Abstract
The opening of sarcolemmal and mitochondrial ATP-sensitive K+ (KATP) channels in the heart is believed to mediate ischemic preconditioning, a phenomenon whereby brief periods of ischemia/reperfusion protect the heart against myocardial infarction. Here, we have applied digital epifluorescent microscopy, immunoprecipitation and Western blotting, perforated patch clamp electrophysiology, and immunofluorescence/laser confocal microscopy to examine the involvement of KATP channels in cardioprotection afforded by preconditioning. We have shown that adult, stimulated-to-beat, guinea-pig cardiomyocytes survived in sustained hypoxia for ∼17 min. An episode of 5-min-long hypoxia/5-min-long reoxygenation before sustained hypoxia dramatically increased the duration of cellular survival. Experiments with different antagonists of KATP channels, applied at different times during the experimental protocol, suggested that the opening of sarcolemmal KATP channels at the beginning of sustained hypoxia mediate preconditioning. This conclusion was supported by perforated patch clamp experiments that revealed activation of sarcolemmal KATP channels by preconditioning. Immunoprecipitation and Western blotting as well as immunofluorescence and laser confocal microscopy showed that the preconditioning is associated with the increase in KATP channel proteins in sarcolemma. Inhibition of trafficking of KATP channel subunits prevented preconditioning without affecting sensitivity of cardiomyocytes to hypoxia in the absence of preconditioning. We conclude that the preconditioning is mediated by the activation and trafficking of sarcolemmal KATP channels.
Keywords: ischemia, heart, Kir6.2, SUR2A, cardioprotection
Brief periods of ischemia/reoxygenation that precede a sustained ischemia lead to a reduction of myocardial infarct size (1). This phenomenon is termed ischemic preconditioning and the underlying mechanisms are still a matter of vigorous debate (2).
It is well established that sarcolemmal ATP-sensitive potassium (KATP) channels are present at high density in the sarcolemma of cardiac myocytes where they link membrane excitability with the cellular bioenergetic state (3). In the early days of preconditioning research, KATP channels were proposed to play an important role in preconditioning-mediated cardioprotection (4). However, a study by Inoue et al. suggested that mitochondria also harbor KATP channels (5), and later research has suggested that the activation of the mitochondrial rather than the sarcolemmal KATP channel underlies the cardioprotective effect of ischemic preconditioning (6, 7). However, the most recent research revived the idea that sarcolemmal KATP channels may play a role in signaling pathways of ischemic preconditioning (8).
The structure of putative mitochondrial KATP channels is still controversial (9-11). However, the structure of sarcolemmal KATP channels is well established, and it is known that these channels are heteromultimers composed of Kir6.2 subunits; an inwardly rectifying K+ channel core primarily responsible for K+ permeance; and SUR2A, a regulatory subunit implicated in ligand-dependent regulation of channel gating (12). Normal expression of functional KATP channels on the cell surface requires coassembly of SUR and Kir subunits into an octameric complex (13).
Several recent studies suggested that the numbers of sarcolemmal KATP channels are an important factor in setting cardiac resistance toward different types of metabolic stress, including hypoxia (14-16). Given that channel activity depends critically on the number of functional channels, KATP channel traffic regulation could be an important mechanism of KATP channel modulation. In this regard, it has been recently reported that activation of protein kinase C (PKC) may regulate trafficking of KATP channels (17). This has raised the possibility that a fast increase or decrease in the number of KATP channels in sarcolemma could play previously unrecognized roles in cardiac physiology under normal and stressful conditions.
This has prompted us to examine whether preconditioning-induced cardioprotective signaling involves regulation of the activity and trafficking of sarcolemmal KATP channels. To address these questions, we applied an experimental model of hypoxic preconditioning that implements adult cardiomyocytes stimulated to beat. Hypoxia was induced solely by reducing partial oxygen tension (PO2) without using additional means to metabolically challenge the cells, which, all together, makes this model closer to in vivo conditions than the majority of previously published cellular models of preconditioning; this model was originally described by Gallagher and Allshire (18) and Mora et al. (19). Thus, we have taken advantage of this experimental setting and examined whether the activity and trafficking of KATP channels are involved in the intracellular signaling of preconditioning in the heart.
MATERIALS AND METHODS
Isolation of single cardiomyocytes
Ventricular cardiomyocytes were dissociated from male guinea pigs (200–250 g), using an established enzymatic procedure (14). In brief, hearts were retrogradely perfused (at 37°C) with medium 199, followed by Ca2+-EGTA-buffered low-Ca2+ medium (pCa=7), and low-Ca2+ medium containing pronase E (8 mg/100 ml), proteinase K (1.7 mg/100 ml), bovine albumin (0.1 g/100 ml, fraction V), and 200 μM CaCl2. Ventricles were cut into fragments in the low-Ca2+ medium enriched with 200 μM CaCl2. Cells were isolated by stirring the tissue (at 37°C) in a solution containing pronase E and proteinase K supplemented with collagenase (5 mg/10 ml). The first aliquot was removed, filtered through a nylon sieve, centrifuged for 60 s (at 300–400 rpm), and washed. Remaining tissue fragments were reexposed to collagenase, and isolation continued for two to three such cycles.
Experimental protocol of hypoxia and preconditioning
Hypoxia of isolated cardiomyocytes has been performed as described by Gallagher and Allshire (18) and Mora et al. (19). Thus, cardiomyocytes were placed into Tyrode's solution (in mM: NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, HEPES-NaOH 5.5, pH 7.4), plated out either on glass coverslips (for digital epifluorescent imaging and laser confocal microscopy) or on six-well plates (for immunoprecipitation and Western blotting), and paced to beat by field stimulation (parameters of the stimulation: 5–20 mV depending on cellular threshold, 5 ms, 0.5 Hz). Beating cardiomyocytes were perfused with Tyrode's solution at a rate of 3 ml/min, and, under these conditions, the partial pressure of O2 (PO2) in perfusate was 140 mmHg (19). To induce preconditioning, we exposed cardiomyocytes to a single episode of 5-min-long hypoxia/5-min-long reoxygenation followed by hypoxia (termed here as long-lasting hypoxia) that was maintained until cell death. To induce hypoxia, we bubbled Tyrode's solution with 100% argon (PO2=20 mm Hg). Some experiments were done in the presence of glybenclamide, 5-hydroxydecanoate (5-HD; both from Sigma, Dorset, UK), HMR 1098 (Aventis Pharma, Frankfurt, Germany), or with a cocktail of protein trafficking inhibitory drugs that contained brefeldin A, colchicine, and nocodazole (all from Sigma). Cells were pretreated with the cocktail for 30 min before the experiment). Cells exposed to this experimental protocol were used for digital epifluoresecent imaging, immunoprecipitation/Western blotting, and laser confocal experiments.
Digital epifluorescence imaging
Cell morphology, size parameters, and intracellular concentration of Ca2+ were continuously monitored in cells exposed to the experimental protocol described previously. To measure intracellular Ca2+ concentration cardiomyocytes were loaded with the esterified form of the Ca2+-sensitive fluorescent probe Fura-2 and imaged using a digital epifluorescence imaging system coupled to an inverted microscope (Image Solutions, Standish, UK). A mercury lamp served as a source of light to excite Fura-2 at 340 and 380 nm. Fluorescence emitted at 520 nm was captured, after crossing dichroic mirrors, by an intensified charge coupled device camera and was digitized using imaging software. The moment of cell death was defined as the point when irreversible cellular hypercontracture associated with the Ca2+ overload occurs (19).
Immunoprecipitation and Western blotting
Anti-SUR2A and anti-Kir6.2 antibodies were used for immunoprecipitation and Western blotting (see refs 14-16). Cardiomyocytes were harvested, when preconditioning was performed, at the time point before brief hypoxia, after the end of brief hypoxia (4 min in hypoxia), at the end of reoxygenation (4 min in reoxygenation), and at the beginning of long-lasting hypoxia (4 min in hypoxia). Cardiomyocytes exposed to the experimental protocol of hypoxia alone were collected at the same time points as those exposed to preconditioning. Cardiac membrane fraction was obtained as described (14-16). Epitope-specific Kir6.2 antibody (10 μg) or 40 μg of the epitope-specific SUR2A antibody was pre-bound to Protein-G Sepharose beads and used to immunoprecipitate from 50 μg of membrane fraction protein extract. The pellets of this precipitation were run on SDS polyacrylamide gels for Western analysis. Western blot probing was performed using 1/1000 dilution of anti-SUR2A and anti-Kir6.2 antibody, respectively, and detection was achieved using Protein-G HRP and ECL reagents. The band intensities were analyzed using Quantiscan software.
Immunofluorescence and laser confocal microscopy
Cardiomyocytes were fixed at the same time points as described previously with 4% paraformaldehyde for 20 min. After they were washed with PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min and washed and blocked with 1% donkey serum for 30 min. Cells were incubated at room temperature in a humidified chamber (for 1 h) with primary antibody (sheep anti-SUR2A) in PBS and incubated in the dark with the secondary antibody (donkey anti-sheep fluorescein [Jackson Immunochemicals, West Grove, PA]) at 1:500 dilution for 1 h (in some experiments, primary antibody was incubated with the antigenic peptide). Samples were washed with PBS, mounted on slides using 50% glycerol containing 1% n-propylgallate, and sealed with nail polish. Slides were examined by laser confocal microscopy (LSM-510, Zeiss, Gottingem, Germany). Images were acquired and analyzed (intensity of fluorescence per unit of sarcolemmal length was determined) using Zeiss Image Examiner software.
Whole cell perforated patch clamp electrophysiology
Cells were superfused with Tyrode's solution. Pipettes (resistance 3–5 MΩ) were filled with (in mM): KCl 140, MgCl2 1, HEPES-KOH 5, and amphotericin B (Sigma, 240 μg/ml) (pH 7.3). The membrane potential was held at −40 mV, and the currents were evoked by a 400 ms current step (to +80 mV) recorded directly to hard disk using an Axopatch-200B amplifier, Digidata-1321 interface, and pClamp8 software (Axon Instruments, Foster City, CA) (20). The whole cell K+ current was monitored every 30 s during the experimental protocol.
Statistical analysis
Data are presented as means ± SE, with n representing the number of experiments or analyzed cells. Mean values were compared by Student's t test, Mann-Whitney rank sum test, or ANOVA on ranks using SigmaStat program (Jandel Scientific, Chicago, IL). P < 0.05 was considered statistically significant.
RESULTS
Hypoxia induces death of cardiomyocytes without affecting levels of sarcolemmal KATP channel subunits
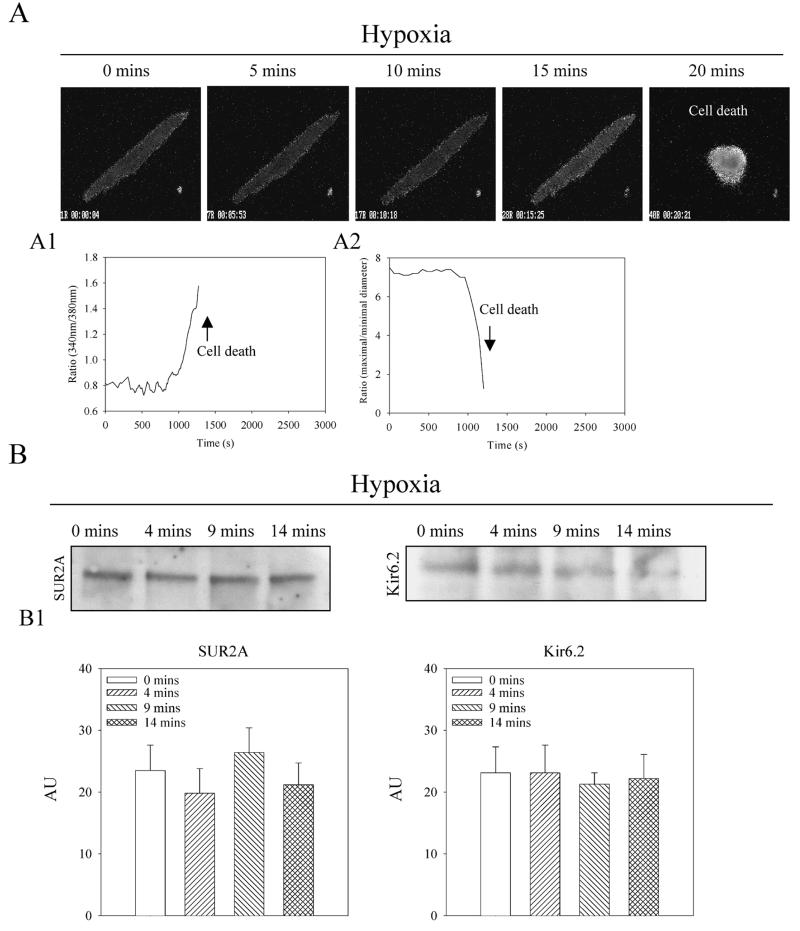
Single beating guinea-pig cardiomyocytes responded to hypoxia with intracellular Ca2+ loading and irreversible hypercontracture, which was indicative of cell death (Fig. 1A). The average time of survival in cells exposed to hypoxia was 16.8 ± 2.8 min (n=10). To assess fluctuations of the number of sarcolemmal KATP channels during hypoxia, membrane fraction of cardiomyocytes was, at different time points, immunoprecipitated with anti-Kir6.2 antibody and probed with the anti-SUR2A antibody and vice versa (immunoprecipitated with anti-SUR2A antibody and probed with anti-Kir6.2 antibody; using this approach only those subunits physically associated with each other were measured, 14–16). These experiments revealed that levels of sarcolemmal KATP channel subunits were steady during hypoxia (Fig. 1B).
Figure 1. Hypoxia induces death of cardiomyocytes without affecting the number of sarcolemmal KATP channels.
A) Epifluorescent images of Fura-2-loaded cardiomyocytes exposed to hypoxia. Magnification was ×40. Time course of Fura-2 ratio (A1) and cell diameters (A2) corresponding to experiments in A. B) Western blot with anti-Kir6.2 and anti-SUR2A of anti-SUR2A and anti-Kir6.2 immunoprecipitate pellets from membrane fractions from cardiomyocytes collected at depicted time points. B1) Graphs corresponding to B. Each bar represents mean ± se (n=3–4).
A single episode of hypoxia/reoxygenation promotes survival of cardiomyocytes in hypoxia and increases the number of sarcolemmal KATP channels
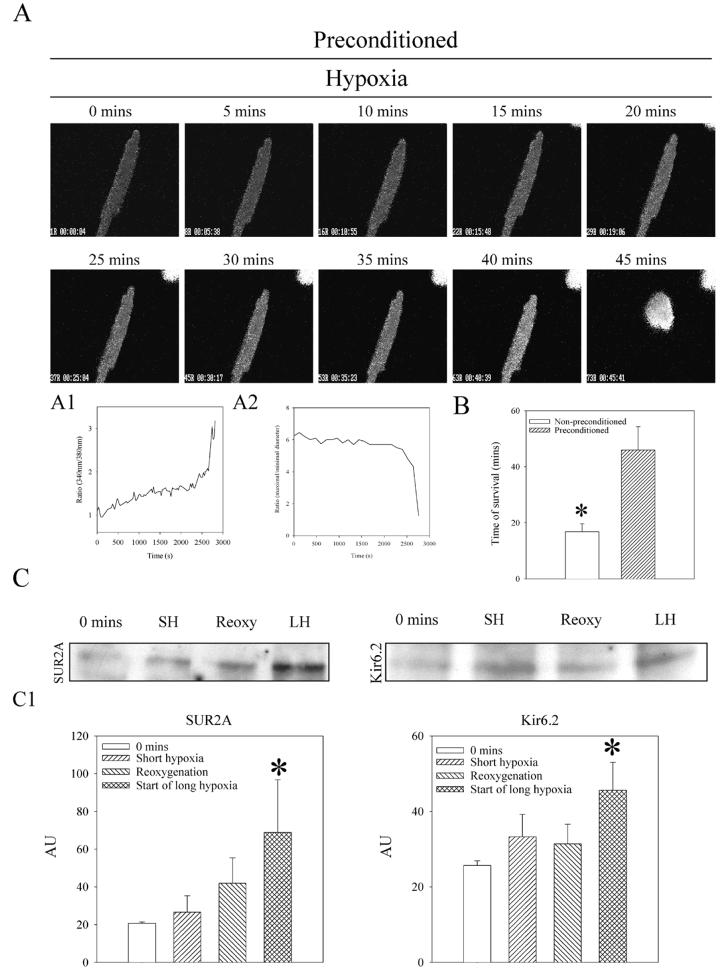
When beating, cardiomyocytes were exposed to an episode of 5-min-long hypoxia/5-min-long reoxygenation (preconditioning) before long-lasting hypoxia. The average time of survival was significantly increased to 46.0 ± 8.3 min (P=0.001 when compared with hypoxia alone, n=10; Fig. 2A, 2B). At the same time, immunoprecipitation followed by Western blotting demonstrated that levels of both subunits were increased specifically at the time point that followed the hypoxia/reoxygenation episode, that is, at the beginning of long-lasting hypoxia (SUR2A: under control conditions, band intensity was 20.7±0.7 AU [arbitrary units] and 68.8±28.0 AU at the beginning of long-lasting hypoxia, n=7, P≤0.001; Kir6.2: under control conditions band intensity was 25.7±1.2 AU and 45.6±7.4 AU at the beginning of long-lasting hypoxia, n=5, P=0.002; Fig. 2C)
Figure 2. Preconditioning significantly delays hypoxia-induced death of cardiomyocytes and increases the level of sarcolemmal KATP channel protein complex.
A) Epifluorescent images of Fura-2-loaded preconditioned cardiomyocytes exposed to hypoxia. Magnification was ×40. Time course of Fura-2 ratio (A1) and cell diameters (A2) corresponding to experiment in A. B) Average survival time of nonpreconditioned and preconditioned cardiomyocytes exposed to hypoxia. Bars represent mean ± se (n=10 for each), *P < 0.01. C) Western blot with anti-Kir6.2 and anti-SUR2A of anti-SUR2A and anti-Kir6.2 immunoprecipitate pellets from membrane fractions from cardiomyocytes collected at depicted time points. C1) Graphs corresponding to C. Each bar represents mean ± se (n=5–7). *P < 0.01.
Preconditioning-induced increase in sarcolemmal KATP channels can be visualized and is synchronized with the channels opening
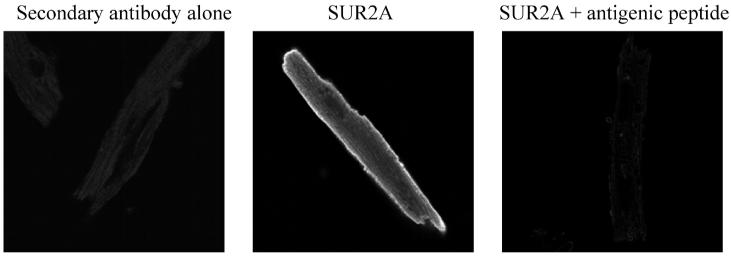
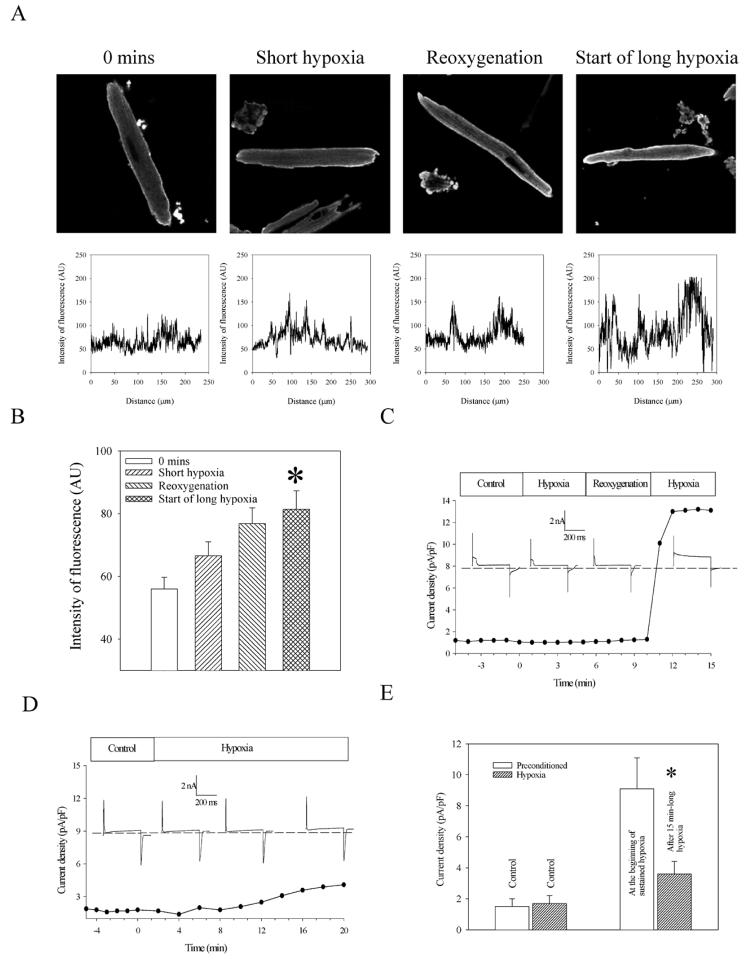
To visualize sarcolemmal KATP channel, we used laser confocal microscopy and anti-SUR2A antibody labeled with anti-sheep fluorescein. When cardiomyocytes were treated with secondary antibody alone, no fluorescence was observed (Fig. 3). In contrast, cardiomyocytes treated with anti-SUR2A antibody labeled with anti-sheep fluorescein emitted fluorescence predominantly from sarcolemma (Fig. 3), and this fluorescence was blocked with the antigenic peptide (Fig. 3). Measurement of intensity of sarcolemma fluorescence revealed a similar pattern to one that was obtained with Western blotting; the level of SUR2A subunit was significantly increased at the time point following the hypoxia/reoxygenation episode, that is, at the beginning of long-lasting hypoxia (under control conditions, fluorescence intensity was 56.0±3.7 AU and 81.4±5.9 AU at the beginning of long-lasting hypoxia, n=15–17, P=0.0008; Fig. 4A, 4B).
Figure 3. Anti-SUR2A antibody.
Original images of cardiomyocytes stained with either secondary antibody alone or anti-SUR2A antibody (without and with antigenic peptide) labeled with fluorescein as indicated.
Figure 4. Preconditioning induces trafficking and opening of sarcolemmal KATP channels in cardiomyocytes.
A) Original images and corresponding graphs of cardiomyocytes stained with anti-SUR2A antibody labeled with fluorescein and collected at depicted stages of experimental protocol. Graphs depict intensity of fluorescence drown against distance points in sarcolemma (each graph corresponds to the image above). B) Graph corresponding to A. Each bar represents mean ± se (n=15–17). *P < 0.01. C) Line and scatter graph: Time course of whole cell current density at membrane potential of +80 mV in a cardiomyocyte under control conditions, during preconditioning (5-min-long hypoxia/5-min-long reoxygenation and in first 5 min of long-lasting hypoxia). Insets: Typical whole cell currents at membrane potential +80 mV during different stages of preconditioning. The membrane potential was held at −40 mV, and the current was evoked by a 400 ms current step (to +80mV). Dotted line represents zero current line. D) Line and scatter graph: Time course of whole cell current density at membrane potential of +80 mV in a cardiomyocyte under control conditions and hypoxia. Insets: Typical whole cell currents at membrane potential +80 mV under control conditions and during hypoxia. The membrane potential was held at −40 mV, and the current was evoked by a 400 ms current step (to +80mV). Dotted line represents zero current line. E) Average current density of nonpreconditioned and preconditioned cardiomyocytes exposed to hypoxia. Bars represent mean ± se (n= 5–6), *P < 0.05 when compared with preconditioned cells.
We applied perforated patch clamp electrophysiology to determine when, in relation to the observed increase in the number of sarcolemmal KATP channels, KATP channels become active. At rest, sarcolemmal KATP channels are closed; the whole K+ current flows through IK1 channels, and the opening of sarcolemma KATP channels is manifested as outward K+ current particularly increased at positive membrane potential. Therefore, we measured K+ current at 80 mV knowing that the activation of KATP channels would be most visible at this voltage. During stages of preconditioning (brief hypoxia and reoxygenation), no changes in steady-state current occurred and the current density varied between 1 and 1.3 pA/pF (Fig. 4C). However, at the beginning of long-lasting hypoxia, current rose from 1.3 pA/pF to 10.1 pA/pF in ∼60 s and then to 13.1 pA/pF in the next 60 s remaining at this level until the end of the experiment (Fig. 4C). On average, current density was 1.5 ± 0.5 pA/pF under control conditions and 9.1 ± 2.1 pA/pF at the beginning of sustained hypoxia (P=0.009, n=5, Fig. 4E). However, when cells were exposed to hypoxia without preconditioning, current density was 3.6 ± 0.9 pA/pF after 15 min of hypoxia, which was significantly lower compared with current densities in preconditioned cells (Fig. 4D, 4E; P=0.028, n=6).
Inhibition of the opening of sarcolemmal KATP channels during sustained hypoxia abolishes preconditioning
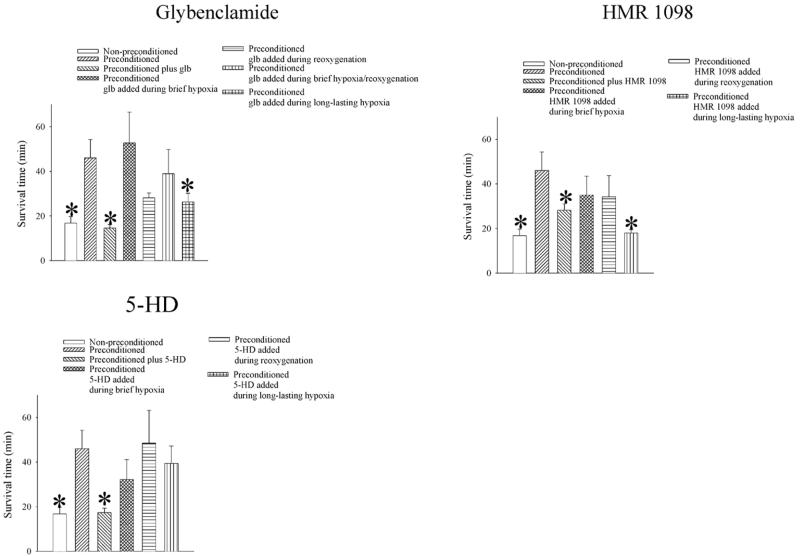
Patch clamp electrophysiology suggested that the opening of these channels takes place following an episode of brief hypoxia/reoxygenation. Therefore, we tested whether inhibition of the activity of sarcolemmal KATP channels at this particular stage, as well as any other, of the experiment would abolish preconditioning. When glybenclamide (10 μM), a prototype antagonist of KATP channels, was present throughout the experiment, the beneficial effect of preconditioning was abolished (survival time decreased from 46.0±8.3 min, n=10, to 14.6±1.5 min, P=0.02, n=5, Fig. 5). The survival time was also significantly shorter when glybenclamide was present during long-lasting hypoxia (in this group, survival time was 26.4±3.8 min, P=0.03, n=11; Fig. 5) but not when it was present during any other stage of experimental protocol (Fig. 5).
Figure 5. Inhibition of KATP channel activity blocks preconditioning.
Average survival time of nonpreconditioned and preconditioned cardiomyocytes exposed to hypoxia when different antagonists of KATP channels (glybenclamide, HMR 1098, and 5-HD) were present throughout the experiments or only during brief hypoxia, only during reoxygenation, or only during long-lasting hypoxia. Bars represent mean ± se (n= 5–11), *P < 0.05 when compared with preconditioned cells.
Similar results were obtained with a selective antagonist of sarcolemmal KATP channels, HMR 1098. When cells were exposed to sustained hypoxia in the presence of HMR 1098 (30 μM), survival time was significantly decreased compared with those obtained in preconditioned cells in the absence of HMR 1098 (survival time was 18.0±1.5 min, n=6, P=0.005 when compared with preconditioning alone; Fig. 5). HMR 1098 (30 μM) did not modify cellular resistance afforded by preconditioning when present during brief hypoxia or brief reoxygenation (survival times were 35.0±14.7 and 34.2±9.4 min when HMR1098 was applied during brief hypoxia or reoxygenation, respectively, P>0.05 when compared with preconditioning alone, n=5 for each; Fig. 4D). When 5-HD (50 μM), an antagonist of putative mitochondrial KATP channels, was present throughout the whole experimental protocol, the beneficial effect of preconditioning was abolished (survival time decreased from 46.0±8.3 min, n=10, to 17.4±1.9 min, P=0.03, n=5; Fig. 5). However, when cells were preconditioned in the absence of 5-HD and then exposed to hypoxia in the presence of 5-HD (50 μM), survival time was not significantly different compared with that obtained in preconditioned cells in the absence of 5-HD (survival time was 39.4±7.8 min, n=5, P=0.622 when compared with preconditioning alone; Fig. 5), and it was significantly different compared with hypoxia alone (P=0.012; Fig. 5). In addition, 5-HD (50 μM) did not modify cellular resistance afforded by preconditioning when present during brief hypoxia or reoxygenation (Fig. 5).
Inhibition of sarcolemmal KATP channel trafficking blocks preconditioning
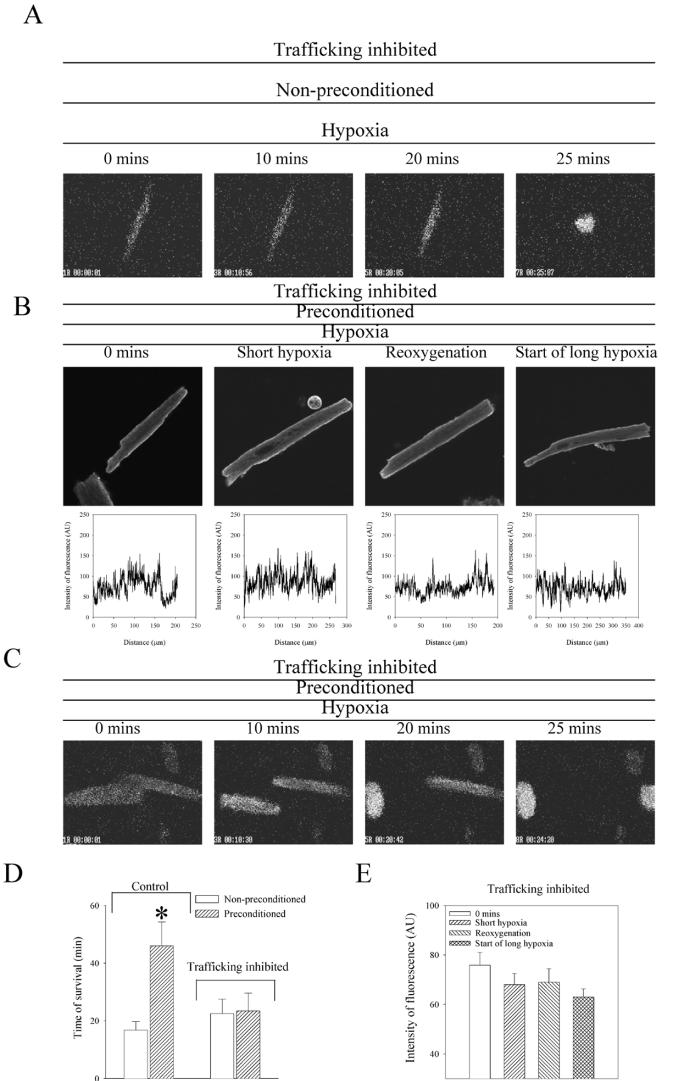
Based on previous studies (14-16), it was expected that the increase in number of sarcolemmal KATP channels would lead to cellular phenotype more resistant to a metabolic stress. To test this hypothesis, we treated cardiomyocytes with a cocktail of drugs known to inhibit protein trafficking (5 μg/ml brefeldin A, 5 μM colchicine, and 20 μM nocodazole; 21). This treatment did not affect the general sensitivity of cardiomyocytes to hypoxia, as pretreated cells survived for 22.5±5.0 min (n=4; Fig. 6A, 6D), which was not significantly different from cells untreated with inhibitory cocktail (16.8±2.8 min, n=10, P=0.32), suggesting that this compound does not increase cardiac sensitivity to hypoxia per se. In treated cardiomyocytes, preconditioning did not increase the number of KATP channels in sarcolemma (Fig. 6B, 6E). Under these conditions, an episode of brief hypoxia/reoxygenation did not promote survival of cardiomyocytes exposed to hypoxia (Fig. 6C, 6D). Treated preconditioned cells survived only 23.4±6.2 min (n=6), which was not significantly different from cells exposed to hypoxia alone, both treated (P=0.92) and untreated (P=0.29) with the protein trafficking inhibitory cocktail.
Figure 6. Prevention of sarcolemmal KATP channels trafficking inhibits preconditioning.
A) Epifluorescent images of Fura-2-loaded nonpreconditioned cardiomyocytes pretreated with protein trafficking inhibitory cocktail (5 μg/ml brefeldin A, 5 μM colchicine, and 20 μM nocodazole) exposed to hypoxia. B) Original images and corresponding graphs of cardiomyocytes pretreated with protein trafficking inhibitory cocktail stained with anti-SUR2A antibody labeled with fluorescein and collected at depicted stages of experiments. Graphs depict intensity of fluorescence drown against distance points in sarcolemma (each graph corresponds to the image above). C) Epifluorescent images of Fura-2-loaded preconditioned cardiomyocytes pretreated with protein trafficking inhibitory cocktail exposed to hypoxia. D) The average survival time ± se of nonpreconditioned and preconditioned cardiomyocytes exposed to hypoxia under control conditions and when pretreated with protein trafficking inhibitory cocktail (n=4–10). *P < 0.01. E) The average intensity of fluorescence per unit of sarcolemma length ± se for cardiomyocytes pretreated with protein trafficking inhibitory cocktail collected at depicted stages of experimental protocol (n=13–19).
DISCUSSION
In the present study, we have demonstrated that stimulation of sarcolemmal KATP channel trafficking and channel activation by an episode of brief hypoxia/reoxygenation are essential for preconditioning in adult beating cardiomyocytes. This is the first account of an ion channel trafficking involved in preconditioning and cardioprotection.
The mechanism underlying preconditioning is seemingly complex and still a matter of vigorous debate. Here, we have applied a model suitable to study intracellular signaling in preconditioning that utilizes adult beating cardiomyocytes, a pure myocardial preparation free of confounding neuronal, vascular, and humoral influences (22). In this study, adult beating cardiomyocytes survived ∼17 min in hypoxia. This survival time was similar to those previously reported for this experimental model (18, 19). For almost a decade, it has been known that an episode of hypoxia/reoxygenation can induce preconditioning in the heart (23). In the present study, we have shown that a single episode of brief hypoxia/reoxygenation can increase the survival time of isolated beating cardiomyocytes in hypoxia, confirming the efficiency of preconditioning in cardioprotection (1, 23). This result also suggests that intercellular communications as well as neuronal, vascular, and humoral factors—all elements lacking in our model—are not required to induce preconditioning and that the mechanism of preconditioning is largely confined to intracellular signaling.
So far, several lines of evidence suggest that the sarcolemmal KATP channels are cardioprotective: 1) Coexpression of Kir6.2 with SUR2A (in combination with KATP channel opener or muscle form of lactate dehydrogenase) confers resistance against metabolic stress in otherwise stress-sensitive cells (24, 25); 2) in mice with genetically disrupted sarcolemmal KATP channel, the heart is more susceptible to metabolic stress (26); 3) KATP channel opener-mediated protection against ischemia is associated with the effect on cardiac membrane potential and with measurable sarcolemmal KATP channels opening (8); 4) an increase in the number of sarcolemmal KATP channels increases cardiac resistance to metabolic stress, and this is blocked by HMR 1098, a selective antagonist of sarcolemmal KATP channels (14-16); and 5) ischemic preconditioning cannot be conferred in transgenic animals lacking sarcolemmal KATP channels (8).
In addition, researchers have recently discovered that trafficking of sarcolemmal KATP channels is regulated by PKC and adenosine, compounds known to be involved in cardioprotective signaling (17). In the present study, using both immunoprecipitation/Western blotting and immunofluorescence/laser confocal microscopy, we have found that preconditioning induced an increase in the number of sarcolemmal KATP channels, which was not observed when cardiomyocytes were exposed to hypoxia in the absence of preconditioning. Bearing in mind that PKC is involved in preconditioning, at first sight, it may seem that the results obtained were in disagreement with Hu et al. (17), who reported PKC-mediated inhibition of KATP channel trafficking. However, it has been reported that the activation as well as inhibition of certain subtypes of PKC may mediate cardioprotection and preconditioning (27). Thus, bearing in mind the complexity of signaling pathways in preconditioning that involve inhibition and activation of different subtypes of PKC, the obtained results are in agreement with the suggestion that PKC may regulate trafficking of KATP channels and that recruitment of sarcolemmal KATP channels contributes to the cardioprotection afforded by preconditioning.
In the present study, preconditioning induced vigorous opening of sarcolemmal KATP channels, and the magnitude of channel activation was significantly higher compared with those in the absence of preconditioning. The observed preconditioning-induced increase in number of sarcolemmal KATP channels had a similar time course as the channel activation. In fact, the opening of the channels was observed at the same time as the increase in the channel number. More than a decade ago, researchers suggested that the activation of sarcolemmal KATP channels mediates preconditioning (4). However, this notion was challenged when it was determined that some KATP channel-opening drugs may affect the membrane potential of the mitochondria, which was ascribed to the effect on putative mitochondrial KATP channels (6). Putative mitochondrial KATP channels were described for the first time in 1991 (5), and it was suggested that the opening of these channels, rather then sarcolemmal KATP channels, may mediate cardioprotection (7). This conclusion was largely based on the use of diazoxide and 5-HD as a selective opener and antagonist of mitochondrial KATP channels, respectively (6, 7). However, more recent studies suggested that diazoxide and 5-HD may have effects on mitochondria that are not associated with changes in mitochondrial membrane permeability (28-32). Here, 5-HD, when present throughout the whole experiment, inhibited preconditioning as has been reported in previous studies (for review, see ref 2). However, when we applied 5-HD during specific stages of the experimental protocol, it had no effect on preconditioning-induced cardioprotection. These results may be interpreted in one of two ways: 1) Putative mitochondrial KATP channels are involved in preconditioning, or 2) 5-HD blocks preconditioning in a KATP channel-independent manner. At present, as the structure of putative mitochondrial KATP channels is still controversial, and sufficiently selective activators and inhibitors of this channel are not available, it is not possible to come up with a definite conclusion regarding the involvement of mitochondrial KATP channels in our cellular model of preconditioning.
However, glybenclamide and HMR 1098, “nonselective” and selective antagonists of sarcolemmal KATP channels, respectively (33), abolished preconditioning when applied throughout the whole experiment or only during sustained hypoxia. These results strongly suggest that the activation of the sarcolemmal KATP channels at the beginning of sustained hypoxia is crucial for preconditioning-induced cardioprotection. This confirms the notion that the opening of these channels is essential for preconditioning, and the timing of the channel opening would suggest that these channels serve primarily as end-effectors rather then triggers of preconditioning. Although a recent study suggested that the opening of sarcolemmal KATP channels is involved in preconditioning (8), this is the first report to determine the exact stage of preconditioning when activity of KATP channels mediates cardioprotection.
It has been recently reported that the number of expressed sarcolemmal KATP channels regulates the resistance to metabolic stress in cardiomyocytes (14-16, 26). Bearing this in mind, it is reasonable to expect that an acute increase in the number of sarcolemmal KATP channels would increase the cellular resistance to hypoxia. In support of this expectation is our result that inhibition of increase in number of sarcolemmal KATP channels abolished preconditioning. This shows that recruitment of KATP channels to sarcolemma is essential for preconditioning-induced cardioprotection. However, the cocktail of drugs we used to block KATP channel trafficking did not affect the susceptibility of nonpreconditioned cells to hypoxia, suggesting that the inhibition of preconditioning was not due to a nonspecific increase in cellular sensitivity to hypoxia.
Why cardiomyocytes expressing more sarcolemmal KATP channels are more resistant to ischaemia/hypoxia has yet to be determined (14-16). One possible explanation of this phenomenon would be that a higher number of functionally active KATP channels would have a more pronounced effect on cardiac membrane excitability. However, excessive KATP channel opening might have deleterious effects on the outcome of ischemia (34, 35), and it is not certain, as it might look on first sight, that a KATP channel-mediated increase in whole cell K+ current underlies cardioprotection afforded by an increased number of sarcolemmal KATP channels. Even now, after many studies, the mechanism of cardioprotection by sarcolemmal KATP channel opening is disputed. Probably the most common view is that the activation of K+ channels leads to shortening of the action membrane potential which, in turn, decreases the Ca2+ influx, consequent hypercontracture, and cell death (8, 36). However, the protective effect of the opening of sarcolemmal KATP channels is also seen when these channels are expressed and activated in cells that do not generate action membrane potential (24, 25), including quiescent cardiomyocytes (37). This suggests a possibility that the mechanism of cardioprotection afforded by sarcolemmal KATP channels may be, at least in part, dissociated from the effect on the action membrane potential (which is a long-standing and controversial issue; see ref 38) or even from the function of these proteins as ion channels.
It has been recently proposed that sarcolemmal KATP channels are composed in vivo, in cardiomyocytes, of more proteins than Kir6.2/SUR2A; that is, it seems that adenylate kinase (AK), creatine kinase (CK), and lactate dehydrogenase (LDH) are also parts of the sarcolemmal KATP channel protein complex (25, 39, 40). Therefore, an increase in the number of sarcolemmal KATP channels also means an increase in sarcolemmal presence of crucial enzymes for cellular metabolism and energy production, which may promote cellular survival under conditions of metabolic stress. In support of this idea are recent reports suggesting that sarcolemmal KATP channels are likely to have functions in addition to their channel property, such as glucose transport (41), which could contribute to cardioprotection evoked by preconditioning. In this respect, regulation of not only channel activity but also channel numbers seems to be an important part of cardioprotective signaling in preconditioning.
In conclusion, we have demonstrated that both sarcolemmal KATP channel activation and channel trafficking are essential for preconditioning-induced cardioprotection. The importance of the channel trafficking in preconditioning has not been observed before, and findings from this study may provide the basis for further research that would lead to a better understanding of endogenous cardioprotective mechanisms and their exploitation in therapy of ischemic heart diseases.
ACKNOWLEDGMENTS
We thank A. M. Davies for helping us to set up the experimental model of preconditioning. We thank Aventis Pharma (Frankfurt, Germany) for HMR 1098. This research was supported by grants from Anonymous Trust, BBSRC, British Heart Foundation, MRC, TENOVUS-Scotland, and the Wellcome Trust.
REFERENCES
- 1.Murray CE, Jennings RB, Reimer KA. Precoditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol. Rev. 2003;83:1113–11151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 3.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 4.Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ. Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Sato T, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, Sasaki N, Seharaseyon J, O'Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal K(ATP) channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seharaseyon J, Ohler A, Sasaki N, Fraser H, Sato T, Johns DC, O'Rourke B, Marban E. Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral Kir gene transfer. J. Mol. Cell. Cardiol. 2000;32:1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- 10.Singh H, Hudman D, Lawrence CL, Rainbow RD, Lodwick D, Norman RI. Distribution of Kir6.0 and SUR2 ATP-sensitive potassium channel subunits in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2003;35:445–459. doi: 10.1016/s0022-2828(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 11.Lacza Z, Snipes JA, Miller AW, Szabo C, Grover G, Busija DW. Heart mitochondria contain functional ATP-dependent K+ channels. J. Mol. Cell. Cardiol. 2003;35:1339–1347. doi: 10.1016/s0022-2828(03)00249-9. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 13.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 14.Ranki HJ, Budas GR, Crawford RM, Jovanovic A. Gender-specific difference in cardiac ATP-sensitive K+ channels. J. Am. Coll. Cardiol. 2001;38:906–915. doi: 10.1016/s0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- 15.Ranki HJ, Budas GR, Crawford RM, Davies AM, Jovanovic A. 17β-estradiol regulates expression of KATP channels in heart-derived H9c2 cells. J. Am. Coll. Cardiol. 2002;40:367–374. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- 16.Crawford RM, Jovanovic S, Budas GR, Davies AM, Lad H, Wenger RH, Robertson KA, Roy DJ, Ranki HJ, Jovanovic A. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channels. J. Biol. Chem. 2003;278:31444–31455. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu K, Huang CS, Jan YN, Jan LY. ATP-sensitive potassium channel traffic regulation by adenosine and protein kinase C. Neuron. 2003;38:417–432. doi: 10.1016/s0896-6273(03)00256-3. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher MM, Allshire AP. Failure of magnesium to protect isolated cardiomyocytes from effects of hypoxia or metabolic poisoning. Clin. Cardiol. 2000;23:530–534. doi: 10.1002/clc.4960230712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton V, Kahn RC, Lucocq JM, Gray GA, et al. Deficiency of PDK1 in heart results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jovanovic S, Crawford RM, Ranki HJ, Jovanovic A. Large conductance Ca2+-activated K+ channels sense acute changes in oxygen tension in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003;28:363–372. doi: 10.1165/rcmb.2002-0101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lhuillier L, Dryer SE. Developmental regulation of neuronal K(Ca) channels by TGFbeta1: an essential role for PI3 kinase signaling and membrane insertion. J. Neurophysiol. 2002;88:954–964. doi: 10.1152/jn.2002.88.2.954. [DOI] [PubMed] [Google Scholar]
- 22.Marber M. Ischemic Preconditioning in Isolated Cells. Circ. Res. 2000;86:926–931. doi: 10.1161/01.res.86.9.926. [DOI] [PubMed] [Google Scholar]
- 23.Cohen MV, Walsh RS, Goto M, Downey JM. Hypoxia preconditions rabbit myocardium via adenosine and catecholamine release. J. Mol. Cell. Cardiol. 1995;27:1527–1534. doi: 10.1016/s0022-2828(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 24.Jovanovic A, Jovanovic S, Lorenz E, Terzic A. Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance towards chemical hypoxia-reoxygenation injury. Circulation. 1998;98:1548–1555. doi: 10.1161/01.cir.98.15.1548. [DOI] [PubMed] [Google Scholar]
- 25.Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, Jovanovic A. M-LDH serves as a sarcolemmal KATP channel subunit essential for cell protection against ischemia. EMBO J. 2002;21:3936–3948. doi: 10.1093/emboj/cdf388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, et al. Kir6.2 is required for adaptation to stress. Proc. Natl. Acad. Sci. USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch. Biochem. Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 28.Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial K(ATP) channel change the function of mitochondria in situ? J. Biol. Chem. 2000;275:37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- 29.Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H531–H539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- 30.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J. Physiol. (Lond.) 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J. Physiol. (Lond.) 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- 33.Gogelein H, Ruetten H, Albus U, Englert HC, Busch AE. Effects of the cardioselective KATP channel blocker HMR 1098 on cardiac function in isolated perfused working rat hearts and in anesthetized rats during ischemia and reperfusion. Naunyn Schmiedebergs Arch. Pharmacol. 2001;364:33–41. doi: 10.1007/s002100000391. [DOI] [PubMed] [Google Scholar]
- 34.Gasser RNA, Vaughan-Jones RD. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J. Physiol. (Lond.) 1990;431:713–741. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilde AAM, Janse MJ. Electrophysiological effects of ATP sensitive potassium channel modulation: implications for arrhythmogenesis. Cardiovasc. Res. 1994;28:16–24. doi: 10.1093/cvr/28.1.16. [DOI] [PubMed] [Google Scholar]
- 36.Lascano EC, Negroni JA, del Valle HF. Ischemic shortening of action potential duration as a result of KATP channel opening attenuates myocardial stunning by reducing calcium influx. Mol. Cell. Biochem. 2002;236:53–61. doi: 10.1023/a:1016198011919. [DOI] [PubMed] [Google Scholar]
- 37.Jovanovic S, Jovanovic A. Pinacidil prevents membrane depolarisation and intracellular Ca2+ loading in single cardiomyocytes exposed to severe metabolic stress. Int. J. Mol. Med. 2001;7:639–643. doi: 10.3892/ijmm.7.6.639. [DOI] [PubMed] [Google Scholar]
- 38.Hamada K, Yamazaki J, Nagao T. Shortening of action potential duration is not prerequisite for cardiac protection by ischemic preconditioning or a KATP channel opener. J. Mol. Cell. Cardiol. 1998;30:1369–1379. doi: 10.1006/jmcc.1998.0701. [DOI] [PubMed] [Google Scholar]
- 39.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc. Natl. Acad. Sci. USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crawford RM, Ranki HJ, Booting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, Minokoshi Y, Renaud JM, Seino S. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1178–E1184. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]