. In particular, meperidine has a short length of effectiveness and a tendency to induce euphoria (Hansen, 2005); propoxyphene similarly provides minimal analgesia while having a high abuse potential (Sullivan, Metzger, Fudala, & Fiellin, 2005). Those patients actively abusing narcotics or those on methadone maintenance programs should not be given agonist–antagonist medications that may precipitate opiate withdrawal, such as buprenorphine, pentazocine, nalbuphine, or butorphanol (Manfredi, Gonzales, Cheville, Kornick, & Payne, 2001).
7. Structuring pain management services
Several authors and the American Academy of Pain Medicine have recommended that a written treatment agreement (“contract”) be signed by both the provider and the patient at the time of starting pharmacological therapy for pain; templates have been created by the American Academy of Pain Medicine for this purpose (Passik & Kirsh, 2004a). The utility of these agreements has been anecdotal, with little literature supporting their actual efficacy. Some expert guidelines suggest that only one physician (or one group if provided within a group practice) should provide all pain medication prescriptions to reduce the possibility of abuse, and this specification can be incorporated into the wording of the contract itself (Prater et al., 2002).
In a number of treatment settings, urine toxicology has been instituted as a routine office procedure for all clients receiving pain medications to avoid discrimination. In one trial, toxicology screening results were found to be positive in 20% of patients who otherwise did not exhibit signs of aberrant behavior (Antoin & Beasley, 2004). Notably, the screening test employs a nonspecific immunoassay technique, and only some laboratories send positive samples for confirmatory mass spectrometry or gas chromatography. According to current literature, even these confirmatory tests can provide false-positive results by poppy seeds (testing positive for opiates), Vicks Vapor Rub (containing l-methamphetamine), and some diet drugs. On the other hand, several opiates are not routinely screened for and must be specifically requested (Butler, Budman, Fernandez, & Jamison, 2004). Oxycodone, for example, does not test positive for opiates until a large-enough daily dose (>320 mg) is ingested. Also, metabolites of medications may be reported on the urine screen, giving a false sense that a patient is taking both codeine and morphine when, in fact, these substances are metabolites of codeine coformulated with acetaminophen (Tylenol No. 3; Passik & Kirsh, 2004b). In contrast, a patient who rapidly metabolizes drugs may receive a false-negative result (Katz & Fanciullo, 2002). Interpreting urine toxicology results requires appropriate laboratory methods, which have been detailed extensively elsewhere (Gourlay et al., 2005).
Recent surveys and case reports suggest that HIV-infected patients who appear to be abusing street drugs while receiving pain treatment are among the most difficult to engage around treatment of both pain and the substance abuse disorder. Many of these patients are fearful that disclosure of abuse will result in the removal of needed pain medications and, in extreme cases, discharge from the clinical setting (Gorman & Newshan, 1998; Larue et al., 1997). It has been suggested, therefore, that the patient should be viewed as having two diagnoses, a substance abuse condition and a pain condition, each of which should be addressed independent of the other (Vogl et al., 1999). The goal of pain treatment would then not be the same as that of substance abuse treatment. For example, if a patient is taking methadone for chronic pain and abusing crack cocaine, the patient would be viewed as having two distinct problems and both conditions would not be treated in the same manner (Turner, Laine, Lin, & Lynch, 2005). The removal of methadone does not improve the pain treatment and is unlikely to enhance treatment for cocaine misuse (Nathan & Karan, 1989; Scimeca, Savage, Portenoy, & Lowinson, 2000). From the perspective of the authors of this article, an analogous situation often arises in internal medicine: A clinician would not be likely to withdraw insulin from a patient who has both diabetes and hypertension if that patient failed to adhere to anti-hypertensive medications.
In the setting of active substance use, it has been suggested that the patient should be instructed on the need to enter into substance abuse treatment to continue with the treatment of pain (Nathan & Karan, 1989). Adequate time should be allowed for entry into drug treatment, as waiting lists are often prohibitive (Jaffe & O'Keeffe, 2003). This information might be included in the patient contract so that the patient will be familiar with this stipulation from the beginning of the treatment of pain.
8. Characterizing “aberrant behavior”
Although many authors have attempted to identify those most likely to abuse medications, such attempts may be viewed with caution given that our review of current data suggests that practitioners in trials are poor at predicting which patients will abuse medications (Drug Enforcement Administration, 2004; Gourlay et al., 2005). No screening instrument for assessing pain medication abuse is currently employed widely. A recent consensus document from the Drug Enforcement Agency, Last Acts Partnership, and Pain and Policy Studies Group at the University of Wisconsin issued joint guidelines for clinicians that may be predictive of addiction. These include multiple “lost” or “stolen” scripts, refusal to comply with requests for random drug tests, concurrent abuse of alcohol or illicit drugs, impairment in social or work functioning, injecting or snorting medication, illegal activities, and seeing multiple physicians and pharmacies (Kirsh et al., 2002). Among a set of HIV-infected patients, the most common aberrant drug-taking behaviors were alcohol use (51%), dose escalation of pain medications (46%), obtaining opiates from street dealers (14%), and seeing multiple physicians without the providers' knowledge (11%; Kirsh et al., 2002).
Whenever a practitioner is suspicious of prescription medication abuse, the problem of pseudoaddiction, or the abuse of medications driven by unrelieved pain, may be considered. Case reports about pseudoaddiction describe patients whose behaviors include attempting to take higher doses of medication or supplement the medication (e.g., with alcohol) during periods of painful distress (Kirsh et al., 2002). Distinguishing pseudoaddiction from addiction in an HIV-infected population may be complicated because definitions of addiction and dependence are typically based on populations without underlying chronic illnesses. Published case reports suggest that the alterations of functioning caused by HIV disease itself and its surrogate infection- and drug-induced conditions may make it difficult to distinguish problematic behaviors from pseudoaddiction or even cognitive changes related to HIV infection (Breitbart et al., 1999; Breitbart & McDonald, 1996; Breitbart et al., 1998; Breitbart et al., 1997; Frich & Borgbjerg, 2000; Passik & Kirsh, 2004a; Passik et al., 2000; Prater et al., 2002). To avoid confusing pseudoaddiction with addiction or other problems, a clinician may use a differential diagnosis list to work through when a patient presents with aberrant drug-taking behaviors; our collection of such a list, based on published literature to date, is shown in Table 4.
Table 4.
Differential diagnosis of aberrant drug-taking behaviors in the HIV-infected patient
| Addiction |
| Pseudoaddiction |
| Metabolic conditions (e.g., producing encephalopathy) |
| Infectious process (e.g., neurocognitive effects of HIV, HCV, or both) |
| Mental Health Axis I (e.g., depression, bipolar disorder) |
| Mental Health Axis II (e.g., borderline personality disorder) |
| Social stressorsa |
Such as family dysfunctions or the desire to divert medications for money.
9. Conclusions
There is no definitive method to approaching pain management in an HIV-infected patient with a history of substance abuse. Our interpretation of the existing data in the field suggests that there are general concepts to assist the physician in providing pain relief while protecting patients from subsequent addiction. HIV clinicians can use a stepwise approach (shown in Figs. 1 and 2) to minimize the potential for addiction when treating patients who present with pain.
There is general agreement in the literature that patients should be approached with a thorough investigation of the details of prior substance use, addressing the possibility of acute withdrawal or ongoing abuse. An organic etiology for pain should always be sought, and patients could begin on the WHO stepladder with nonopiate medications before moving onto opiate therapy. Long-acting opiates appear to serve better than those acting over shorter periods, although short-acting drugs can be used during the initiation of long-acting therapy, and to treat breakthrough pain. Chronic pain therapy can incorporate treatment contracts or otherwise explicit declarations of the goals of treatment. For HIV-infected patients in particular, specific drug–drug interactions must be considered prior to initiating medications such as methadone. HIV-infected patients who do manifest “aberrant” behaviors while taking opiates should be worked up for possible infection-related etiologies (Table 4) and should be evaluated for the possibility of pseudoaddiction.
Further research is necessary to probe how to best treat pain in the growing and aging population of HIV-infected patients who face comorbid diseases and syndromes of the elderly. These include neuropathic pain syndromes, coinfections with hepatitis or herpes, and neurological syndromes that may be associated with prolonged use of antiretroviral therapies. Another subject of growing research is the management of pain in patients with HIV and hepatitis C coinfection. These patients may face painful experiences both before and during therapies for hepatitis C; the treatment course for this disease also involves psychiatric side effects that may further complicate the pain picture. These pressing research issues highlight the complicated territory under which HIV-infected patients experience pain syndromes, as well as the challenges that practitioners face in attempting to navigate the territory between pain management and pharmacotherapeutic complexities in HIV-infected patients.
Acknowledgments
The authors would like to thank the National Institute on Drug Abuse (R.D.B.: K23 DA 022143; F.L.A.: K24 DA 017072) and the Substance Abuse and Mental Health Services Agency (H79 TI 15767) for their funding in these clinical and research efforts. S.B. is also funded by the NIH Medical Scientist Training Program (T23 GM 07205).
References
- Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Annals of Internal Medicine. 2006;144:127–134. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoin H, Beasley RD. Opioids for chronic noncancer pain. Tailoring therapy to fit the patient and the pain. Postgrad Med. 2004;116(3):37–40. 43–44. doi: 10.3810/pgm.2004.09.1581. [DOI] [PubMed] [Google Scholar]
- Ballantyne J. The Massachusetts General Hospital handbook of pain management. Philadelphia: Lippincott, Williams & Wilkins; 2002. p. 562. [Google Scholar]
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. New England Journal of Medicine. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Basu S, Chwastiak LA, Bruce RD. Clinical management of depression and anxiety in HIV-infected adults. AIDS. 2005;19:2057–2067. doi: 10.1097/01.aids.0000182518.84407.32. [DOI] [PubMed] [Google Scholar]
- Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clinical Infectious Diseases. 2006;42:716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Bloodworth D. Issues in opioid management. American Journal of Physical Medicine and Rehabilitation. 2005;84 3:S42–S55. [PubMed] [Google Scholar]
- Breitbart W, Kaim M, Rosenfeld B. Clinicians' perceptions of barriers to pain management in AIDS. Journal of Pain and Symptom Management. 1999;18:203–212. doi: 10.1016/s0885-3924(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Breitbart W, McDonald MV. Pharmacologic pain management in HIV/AIDS. Journal of the International Association of Physicians in AIDS Care. 1996;2:17–26. [PubMed] [Google Scholar]
- Breitbart W, Passik S, McDonald MV, Rosenfeld B, Smith M, Kaim M, et al. Patient-related barriers to pain management in ambulatory AIDS patients. Pain. 1998;76:9–16. doi: 10.1016/s0304-3959(98)00018-9. [DOI] [PubMed] [Google Scholar]
- Breitbart W, Rosenfeld B, Passik S, Kaim M, Funesti-Esch J, Stein K. A comparison of pain report and adequacy of analgesic therapy in ambulatory AIDS patients with and without a history of substance abuse. Pain. 1997;72:235–243. doi: 10.1016/s0304-3959(97)00039-0. [DOI] [PubMed] [Google Scholar]
- Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: Implications and management for clinical practice. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;41:563–572. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, et al. Pain and its treatment in outpatients with metastatic cancer. New England Journal of Medicine. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: Correlates of drug type and use status. Journal of Pain and Symptom Management. 1994;9:462–473. doi: 10.1016/0885-3924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs. amitriptyline in painful diabetic neuropathy: An open-label pilot study. Journal of Pain and Symptom Management. 2000;20:280–285. doi: 10.1016/s0885-3924(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Dews TE, Mekhail N. Safe use of opioids in chronic noncancer pain. Cleveland Clinic Journal of Medicine. 2004;71:897–904. doi: 10.3949/ccjm.71.11.897. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Last Acts, Pain and Policy Studies Group. Prescription pain medications: Frequently asked questions and answers for health care professionals and law enforcement personnel. Washington, DC: Drug Enforcement Association; 2004. [PubMed] [Google Scholar]
- Fishman SM, Wilsey B, Mahajan G, Molina P. Methadone reincarnated: Novel clinical applications with related concerns. Pain Medicine. 2002;3:339–348. doi: 10.1046/j.1526-4637.2002.02047.x. [DOI] [PubMed] [Google Scholar]
- Frich LM, Borgbjerg FM. Pain and pain treatment in AIDS patients: A longitudinal study. Journal of Pain and Symptom Management. 2000;19:339–347. doi: 10.1016/s0885-3924(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Galer BS, Coyle N, Pasternak GW, Portenoy RK. Individual variability in the response to different opioids: Report of five cases. Pain. 1992;49:87–91. doi: 10.1016/0304-3959(92)90192-E. [DOI] [PubMed] [Google Scholar]
- Gorman M, Newshan G. Pain management needs of patients with HIV or AIDS who also have a history of chemical dependency. American Journal of Nursing. 1998;98:61–63. [PubMed] [Google Scholar]
- Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: A rational approach to the treatment of chronic pain. Pain Medicine. 2005;6:107–112. doi: 10.1111/j.1526-4637.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- Gross R, Bilker WB, Friedman HM, Coyne JC, Strom BL. Provider inaccuracy in assessing adherence and outcomes with newly initiated antiretroviral therapy. AIDS. 2002;16:1835–1837. doi: 10.1097/00002030-200209060-00021. [DOI] [PubMed] [Google Scholar]
- Hansen GR. The drug-seeking patient in the emergency room. Emergency Medicine Clinics of North America. 2005;23:349–365. doi: 10.1016/j.emc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- HIVpositive.com. [17 Nov 2006];Pharmacologic Management of Pain in HIV/AIDS. 2005 Available at: http://www.hivpositive.com/f-PainHIV/Pain/PainMenu3.html.
- Hughes JR, Bickel WK, Higgins ST. Buprenorphine for pain relief in a patient with drug abuse. American journal of drug and alcohol abuse. 1991;17:451. doi: 10.3109/00952999109001604. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, O'Keeffe C. From morphine clinics to buprenorphine: Regulating opioid agonist treatment of addiction in the United States. Drug and Alcohol Dependence. 2003;70 2:S3–S11. doi: 10.1016/s0376-8716(03)00055-3. [DOI] [PubMed] [Google Scholar]
- Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clinical Journal of Pain. 2002;18 4:S76–S82. doi: 10.1097/00002508-200207001-00009. [DOI] [PubMed] [Google Scholar]
- Kimball LR, McCormick WC. The pharmacologic management of pain and discomfort in persons with AIDS near the end of life: Use of opioid analgesia in the hospice setting. Journal of Pain and Symptom Management. 1996;11:88–94. doi: 10.1016/0885-3924(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Kirsh KL, Whitcomb LA, Donaghy K, Passik SD. Abuse and addiction issues in medically ill patients with pain: Attempts at clarification of terms and empirical study. Clinical Journal of Pain. 2002;18 4:S52–S60. doi: 10.1097/00002508-200207001-00006. [DOI] [PubMed] [Google Scholar]
- Koo PJ. Acute pain management. Journal of Pharmacy Practice. 2003;16:231–248. [Google Scholar]
- Larue F, Fontaine A, Colleau SM. Underestimation and undertreatment of pain in HIV disease: Multicentre study. British Medical Journal. 1997;314:23–28. doi: 10.1136/bmj.314.7073.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovits AH, Lefkowitz M, McCarthy D, Simon R, Wilpon H, Jung R, et al. The prevalence and management of pain in patients with AIDS: A review of 134 cases. Clinical Journal of Pain. 1989;5:245–248. doi: 10.1097/00002508-198909000-00009. [DOI] [PubMed] [Google Scholar]
- Manfredi PL, Gonzales GR, Cheville AL, Kornick C, Payne R. Methadone analgesia in cancer pain patients on chronic methadone maintenance therapy. Journal of Pain and Symptom Management. 2001;21:169–174. doi: 10.1016/s0885-3924(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Academic Medicine. 2001;76:410–418. doi: 10.1097/00001888-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Archives of Internal Medicine. 1999;159:1931–1937. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- Nathan JA, Karan LD. Substance abuse treatment modalities in the age of HIV spectrum disease. Journal of Psychoactive Drugs. 1989;21:423–429. doi: 10.1080/02791072.1989.10472188. [DOI] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL. Assessing aberrant drug-taking behaviors in the patient with chronic pain. Current Pain and Headache Reports. 2004a;8:289–294. doi: 10.1007/s11916-004-0010-3. [DOI] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL. Opioid therapy in patients with a history of substance abuse. Central Nervous System Drugs. 2004b;18:13–25. doi: 10.2165/00023210-200418010-00002. [DOI] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL, McDonald MV, Ahn S, Russak SM, Martin L, et al. A pilot survey of aberrant drug-taking attitudes and behaviors in samples of cancer and AIDS patients. Journal of Pain and Symptom Management. 2000;19:274–286. doi: 10.1016/s0885-3924(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Penttila J, Syvalahti E, Hinkka S, Kuusela T, Scheinin H. The effects of amitriptyline, citalopram and reboxetine on autonomic nervous system. A randomised placebo-controlled study on healthy volunteers. Psychopharmacology (Berl) 2001;154:343–349. doi: 10.1007/s002130000664. [DOI] [PubMed] [Google Scholar]
- Prater CD, Zylstra RG, Miller KE. Successful pain management for the recovering addicted patient. Primary Care Companion to the Journal of Clinical Psychiatry. 2002;4:125–131. doi: 10.4088/pcc.v04n0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. Journal of the American Medical Association. 2003;289:2370–2378. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews. 2005:CD005454. doi: 10.1002/14651858.CD005454. [DOI] [PubMed] [Google Scholar]
- Schweiger FJ, Kelton JG, Messner H, Klein M, Berger S, McIlroy WJ, et al. Anticonvulsant-induced marrow suppression and immune thrombocytopenia. Acta Haematologica. 1988;80:54–58. doi: 10.1159/000205599. [DOI] [PubMed] [Google Scholar]
- Scimeca MM, Savage SR, Portenoy R, Lowinson J. Treatment of pain in methadone-maintained patients. Mount Sinai Journal of Medicine. 2000;67:412–422. [PubMed] [Google Scholar]
- Streltzer J. Pain management in the opioid-dependent patient. Current Psychiatry Reports. 2001;3:489–496. doi: 10.1007/s11920-001-0043-9. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Metzger DS, Fudala PJ, Fiellin DA. Decreasing international HIV transmission: The role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100:150–158. doi: 10.1111/j.1360-0443.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Lin YT, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Archives of Internal Medicine. 2005;165:1769–1776. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- Umbricht A, Hoover DR, Tucker MJ, Leslie JM, Chaisson RE, Preston KL. Opioid detoxification with buprenorphine, clonidine, or methadone in hospitalized heroin-dependent patients with HIV infection. Drug and Alcohol Dependence. 2003;69:263. doi: 10.1016/s0376-8716(02)00325-3. [DOI] [PubMed] [Google Scholar]
- Vogl D, Rosenfeld B, Breitbart W, Thaler H, Passik S, McDonald M, et al. Symptom prevalence, characteristics, and distress in AIDS outpatients. Journal of Pain and Symptom Management. 1999;18:253–262. doi: 10.1016/s0885-3924(99)00066-4. [DOI] [PubMed] [Google Scholar]
- Walker EA, et al. Dissociation in women with chronic pelvic pain. Am J Psychiatry. 1992;149:534–537. doi: 10.1176/ajp.149.4.534. [DOI] [PubMed] [Google Scholar]
- Wood DP, Wiesner MG, Reiter RC. Psychogenic chronic pelvic pain: Diagnosis and management. Clinics in Obstetrics and Gynaecology. 1990;33:179–195. doi: 10.1097/00003081-199003000-00024. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cancer pain relief: Report of a WHO expert committee. Geneva, Switzerland: World Health Organization; 1986. [PubMed] [Google Scholar]


