Abstract
Background
Bile-pancreatic duct ligation in rats causes acute pancreatic inflammation. We performed serial morphologic evaluation of the exocrine pancreas after duct ligation to facilitate further investigations using the model.
Methods
The pancreas was excised from 74 rats after 0, 1, 3, 5, 24 or 48 h of duct ligation or sham operation. A pathologist evaluated one hematoxylin- and eosin-stained slide from each rat. Confirmatory immunostaining was performed with markers for apoptosis (activated caspase-3), proliferation (cyclin D3), neutrophils (myeloperoxidase), and macrophages (CD68).
Results
Interstitial edema and WBC infiltration were apparent at 24 h and increased at 48 h. Progressive periods of duct ligation were characterized by ductular ectasia (1–3 h), acinar vacuolization (5–48 h), leukocytic margination and neutrophil exocytosis (5–48 h), ductule epithelium hypertrophy and proliferation (24–48 h), and discernible loss of zymogen granules (48 h).
Conclusion
Ligation-induced acute pancreatitis in rats is a useful model to investigate early events in disease pathogenesis.
Keywords: Acute pancreatitis, rat, bile and pancreatic juice, pancreatic duct, morphology, acinar cell
THE INTRODUCTION
To develop novel therapeutic strategies for acute pancreatitis, the elucidation of mechanisms of disease pathogenesis is essential. The practical difficulties encountered in the investigation of the early stages of disease pathogenesis in clinical acute pancreatitis have resulted in a dependence on experimental models. Duct ligation-induced acute pancreatitis in animals is a useful experimental model of gallstone pancreatitis and the most frequently used species have been rats and opossums (1–9). Although duct ligation in opossums is associated with severe acute necrotizing pancreatitis and mortality (10), the use of the model is expensive and the availability of reagents is very limited. The rat model has the advantages of convenience, affordability, and availability of a wide variety of common reagents. Although not associated with either mortality or substantial necrosis, the duct ligation model of acute pancreatitis in rats is useful to investigate the early stages of disease pathogenesis with a focus on acute pancreatic inflammation and acinar injury. Furthermore, other experimental models such as cerulein-induced acute pancreatitis in rodents and choline-deficient ethionine-supplemented (CDE) diet-induced acute pancreatitis in mice do not correspond to clinical acute pancreatitis due to gallstones, alcohol consumption or other cause.
To facilitate further investigations using the rat model of duct ligation, we performed detailed morphological characterization of exocrine pancreatic changes that involved a time-course of duct ligation ranging from one hour to 48 hours. This time-course study of early morphological changes in ligation-induced acute pancreatitis in rats is a valuable baseline to evaluate benefits of various treatment strategies and to investigate mechanisms of disease pathogenesis during the early stages.
MATERIALS AND METHODS
Animal surgery and specimen collection
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Iowa. Male Sprague-Dawley rats (250–300 gm) were purchased from Harlan Sprague-Dawley, Inc., Indianapolis, IN. Midline laparotomy was performed on 74 rats under general anesthesia induced with ketamine hydrochloride (87 mg/kg) and xylazine hydrochloride (13 mg/kg) and the distal bile-pancreatic duct was identified at its point of entry into the duodenum. In the diseased groups, the distal bile-pancreatic duct was ligated at its junction with the duodenum. In sham-operated controls, the duct was only dissected but not ligated. The rats were killed after 0, 1, 3, 5, 24 or 48 hours. Ten non-operated rats comprised the zero-hour surgical control group. In the sham-operated and duct-ligated groups, the number of rats studied were, respectively, five and eight at one hour, six and ten at three hours, five each at five hours, six and nine at 24 hours, and five each at 48 hours. All surgical procedures were performed by one investigator (IS). The pancreas was quickly excised and immersed immediately into 10% phosphate-buffered formaldehyde for morphologic studies. All morphologic evaluations were performed by one investigator (DKM) who is a pathologist with expertise in comparative morphology, and images and findings were then discussed with the co-author (IS).
Histopathology
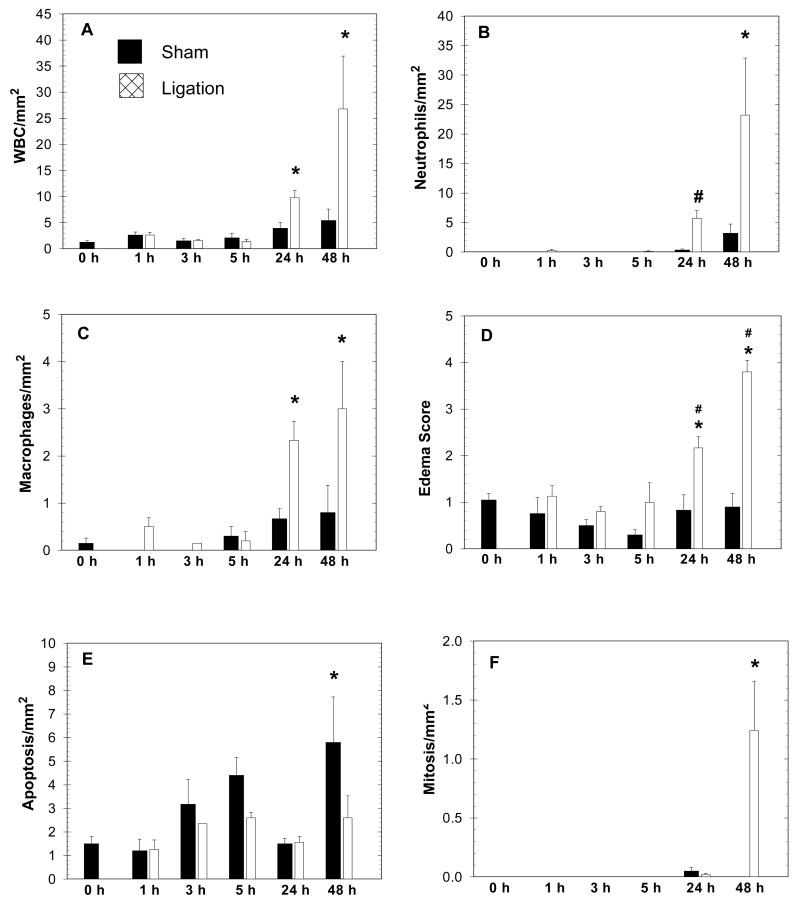
One hematoxylin- and eosin-stained section was prepared from each formaldehyde-fixed, paraffin-embedded portion of pancreas and examined on a light microscope (BX41TF, Olympus, Tokyo, Japan) for morphological studies. Total white blood cell (WBC) count was evaluated by examining two randomly selected 1 mm2 areas of exocrine pancreas and averaging the scores (Figure 1A). A differential WBC count was also obtained from the same areas and the neutrophil and macrophage counts are reported (Figures 1B and 1C). Interstitial edema was scored using a range of 1 (mild) to 5 (severe) (Figure 1D). Apoptosis and mitosis of acinar cells were also evaluated by similarly examining two 1 mm2 areas and studying the morphological characteristics of cells in the selected field (Figures 1E and 1F). Representative photomicrographs of additional findings presented in descriptive form are presented in Figures 2, 3 and 4. To corroborate interpretations of hematoxylin- and eosin-stained sections, we performed immunohistochemistry on selected slides as described below (Figure 4).
Figure 1.
Graphs depict morphologic changes in 74 rats observed during a time-course (1, 3, 5, 24 and 48 hrs) following sham operation (solid bars) or duct ligation (cross-hatched bars), compared to non-operated rats (0-hr group). Values are Mean+/−SEM. Asterisk (*) indicates significance versus 0-hr group, ANOVA, p<0.05; Pound sign (#) indicates significance versus the corresponding sham group at the same time point (unpaired Student’s t test, p<0.05).
Figure 2.
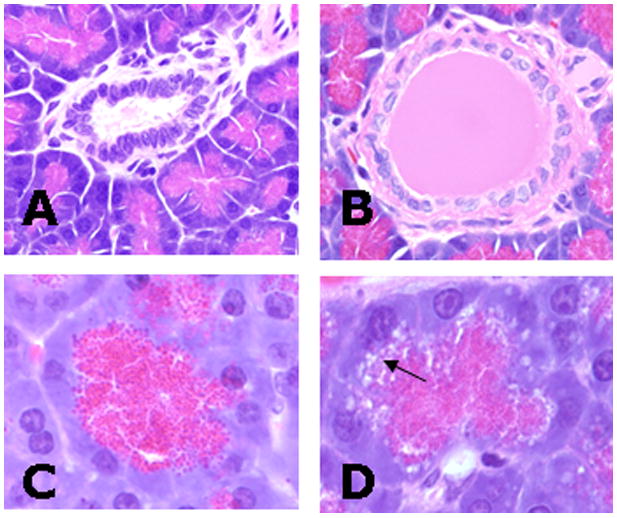
Rat pancreas following sham-operation (A, C) or duct ligation (B, D) (Hematoxylin-Eosin stain). A) Duct from sham pancreas after 1 hour. B) After 1 hour of ligation, ducts were often dilated with eosinophilic proteinaceous fluid. C) Pancreatic acinus five hours after sham operation. D) After 5 hours of ligation, acinar cells had clear to lightly eosinophilic and gray vacuoles (arrow) that were prominent at the basilar layer of the zymogen granules and were associated with morphologic degeneration and loss of the zymogen granules.
Figure 3.
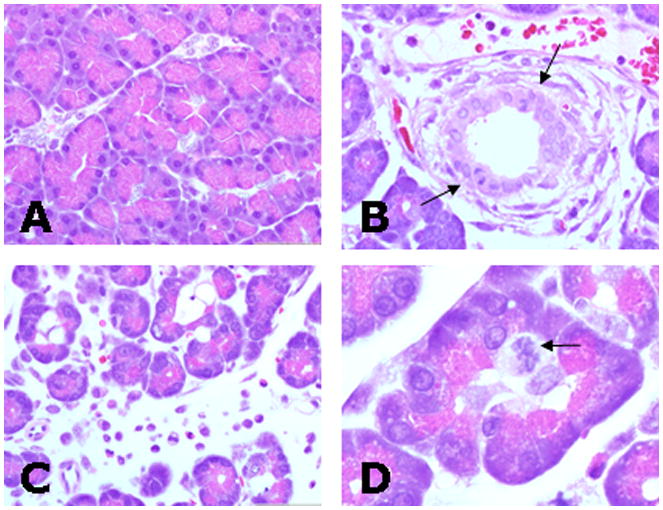
Pancreas from sham-operated (A) and duct ligated rats (B–D) after 48 hours (Hematoxylin-Eosin stain). A) Pancreas of sham-operated rat shows minimal changes after 48 hours. B) Duct epithelia frequently had mitotic figures (arrows) and ducts were surrounded by edema and infiltrating leukocytes. C) Inflammatory edema and leukocytes expanded interstitial space separating lobules and acini. D) Acini often contained proliferating centroacinar cells characterized by swollen nuclei and cytoplasm with multiple mitotic figures (arrow). Note the relative amount of zymogen granules from ligated groups (B and D) is reduced compared to the sham group (A).
Figure 4.
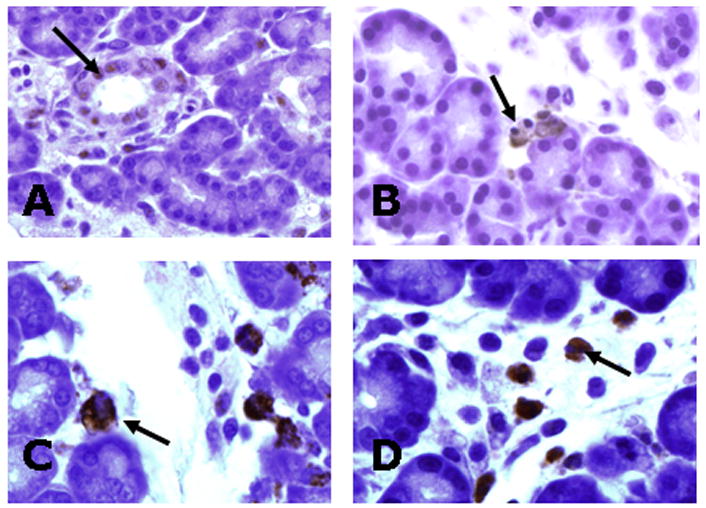
Immunohistochemical staining (arrows) for markers of: A) Proliferation (cyclin D3). B) Apoptosis (activated caspase-3). C) Macrophages (CD68). D) Neutrophils (myeloperoxidase).
Immuno-histochemistry
These studies were purely qualitative and not quantitative as the purpose was only to confirm the impression obtained with light microscopic examination of hematoxylin- and eosin-stained sections. Unstained paraffin sections were warmed, placed in xylene followed by a series of graded ethanol baths, and then assessed by immunohistochemistry using the peroxidase/antiperoxidase method. Immunostaining was performed on selected slides with primary antibodies for the following markers: apoptosis (rabbit anti-activated caspase-3, cat. #9664L, Cell Signalling Technology), proliferation (rabbit anti-cyclin D3, cat. # SC-182, Santa Cruz Biotechnology, Inc), neutrophils (rabbit anti-myeloperoxidase, cat. # A0398, DAKO), and macrophages (mouse anti-rat CD68, ED1 clone, Serotec). Detection of primary antibodies was performed using commercial kits (Rabbit Envision Plus or Mouse Envision Plus, as appropriate, DAKO) and chromogen (DAB, DAKO). Slides were then counterstained with hematoxylin, dehydrated through a series of ethanol and xylenes baths, and cover-slipped for microscopic examination.
Electron microscopy
The relationship between zymogen granules and autophagic vacuoles was examined in acinar cells of rats after five hours of duct ligation using transmission electron microscopy. From the freshly excised pancreas, small cubes less than 1 mm in size were sliced and immediately fixed in Karnovski’s fixative (2.5% glutaraldehyde, 4% paraformaldehyde, 1 M Na cacodylate buffer, pH 7.4). The samples were post-fixed in 1% osmium tetroxide, dehydrated in graded concentrations of ethanol, incubated in ascending concentrations of propylene oxide, and embedded in Spurr’s epoxy resin. Ultrathin sections were stained with uranyl acetate and bismuth subnitrate and viewed on a Hitachi transmission electron microscope (H-7000) at the University of Iowa Central Microscopy Research Facility.
Statistical methods
Statistical analyses were performed using the SigmaStat software. One way analysis of variance (ANOVA) tests, as appropriate for parametric or non-parametric data, were used for multiple comparisons. Student’s t test was performed for two-sample comparisons.
RESULTS
Quantitative histopathological evaluation
Morphometry and scoring systems were applied to the pancreata throughout the time course to assess quantitative changes in inflammation (cellular infiltration and edema), apoptosis and proliferation. Compared to the 0-hour baseline group, total WBC infiltration was significantly increased in the ligated but not the sham operated group by 24 and 48 hours (Figure 1A). The lack of statistical significance between the sham controls versus ligated group at 24 and 48 hours (or the sham groups versus the 0-hour group) probably reflects the large standard deviations observed with this method of quantitative analysis. However, it is important for the purposes of the present study that 24 and 48 hours of duct ligation result in significant changes compared to the 0-hour baseline. The cellular infiltrate was composed primarily of neutrophils and, to a lesser extent, macrophages (Figures 1B and 1C). After 24 hours of duct ligation the percentage distribution of WBC’s was 58% neutrophils and 24% macrophages, and after 48 hours there were 87% neutrophils and 11% macrophages. No eosinophils were seen in the parenchymal areas examined, while lymphocytes and mast cells were seen occasionally with a similar distribution amongst various experimental groups (data not shown). Edema scores for the ligated group correspondingly increased at 24 and 48 hrs while the sham group edema scores were unchanged (Figure 1D). The effects of surgical manipulation caused a significant increase in apoptotic activity in the 48-hour sham-operated group while the duct ligated groups showed no significant change (Figure 1E). Interestingly, the ligated group by 48 hrs showed significant evidence of proliferation whereas the sham group was unaffected (Figure 1F).
Qualitative histopathological evaluation – early phase
In addition to quantitative comparisons as described above, qualitative morphological evaluations of the pancreas was also performed. On the whole, the duct-ligated and sham-operated groups had minimal morphological changes one to three hours after surgery. There was mild vascular congestion mainly noted in the first hour of both groups, and this generally diminished at the three-hour time point. The duct ligation groups had multifocal moderate ectasia of the ducts and ductules by eosinophilic proteinaceous material and this was lacking in the sham groups (Figures 2A and 2B). Five hours after surgery, the ligation group frequently had morphologic disruption of the acinar cells by cytoplasmic vacuolization at the basal level of the zymogen stores (Figures 2C and 2D). At this level, the zymogen granules were morphologically degenerate having indistinct ragged edges, faint eosinophilic to gray coloration and remnant granules were often located adjacent to or within clear spaced vacuoles. This process, which was most apparent at five hours, was seen through the remaining times points and seemed to correspond to a general decrease in the relative cytoplasmic stores of zymogen granules by 48 hours (Figure 3). In addition, at the 5-hr time point, the ligation group had scattered evidence of early inflammation including leukocytic margination in vessels with mild neutrophilic exocytosis and perivascular edema.
Qualitative histopathological evaluation – late phase
After 24 hours, inflammatory and cellular changes became more readily apparent and generally progressed through 48 hours (Figure 3). During these later time points, the ligation group had increased edema evidenced by clear space separation of collagen fibers, lobules and acini. Much of the edema was focused on the perilobular interstitium with expansion into the periacinar space causing distinct separation of acinar structures by 48 hours. The cellular infiltrate was composed primarily of neutrophils and macrophages, with neutrophils being more prevalent. The infiltrate was predominantly seen in the interstitium surrounding medium to large ducts and vessels of the interlobular matrix and extended into the parenchymal periacinar spaces (Figures 3B and 3C). Interestingly, the epithelium lining ducts and ductules, centriacinar cells, and occasionally acinar cells, showed evidence (mitoses, anisokaryosis, anisocytosis, etc) of increased proliferation after duct ligation. At 48 hours of ligation, centroacinar cells frequently had swollen anisokaryotic nuclei with clear cytoplasm that expanded the centriacinar space and often contained mitotic figures. Multiple, and occasionally bizarre, mitotic figures were seen from centriacinar cells to larger ducts (Figures 3 B and 3D). Furthermore, the prevalence of apoptotic cells seen in the pancreas was increased after 48 hrs in sham operated rats, but remained relatively unchanged in the ligation group. Immunohistochemistry in selected slides confirmed histological interpretation of hematoxylin- and eosin-stained slides (Figure 4).
Electron microscopic studies
Transmission electron microscopy of pancreata after 5 hours of duct ligation showed the formation of cytoplasmic autophagic vacuoles (secondary lysosomes) in acinar cells (Figure 5). Interestingly, zymogen granules were every so often observed within these autophagic vacuoles. Swelling of the endoplasmic reticulum was an additional feature observed after duct ligation.
Figure 5.
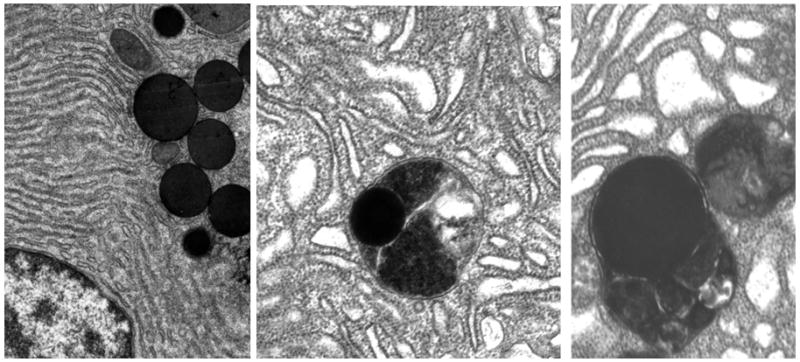
Transmission electron micrograph of rat pancreatic acinar cell. Left panel: Control with 0-hour ligation - normal appearing endoplasmic reticulum and zymogen granules. Center panel: Acinar cell after 5 hours of duct ligation showing dilated endoplasmic reticulum and a zymogen granule engulfed within an autophagic vacuole (secondary lysosome). Right panel: Acinar cell after 5 hours of duct ligation showing dilated endoplasmic reticulum and two autophagic vacuoles, with the one on the left containing a zymogen granule. These electron microscopic changes observed after 5 hours of duct ligation corroborate the findings observed with H&E stain and light microscopy (Figure 2D).
DISCUSSION
Our studies show that acute inflammatory changes after ligation of the distal rat pancreatic duct, such as interstitial edema and infiltration by neutrophils and macrophages, become prominent at 24 hours and increase further at 48 hours. Apoptosis was baseline to somewhat repressed in duct-ligated versus sham-operated rats and this corresponded with increased cellular proliferation in the ligated group that was most apparent at 48 hours. Compared to sham controls, progressive periods of duct ligation were characterized by appearance of ductular ectasia, acinar vacuolization around basal cytoplasmic zymogen granules, leukocytic margination and neutrophil exocytosis, ductule epithelium hypertrophy and proliferation, and discernible loss of zymogen granules. Transmission electron microscopy confirmed the impression on light microscopy that several zymogen granules are within autophagic vacuoles.
Autophagic vacuoles, also called secondary lysosomes, have the function of degrading aged or injured cell organelles and transporting the component macromolecules to the cytosol. Once in the cytosol, these macromolecules (e.g., amino acids, carbohydrates, nucleotides) are either reutilized by the cell to build new cellular organelles or are excreted by the cell. The molecular degradation of cell organelles within these vacuoles is accomplished by several lysosomal enzymes called acid hydrolases (e.g., cathepsin B, acid phosphatase, N-acetylglucosaminidase) (11). When the distal duct is ligated in rats there is exclusion of bile and pancreatic juice from the enteral lumen (11). Enteral exclusion of bile and pancreatic juice is associated with feedback hyperstimulation of the exocrine pancreas via a neurohormonal response initiated in the duodenum and proximal jejunum (12–14). Exclusion-induced acinar hyperstimulation promotes increased digestive enzyme production and the digestive enzymes are packaged in zymogen granules. In duct ligation-induced acute pancreatitis, the increased digestive enzyme production from hyperstimulation occurs in parallel with mechanical duct obstruction, a situation that would logically favor lysosomal autophagocytosis of the digestive zymogens rather than their secretion into the centriacinar lumen. In our study, we present light microscopic evidence that zymogen granule autophagocytosis and degradation begins at the five-hour time point after ligation with reduced or altered zymogen granules still detectable through 48 hours. Electron microscopic studies at the five-hour time point confirm that zymogen granules are engulfed within autophagic vacuoles after duct ligation and provide corroborative evidence to support our light microscopic observations. Interestingly, the lysosomal enzyme cathepsin B is capable of activating trypsinogen to trypsin, as suggested by in vitro and in vivo evidence (15–17). Active trypsin can activate other digestive proenzymes packaged within zymogen granules, potentially resulting in acinar cell “autodigestion” and necrosis. On the other hand, lysosomal enzymes could destroy — rather than activate — digestive zymogen enzymes, a possibility that would question a central role for cathepsin B in disease pathogenesis.
The time-course of inflammatory events in the present morphologic study provides guidance while designing experiments that investigate mechanisms of disease pathogenesis. For instance, interstitial edema, neutrophil infiltration and macrophage invasion into the pancreatic parenchyma are best investigated at the 24- and 48-hour time points in this duct ligation experimental model of acute pancreatitis. Early neutrophil changes to be looked for within the first five hours include scattered margination and peri-ductal infiltration. Additional early changes appreciated between one and five hours of duct ligation include acinar vacuolation and early proliferative changes in acini and duct epithelial cells. In previous studies, we have shown that intracellular molecular events such as stress kinase activation, nuclear translocation of nuclear factor kappa-B (NFkB) and cytokine production occur as early as within one hour of duct ligation, at which time point pancreatic morphologic changes are minimal as shown in the present study (18;19).
The proliferative changes observed in the present study could be a cellular response to growth stimulating hormones such as cholecystokinin (CCK) and secretin that are released by the duodenum into the systemic circulation when bile and pancreatic juice are excluded (20;21). The subtle acinar cell proliferative changes may be attributable to CCK stimulation while the more detectable ductal epithelial proliferative changes may be attributable to secretin. The mitotic changes are well established at the 48 hour time point. Interestingly, progressive apoptotic changes are seen in sham-operated rat pancreata but not in the diseased group. Other investigators have shown that apoptosis of acinar cells after duct ligation in rats is evident on the third post-operative day (3;4), a time point not evaluated in our study. This may be due to the recognized effect of growth hormones, which can regulate apoptotic activity while promoting proliferation (22). Our finding of significantly increased apoptosis after 48 hours in sham controls but not in duct ligated rats needs further evaluation, as other studies that looked at this feature are limited. One group that studied the rat model in detail began their examination only after three days of duct ligation (3), while another group that examined shorter time points used only a zero-hour control for comparison but did not use sham-operated controls of the same duration as the duct ligated rats (4).
A better understanding of the mechanisms of disease pathogenesis is needed to advance the development of new treatment alternatives for acute pancreatitis. As investigations into the pathogenesis of the early stages of clinical acute pancreatitis are difficult, we rely on the use of experimental models to elucidate salient mechanistic events and to test the value of novel treatment regimens. The duct ligation model of acute pancreatitis in rats is a useful experiment model as it is economical and convenient and it mimics early stages of gallstone pancreatitis. The detailed morphological characterization of the early stages of ligation-induced acute pancreatitis in rats in the present study is a prerequisite to further investigations into the evolution of the disease during the first 48 hours in this experimental model. A novel aspect of our study is the inclusion of the time course of pancreatic morphological changes within the first 24 hours of duct ligation ( 1, 3, 5 and 24 hrs of duct ligation), as most previous investigators have studied changes in later stages beginning only after 24 hours or more of duct ligation (3;4;23). Also, our studies have correlated the findings using immunohistochemistry, the surgical operations were performed by a surgeon, and the histopathologic studies were undertaken by a pathologist. Finally, compared to other studies, we have utilized a large number of rats and have included sham-operated controls at each time point.
The classic finding by Opie in 1901 of ampullary obstruction by a gallstone during the autopsy of a patient that succumbed to acute hemorrhagic pancreatitis is a historical landmark in the field (24). Acosta demonstrated that the passage of a stone through the ampulla, even without persistent obstruction, can initiate acute pancreatitis (25). Furthermore, Lee emphasized that biliary sludge is sufficient to cause acute pancreatitis (26). In 1971, Churg performed a detailed light and electron microscopic study of duct ligation-induced acute pancreatitis in rats and dogs with durations ranging from 24 hours to several days and emphasized the atrophy, fibrosis and degeneration of exocrine pancreatic tissue observed with increasing periods of duct ligation (23). Sanfey used Cameron’s well known model of the isolated, perfused ex vivo canine pancreas preparation and performed secretin stimulation in the presence of partial duct obstruction to induce pancreatitis (9). Since then, several groups have studied the duct ligation model of acute pancreatitis in rats and opossums (1–4;6;10;11;27–31). A major short-coming of the rat model of duct ligation is the predominance of atrophic and fibrotic changes over a period of time. In later stages of acute pancreatitis in humans, there are several features such as peripancreatic fat necrosis, extensive exocrine pancreatic necrosis, areas of thrombosis and hemorrhage, and peripancreatic fluid collections, that are not observed in the rat model. However, the early phase changes characterized by acute inflammation and acinar injury following duct ligation in rats are features that can be investigated with regard to early events in disease pathogenesis, as emphasized in the present study. This is especially important as patients with acute pancreatitis do not seek medical attention during the early stages and therefore the early events in disease pathogenesis cannot be investigated in humans.
Conclusion
Ligation-induced acute pancreatitis in rats is a useful model to investigate early events in disease pathogenesis such as acinar cell injury and acute pancreatic inflammation.
Acknowledgments
Dr. I. Samuel was supported for this work by an American College of Surgeons Faculty Research Fellowship and a National Institutes of Health NIDDK Career Development Award (Grant #K08-DK062805).
Footnotes
Abstract presented at the annual meeting of the Association of VA Surgeons, Little Rock, Arkansas, May 10-12, 2007.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David K. Meyerholz, Department of Pathology, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA 52242.
Isaac Samuel, Department of Surgery, VAMC & University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA 52242.
References
- 1.Ohshio G, Saluja A, Steer ML. Effects of short-term pancreatic duct obstruction in rats. Gastroenterology. 1991;104:1768–79. doi: 10.1016/0016-5085(91)90601-g. [DOI] [PubMed] [Google Scholar]
- 2.Lerch MM, Saluja AK, Runzi M, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993;104:853–61. doi: 10.1016/0016-5085(93)91022-a. [DOI] [PubMed] [Google Scholar]
- 3.Gukovskaya AS, Perkins P, Zaninovic V, et al. Mechanisms of cell death after pancreatic duct obstruction in the opossum and the rat. Gastroenterology. 1996 Mar;110(3):875–84. doi: 10.1053/gast.1996.v110.pm8608898. [DOI] [PubMed] [Google Scholar]
- 4.Kaiser AM, Saluja AK, Sengupta A, et al. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995 Nov;269(5 Pt 1):C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 5.Samuel I, Toriumi Y, Zaheer A, Joehl RJ. Mechanism of acute pancreatitis exacerbation by enteral bile-pancreatic juice exclusion. Pancreatology. 2004;4(6):527–32. doi: 10.1159/000080527. [DOI] [PubMed] [Google Scholar]
- 6.Samuel I, Toriumi Y, Yokoo H, et al. Ligation-induced acute pancreatitis in rats and opossums: a comparative morphologic study of the early phase. J Surg Res. 1994;57:001–13. doi: 10.1006/jsre.1994.1149. [DOI] [PubMed] [Google Scholar]
- 7.Hamamoto N, Ashizawa N, Niigaki M, et al. Morphological changes in the rat exocrine pancreas after pancreatic duct ligation. Histol Histopathol. 2002 Oct;17(4):1033–41. doi: 10.14670/HH-17.1033. [DOI] [PubMed] [Google Scholar]
- 8.Saluja A, Saluja M, Villa A, et al. Pancreatitic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest. 1999 Oct 19;84:1260–6. doi: 10.1172/JCI114293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanfey H, Bulkley GB, Cameron JL. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985 May;201(5):633–9. doi: 10.1097/00000658-198505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senninger N, Moody FG, Coelho JCU, Van Buren DH. The role of biliary obstruction in the pathogenesis of acute pancreatitis in the opossum. Surgery. 1986;99:688–93. [PubMed] [Google Scholar]
- 11.Samuel I, Toriumi Y, Wilcockson DP, et al. Bile and pancreatic juice replacement ameliorates early ligation-induced acute pancreatitis in rats. Am J Surg. 1995 Apr;169(4):391–9. doi: 10.1016/s0002-9610(99)80183-4. [DOI] [PubMed] [Google Scholar]
- 12.Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004 Sep;127(3):957–69. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura R, Miyasaka K, Funakoshi A, Kitani K. Interactions between bile and pancreatic juice diversions on cholecystokinin release and pancreas in conscious rats. Proc Soc Exp Biol Med. 1989;192:182–6. doi: 10.3181/00379727-192-42976. [DOI] [PubMed] [Google Scholar]
- 14.Samuel I, Joehl RJ. Bile-pancreatic juice replacement, not cholinergic and cholecystokinin-receptor blockade, reverses acinar cell hyperstimulation after bile-pancreatic duct ligation. Am J Surg. 1996 Jan;171(1):207–11. doi: 10.1016/S0002-9610(99)80101-9. [DOI] [PubMed] [Google Scholar]
- 15.Saluja A, Hashimoto S, Saluja M, et al. Subcellular redistribution of lysomal enzymes during caerulein-induced pancreatitis. Am J Physiology. 1987;253(16):G508–G516. doi: 10.1152/ajpgi.1987.253.4.G508. Gastrointest LIver Physiol. [DOI] [PubMed] [Google Scholar]
- 16.Steer ML, Meldolesi J, Figarella C. Pancreatitis. The role of lysosomes. [Review] [22 refs] Digestive Diseases & Sciences. 1984 Oct;29(10):934–8. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- 17.Figarella C, Miszczuk-Jamska B, Barrett AJ. Possible lysosomal activation of pancreatic zymogens. Biol Chem Hoppe-Seyler. 1988 May;369(Suppl):293–8. [PubMed] [Google Scholar]
- 18.Samuel I, Zaheer S, Zaheer A. Bile-pancreatic juice exclusion increases p38MAPK activation and TNF-alpha production in ligation-induced acute pancreatitis in rats. Pancreatology. 2005;5(1):20–6. doi: 10.1159/000084486. [DOI] [PubMed] [Google Scholar]
- 19.Samuel I, Yorek MA, Zaheer A, Fisher RA. Bile-pancreatic juice exclusion promotes Akt/NF-kappaB activation and chemokine production in ligation-induced acute pancreatitis. J Gastrointest Surg. 2006 Jul;10(7):950–9. doi: 10.1016/j.gassur.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Bourassa J, Laine J, Kruse ML, et al. Ontogeny and species differences in the pancreatic expression and localization of the CCK(A) receptors. Biochem Biophys Res Commun. 1999 Jul 14;260(3):820–8. doi: 10.1006/bbrc.1999.0988. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Lee KY, Chang TM, Chey WY. Effect of pancreatic juice diversion on secretin release in rats. Gastroenterology. 1989;96:1173–9. doi: 10.1016/0016-5085(89)91638-7. [DOI] [PubMed] [Google Scholar]
- 22.Berna MJ, Hoffmann KM, Tapia JA, et al. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta. 2007 Apr;1773(4):483–501. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churg A, Richter WR. Early changes in the exocrine pancreas of the dog and rat after ligation of the pancreatic duct. A light and electron microscopic study. Am J Pathol. 1971 Jun;63(3):521–46. [PMC free article] [PubMed] [Google Scholar]
- 24.Opie EL. The etiology of acute hemorrhagic pancreatitis. Johns Hopkins Hosp Bulletin. 1901;12:182–8. [Google Scholar]
- 25.Acosta J, Ledesma C. Gallstone Migration As A Cause Of Acute Pancreatitis. N Eng J Med. 1974 Feb 28;290(9):484–7. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- 26.Lee SP, Nicholls JF, Park HZ. Biliary Sludge As A Cause of Acute Pancreatitis. N Eng J Med. 1992;236:589–93. doi: 10.1056/NEJM199202273260902. [DOI] [PubMed] [Google Scholar]
- 27.Samuel I, Wilcockson DP, Regan JP, Joehl RJ. Ligation-induced acute pancreatitis in opossums: acinar cell necrosis in the absence of colocalization. J Surg Res. 1995 Jan;58(1):69–74. doi: 10.1006/jsre.1995.1011. [DOI] [PubMed] [Google Scholar]
- 28.Runzi M, Lerch MM, Saluja A, et al. Early ductal decompression prevents the progression of biliary pancreatitis: an experimental study in the opossum. Gastroenterology. 1993;105:157–64. doi: 10.1016/0016-5085(93)90021-4. [DOI] [PubMed] [Google Scholar]
- 29.Larsen F, Schlarman D, Andrus CC, Kaminski DL. The effect of the CCK Receptor Antagonist CR 1409 on Bile Reflux Pancreatitis in the Opossum. Pancreas. 1991;6(3):291–7. doi: 10.1097/00006676-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Murayama KM, Drew JB, Yokoo H, Joehl RJ. Bile exclusion from gut exacerbates acute pancreatitis caused by pancreatic duct obstruction in rats. Pancreas. 1991;6:175–81. doi: 10.1097/00006676-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Toriumi Y, Samuel I, Wilcockson DP, et al. Increased circulating CCK in obstruction-induced acute pancreatitis: II - Pancreatic duct obstruction with and without bile duct obstruction. J Surg Res. 1993;54:132–5. doi: 10.1006/jsre.1993.1020. [DOI] [PubMed] [Google Scholar]