Abstract
Brief periods of ischemia and reperfusion that precede sustained ischemia lead to a reduction in myocardial infarct size. This phenomenon, known as ischemic preconditioning, is mediated by signaling pathway(s) that are yet to be fully defined. 3′-phosphoinositide-dependent kinase-1 (PDK1) has been implicated in numerous cellular processes. However, the involvement of PDK1 in preconditioning has yet to be elucidated. Studying PDK1 is not as straightforward as it is for the majority of kinases, due to the lack of a specific inhibitor of PDK1. Therefore, we have taken advantage of PDK1 hypomorphic mutant mice with reduced expression of PDK1 to study the role of PDK1 in preconditioning. Whole heart and single cell models of preconditioning demonstrated that the hearts and cardiac cells from PDK1 hypomorphic mice could not be preconditioned. The cardioprotective effect of PDK1 was not related to the effect that preconditioning has on sarcolemmal membrane action potential as revealed by di-8-ANEPPS, a sarcolemmal-potential sensitive dye, and laser confocal microscopy. In contrast, experiments with JC-1, a mitochondrial membrane potential-sensitive dye, has demonstrated that intact PDK1 levels were required for preconditioning-mediated regulation of mitochondrial membrane potential. Western blotting combined with functional experiments have shown that intact PDK1 levels were required for preconditioning-induced phosphorylation of protein kinase B (PKB), glycogen synthase kinase-3β (GSK-3β), and cardioprotection. We conclude that PDK1 mediates preconditioning in the heart by regulating activating PKB-GSK-3β to regulate mitochondrial but not sarcolemmal membrane potential. 3′phosphoinositide-dependent kinase-1 (PDK1) is essential for ischemic preconditioning of the myocardium.
Keywords: GSK-3, hypoxia, ischemia, mitochondria, cardioprotection
The recently discovered 3′-phosphoinositide-dependent kinase-1 (PDK1) is a serine/threonine protein kinase that phosphorylates several members of the automatic gain control (AGC) family of protein kinases, including isoforms of protein kinase B (PKB)/Akt, p70 ribosomal S6 kinase (S6K), serum- and glucocorticoid-induced protein kinase (SGK), and protein kinase C (PKC) (1). So far, it has been shown that the PDK1/automatic gain control kinase-signaling pathway regulates diverse cellular processes, including those relevant to cell survival, proliferation, and metabolic responses to insulin (2). Inadequate regulation of AGC kinase members could contribute to many diseases. A deficiency in the activation of AGC kinases may be a primary cause of the insulin-resistant form of diabetes (3), neuronal cell death following a stroke (4), and heart failure (5). However, hyperactivation of this pathway has been implicated in inducing cardiac hypertrophy (6) and promoting the survival and proliferation of a significant number of cancers (7).
In 1986, Murry and colleagues discovered that brief periods of blood vessel occlusion and reperfusion administered prior to a sustained ischemic episode led to a reduction in infarct size (8). This cardioprotective phenomenon (now called ischemic preconditioning) enhances the survival of cardiac cells under conditions that induce myocardial infarction. This and subsequent studies have indicated that brief ischemia or hypoxia are likely to switch on intracellular signaling pathways that ultimately result in an increased cellular tolerance to metabolic stress (9).
The underlying mechanism of preconditioning is still a matter of intense research and vigorous debate. Some of the AGC enzymes, in particular PKC and PKB, have been suggested to mediate ischemic preconditioning in the heart (10-13). However, the involvement of PDK1 in preconditioning has yet to be elucidated. Studying PDK1 is not as straightforward as it is for the majority of kinases, due to the lack of a specific inhibitor of PDK1. Furthermore, a total deficiency of PDK1 is incompatible with life, while a targeted deficiency of PDK1 in the heart results in heart failure and sudden cardiac death in first few weeks of life (5, 14). Recently, PDK1 hypomorphic mice (PDK1fl/−) were generated, and in these mice a neomycin resistance gene was inserted into an intron of the PDK1 gene, which resulted in an 80–90% reduction of PDK1 expression in all tissues including the heart (14). These animals are healthy and fertile and are an ideal model to study the involvement of PDK1 in cardioprotective signaling.
Therefore, we have taken advantage of PDK1 hypomorphic mutant mice to study the role of PDK1 in preconditioning. We report that the PDK1/PKB/GSK-3β signaling cascade underlies the cytoprotective outcome of preconditioning through regulation of mitochondrial, but not sarcolemmal, membrane potential.
MATERIALS AND METHODS
PDK1fl/+ and PDK1fl/− mice. All experiments have been done on PDK1fl/+ (littermate controls and mice expressing normal levels/activity of PDK1) and PDK1fl/− (mice expressing 20% of normal PDK1 levels/activity in the heart). Generation, breeding, and genotyping of these mice have previously been described in detail (14).
Ischemia-reperfusion injury and measurement of myocardial infarction size
Mice were killed by cervical dislocation (according to UK Home Office procedures), and the hearts rapidly removed and placed in ice-cold Tyrode's solution at 4°C. The aorta was then cannulated and secured using 4–0 silk suture and the hearts were attached to a Langendorff perfusion apparatus. Hearts were perfused at a constant flow rate of 5 ml/min at 37°C with oxygenated (95% O2, 5% CO2) Tyrode's solution [in mM: NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose (Glc) 5.5, HEPES-NaOH 5.5, pH 7.4] for a stabilization period of 70 or 30 min for nonpreconditioned and preconditioned hearts, respectively. Preconditioning was induced by 4 cycles of 5 min ischemia (achieved by placing the heart into Tyrode's solution degassed with 100% Ar at 37°C and switching off perfusion) and 5 min reperfusion. After the stabilization period or preconditioning protocol, the heart was subjected to 30 min of global ischemia at 37°C followed by 30 min of reperfusion. After reperfusion, hearts were snap-frozen in liquid nitrogen and stored at −80°C. Frozen hearts were divided into ∼4–5 transverse sections, which were weighed before staining for 1 h in 10% triphenyltetrazolium chloride (TTC) in phosphate buffer saline (PBS; both Sigma-Aldrich, Dorset, UK) at 37°C. The stain was fixed in 4% Paraformaldehyde (Sigma-Aldrich) for 30 min, after which the tissue was photographed and the area of infarcted tissue measured using Zeiss (Gottingem, Germany) LSM Image Analysis software. Infarct sizes were calculated as (A1×W1) + (A2×W2) + (A3×W3) + (A4×W4) + (A5×W5), where A is the area of infarct for the slice and W is the wt of the respective section (15).
Western blot analysis
For Western blot analysis, hearts were perfused: 1) for 80 min (control); 2) for 70 min and then for 10 min in 25 mU/ml insulin (Sigma) as a positive control; 3) 60 min and then 20 min in ischemia (20-min-long ischemia in nonpreconditioned heart); 4) 30 min plus four cycles of 5 min ischemia/5 min reperfusion (time 0 in preconditioning); or 5) 30 min plus four cycles of 5 min ischemia/5 min reperfusion plus 20 min in ischemia (20-min-long ischemia in preconditioned heart) were snap-frozen and homogenized in lysis buffer (50 mM Tris–HCl, pH 7.5; 1 mM EDTA; 1 mM EGTA; 1% (w/v) Triton X-100; 0.1% (v/v) β-mercaptoethanol; 1 mM sodium orthovanadate; 50 mM sodium fluoride; 5 mM sodium pyrophosphate; 1 μM microcystin-LR; and one tablet of “complete” proteinase inhibitor per 50 ml of buffer). A 10-fold mass excess of ice-cold lysis buffer was added to the powdered tissue, briefly vortexed, and then centrifuged at 4°C for 10 min at 13000 g to remove insoluble material. The supernatant was snap-frozen in aliquots in liquid nitrogen and stored at −80°C. Protein concentration was determined by Bradford Assay. From each sample, 10 μg of protein was subjected to SDS/PAGE and transferred to nitrocellulose. For all blots, the nitrocellulose membranes were incubated at 4°C for 16 h using the antibodies against PKB and GSK-3 (1000-fold dilution). The PKB total antibody (Ab), GSK-3 total Ab, phospho-PKB (Thr308), and Phospho-GSK-3 (Ser-9/21) antibodies were purchased from Cell Signaling Technology (Hitchin, UK). The blots were incubated in 50 mM Tris/HCl, pH 7.5; 0.15 M NaCl; and 0.2% (by vol) Tween containing 5% (by mass) skimmed milk. Detection of total or phosphorylated protein was performed using horse radish peroxidase conjugated secondary antibodies (Pierce, Rockford, IL, USA) and enhanced chemiluminescence (ECL) reagent (Upstate, Dundee, UK). The band intensities were analyzed using Quantiscan software (Cambridge, UK) (14).
Isolation of cardiomyocytes
Ventricular cardiomyocytes were dissociated from PDK1fl/+ and PDK1fl/− mice using an established enzymatic procedure (5, 15). In brief, hearts were retrogradely perfused (at 37°C) with medium 199, followed by Ca2+-EGTA-buffered low-Ca2+ medium (pCa=7), and finally low-Ca2+ medium containing Pronase E (8 mg per 100 ml), proteinase K (1.7 mg per 100 ml), bovine albumin (0.1 g per 100 ml, fraction V), and 200 μM CaCl2. Ventricles were cut into fragments in the low-Ca2+ medium enriched with 200 μM CaCl2. Cells were isolated by stirring the tissue (at 37°C) in a solution containing Pronase E and proteinase K supplemented with collagenase (5 mg per 10 ml). The first aliquot was removed, filtered through a nylon sieve, centrifuged for 60 s (at 300–400 rpm), and washed. Remaining tissue fragments were re-exposed to collagenase, and isolation continued for 2–3 such cycles.
Experimental protocol of cellular hypoxia and preconditioning
Hypoxia of isolated cardiomyocytes has been performed as described previously (5, 15, 16). Thus, cardiomyocytes were placed into Tyrode's solution (in mM: NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, Glc 5.5, HEPES-NaOH 5.5, pH 7.4), plated out on glass coverslips, and paced to beat by field stimulation (parameters of the stimulation: 5–20 mV depending on cellular threshold, 5 ms, 1 Hz). Beating cardiomyocytes were perfused with Tyrode solution at a rate of 3 ml/min and, under these conditions, the partial pressure of O2 (PO2) in perfusate was 140 mmHg (5). To induce preconditioning we have exposed cardiomyocytes to a single episode of 5-min-long hypoxia/5-min-long reoxygenation followed by hypoxia (termed here as long-lasting hypoxia) that was maintained until cell death. To induce hypoxia, Tyrode solution was bubbled with 100% argon (PO2=20 mm Hg). Cells exposed to this experimental protocol were used for laser confocal experiments.
Laser confocal microscopy
Cell morphology, size parameters, and sarcolemmal/mitochondrial membrane potential were monitored in cells exposed to the experimental protocol described in the previous section. To measure sarcolemmal membrane potential, cells were loaded with di-8-ANEPPS according to the manufacturer's instruction (Invitrogen, Paisley, UK) and the sarcolemmal membrane was imaged using laser confocal microscopy in line-scan mode (LSM-510, Zeiss). Cells were scanned under control conditions and then exposed to hypoxia with or without preconditioning. Nonpreconditioned cells were scanned every 10 min, while preconditioned cells were scanned at 4 min after the beginning of sustained hypoxia (a time point when sarcolemmal KATP channels are activated by preconditioning; ref. 16). This particular time point of 4 min was specifically chosen as we have previously determined that 4-min-long hypoxia following preconditioning activates sarcolemmal KATP channels, which induces shortening of the action membrane potential (16). The duration of action membrane potential was defined as the time to full repolarization. Fluorescence was detected/imaged at 488 nm excitation wavelength, and emission was captured at >505 nm. To measure the mitochondrial membrane potential, cells were loaded with JC-1 according to the manufacturer's instructions (Invitrogen) and continuously monitored with LSM-510. Fluorescence was imaged at 488 nm excitation wavelength, and emission was captured at 530 and 590 nm for green and red channels, respectively; the JC-1 ratio always refers to green/red ratio. The moment of cell death was defined as the point when the cell has become rounded (ratio of diameters <2).
Statistics
Data are presented as mean ± sem, with n representing the number of experiments. The difference between means where assessed using t test, χ2 test, or ANOVA followed by t test using SigmaStat program (Jandel Scientific, Chicago, IL, USA). P < 0.05 was considered statistically significant.
RESULTS
Preconditioning fails to protect PDK1fl/− mice against myocardial infarction
Ischemia-reperfusion-induced myocardial infarction in PDK1fl/+ mice that was 52.3 ± 8.2% of the area at risk zone (in this particular case this area equals to the whole heart, n=5, Fig. 1). Preconditioning (four cycles of 5 min ischemia and 5 min reperfusion) significantly decreased the size of myocardial infarction induced by ischemia-reperfusion in these mice (28.8±5.0% of area at risk zone, n=5, P = 0.04, Fig. 1). When hearts from PDK1fl/− were exposed to ischemia-reperfusion, the size of myocardial infarction was similar to those in PDK1fl/+ mice (51.5±6.3% of area at risk zone, n=5, P = 0.86. Fig. 1). However, as opposed to PDK1fl/+ mice, ischemic preconditioning was ineffective in hearts from PDK1fl/− mice (50.3±6.2% of area at risk zone, n=5, P = 0.90, Fig. 1).
Figure 1.

PDK1fl/− hearts cannot be preconditioned against ischemia. Typical photographs of myocardial slices and corresponding graphs from PDK1fl/+ and PDK1fl/− exposed to ishemia-reperfusion. Infarcted areas are pale/gray, whereas viable myocardium is dark/red. In graphs, myocardial infarct size is expressed as a percentage of area at risk zone. Each bar represent mean ± sem. *P < 0.05 (as determined by the t test).
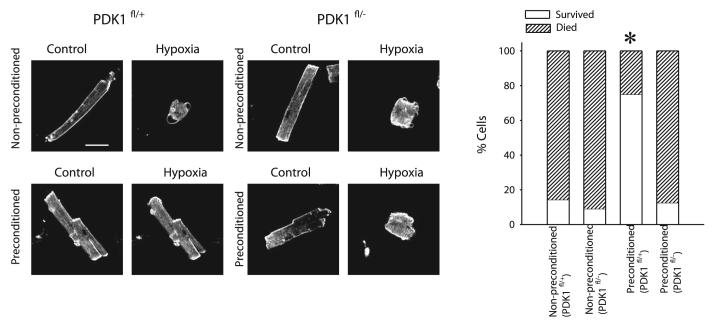
Cardiomyocytes from PDK1fl/− mice can not be preconditioned
We have previously established an experimental model of hypoxia and preconditioning that utilizes field-stimulated, adult cardiomyocytes (5, 15, 16). We used this model to test whether the failure of preconditioning to protect against myocardial infarction in PDK1fl/− hearts was due to a failure of preconditioning in PDK1fl/− cardiomyocytes. In PDK1fl/+ mice, only 1 out of 7 nonpreconditioned cells has survived 30-min-long hypoxia (Fig. 2). Similar results were obtained with PDK1fl/− cells (1 cell out 11 survived 30 min hypoxia, P=1.000, Fig. 2). An episode of 5-min-long hypoxia/5-min-long reoxygenation (preconditioning) administered prior to sustained hypoxia significantly increased the survival of cardiomyocytes from PDKfl/+ mice (6 out of 8 cardiomyocytes survived 30 min hypoxia, P=0.04, Fig. 2). In contrast, preconditioning failed to protect PDK1fl/− cardiomyocytes against hypoxia (only 1 out of 8 cells survived 30 min hypoxia, P=1.000, Fig. 2).
Figure 2.
PDK1fl/− cells cannot be preconditioned against hypoxia. Laser confocal images and a corresponding graph of PDK1fl/+ and PDK1fl/− cells exposed to 30-min-long hypoxia. Rod and rounded shape indicates that cell is alive or dead, respectively. White bar represents 25 μm. Graph depicts number of cells that survived/died in hypoxia. *P < 0.05 (as determined by χ2 test).
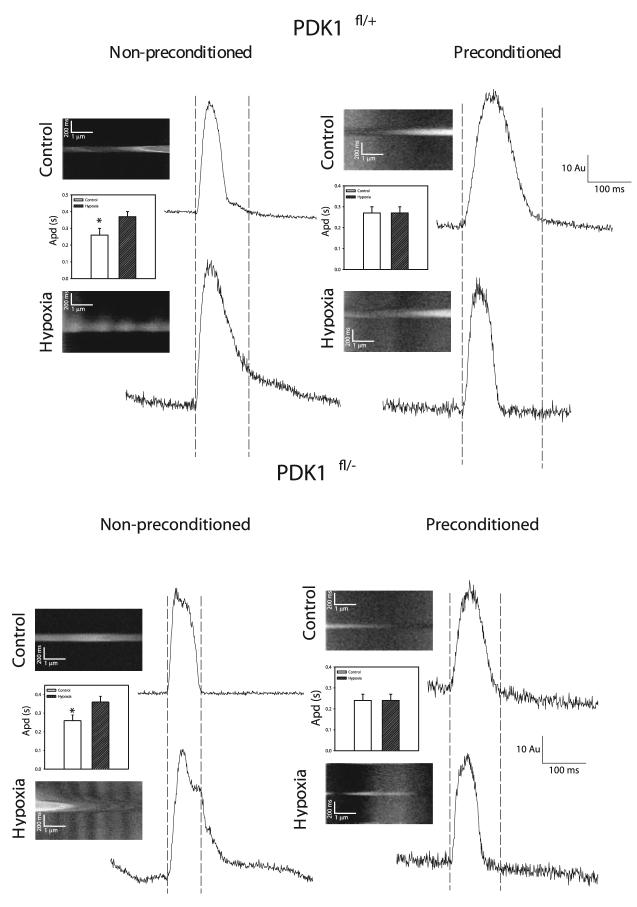
PDK1-mediated cardioprotection is not associated with changes in sarcolemmal membrane potential
It has been shown that preconditioning induces activation of sarcolemmal KATP channels, which would shorten action membrane potential duration and decrease intracellular Ca2+ loading, leading to cardioprotection (16, 17). Preconditioning activates sarcolemmal KATP channels after 4 min of hypoxia in single beating cardiomyocytes (16). Therefore, we have measured membrane potential under control conditions and after 4 min of hypoxia with and without preconditioning. In cardiomyocytes from both PDK1fl/+ and PDK1fl/− mice, prolongation of action membrane potential occurred at the beginning of hypoxia [PDK1fl/+: the action potential duration (apd) was 0.26±0.04 s under control conditions and 0.37±0.03 s in hypoxia, in all 8 examined cells action potential prolongation has been observed, P=0.04; PDK1fl/−: apd was 0.26±0.03 s under control conditions and 0.36±0.03 s in hypoxia, in all 8 examined cells action potential prolongation has been observed, P=0.02, Fig. 3]. After an initial prolongation, the action potential duration decreased after longer (20 min) hypoxia (apd duration from 0.36±0.03 s in early hypoxia has decreased to 0.26±0.03 s in late hypoxia for both PDK1fl/+ and PDK1fl/+ cells, n=7 for both, data not shown). At the time point when the opening of sarcolemmal KATP channels occurs, preconditioning prevented hypoxia-induced prolongation of action membrane potential in both phenotypes (PDK1fl/+: control apd was 0.27±0.03 msec and apd in hypoxia after preconditioning was 0.27±0.03 s; in 4 out of 8 cells action potential shortening was observed; P=0.87; PDK1fl/+, control apd was 0.24±0.03 s and apd in hypoxia after preconditioning was 0.24±0.03 s; P=1.00; in 4 out of 9 cells action potential shortening was observed, Fig. 3).
Figure 3.
The response of cardiac action membrane potential to hypoxia with and without preconditioning does not differ between PDK1fl/+ and PDK1fl/− mice. Original line-scans of di-8-ANEPPS-loaded cardiomyocytes and corresponding time-courses and bar graphs for nonpreconditioned and preconditioned PDKfl/+ and PDK1fl/− cardiomyocytes under control conditions and hypoxia. AU = arbitrary units. Each bar represents mean ± sem. *P < 0.05 (as determined by t test). Doted lines visualize action potential duration under control conditions.
PDK1-mediated cardioprotection in preconditioning is associated with regulation of mitochondrial membrane potential
It has been proposed that preconditioning may activate putative mitochondrial KATP channels and/or inhibit the mitochondrial permeability transition pore (18, 19). In both cases, the mitochondrial membrane potential would change. Therefore, we have monitored mitochondrial membrane potential in vivo in PDK1fl/+ and PDK1fl/− mice using the fluorescent dye JC-1, a ratiometric dye that is a reliable indicator of mitochondrial membrane potential (20). During first 20 min of hypoxia, the JC-1 ratio did not significantly change in nonpreconditioned cardiomyocytes regardless of their phenotype (for PDK1fl/+ JC-1 ratio increased for only 12.4±14.8% after 20 min of hypoxia, n=5, P=0.44, Fig. 4; for PDKfl/− this parameter was 7.2±5.1% after 20 min of hypoxia, P=0.67, n=5, Fig. 4). However, in preconditioned PDK1fl/+ cardiomyocytes, the JC-1 ratio steadily rose during hypoxia to reach statistical significance after 20 min (JC-1 ratio rose for 50.5±18.0% after 20 min-long hypoxia, P=0.02, n=6, Fig. 4). This effect of preconditioning was not observed in PDK1fl/− mice (increase in JC-1 ratio was only 14.7±7.1% after 20 min hypoxia, P=0.31, n=5, Fig. 4). In preconditioned PDK1fl/+ cells, the rate of mitochondrial membrane depolarization was slow and associated with prolonged cell survival (Fig. 5, n=7). In a typical example shown on Fig. 5, JC-1 ratio rose 0.65% per min in the first 20 min and 0.40% per min during 30–40 min period. This finding was in contrast to preconditioned PDK1fl/− cells where mitochondrial membrane depolarization did not occur in the first 20 min (Fig. 4), but when it did it was fast and associated with cell death (Fig. 5). Thus, in a PDK1fl/− cell depicted on Fig. 5, JC-1 ratio rose less then 0.20% per min in the first 20 min and 8.30% per min during 30–40 min period.
Figure 4.
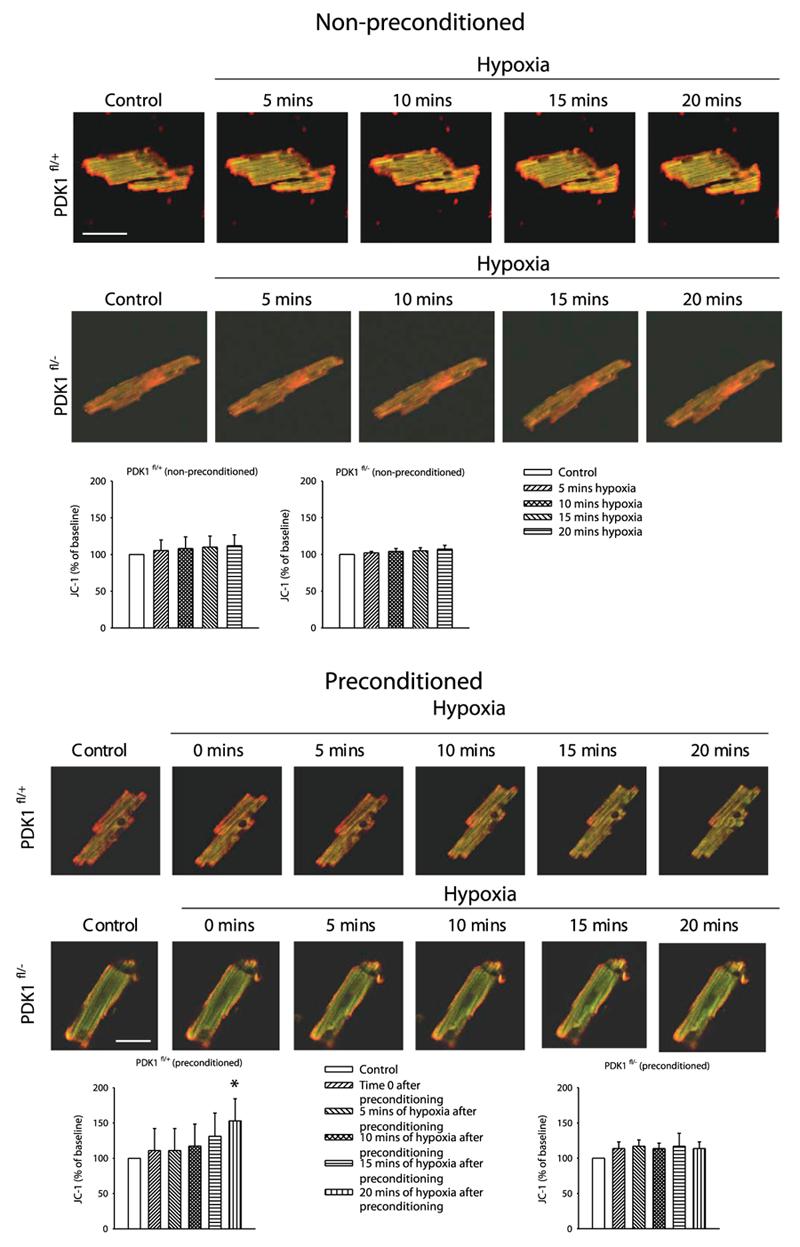
Preconditioning induces depolarization of cardiac mitochondrias in PDK1fl/+, but not in PDK1fl/− mice. Original images of cardiomyocytes loaded with JC-1 and corresponding graphs under depicted conditions. The disappearance of red color is indicative of mitochondrial membrane depolarization. Horizontal white bar represents 35 μm. Each bar represent mean ± sem. *P < 0.05 (as determined by ANOVA followed by t test).
Figure 5.
Preconditioning inhibits late and dramatic mitochondrial membrane depolarization and cell death in PDK1fl/+, but not PDK1fl/−, mice. A) Original images of preconditioned PDK1fl/+ and PDK1fl/− cardiomyocytes loaded with JC-1 exposed to hypoxia. Horizontal white bar represents 30 μm. B) Time course corresponding to (A).
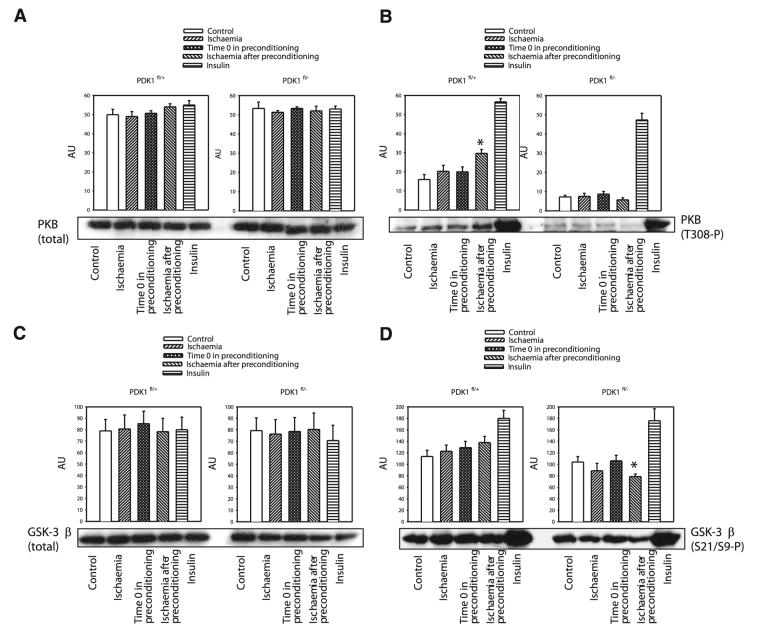
PDK1/PKB/GSK-3β cascade mediates preconditioning
PKB, a PDK1 substrate, has been previously implicated in ischemic preconditioning in the heart (12, 13). Western blotting with PKB total Ab demonstrated that levels of PKB in the heart remained steady under any of the experimental conditions and in both types of mice (Fig. 6). When phospho-PKB (Thr308) Ab was used it was revealed that preconditioning induces phosphorylation of PKB in PDK1fl/+ (Fig. 6) but not in PDK1fl/− mice (Fig. 6). The main target of PKB is GSK-3, an enzyme that has also been involved in cardioprotection (12). In PDK1fl/+ mice, preconditioning was successful to maintain the degree of phosphorylation of GSK-3β seen in control hearts (Fig. 6). In contrast, in PDK1fl/− mice, preconditioning was associated with a significant decrease in levels of phosphorylated form of GSK-3β (Fig. 6). PDK1 is a activated by phosphoinositide 3-kinase (PI3K) (21), and the obtained results would suggest that PDK1 is involved in preconditioning as a part of PI3K-PDK1-PKB-GSK-3β signaling cascade. To further test this hypothesis, we have tested the effect of known activator of this signaling cascade, insulin (22), on PDK1fl/+ and PDK1fl/−cells exposed to hypoxia. It has been shown previously as well as here (Fig. 6) that insulin activate PI3K-PDK1-PKB-GSK-3β signaling pathway cascade even in PDK1fl/− mice (14). In both cell types insulin (25 mU/ml) has significantly increased cell survival in hypoxia (all cells tested has survived hypoxia >90 min), which was associated with gradual mitochondrial membrane depolarization (Fig. 7). Conversely, wortmannin (5 μM), an inhibitor of PI3K (23; Fig. 7), has abolished any difference in response of preconditioned PDK1fl/+ and PDK1fl/−cells to hypoxia (Fig. 7).
Figure 6.
Preconditioning phosphorylates PKB in PDK1fl/+ hearts and inhibits dephosphorylation of GSK-3β. The effect of ischemia on phosphorylation of PKB and GSK-3β. Original Western blots with total PKB (A), phospho-PKB (B), total GSK-3β (C), and phospho-GSK-3β (D) antibodies applied on extracts from hearts under control conditions (control), and those exposed to 20-min-long ischemia without preconditioning (ischemia), to four cycles of ischemia/reperfusion (time 0 in preconditioning), 20-min-long ischemia after the preconditioning (ischemia after preconditioning) and treated with insulin (insulin), and corresponding graphs. Each bar represents mean ± sem. *P < 0.05 (as determined by ANOVA followed by t test).
Figure 7.
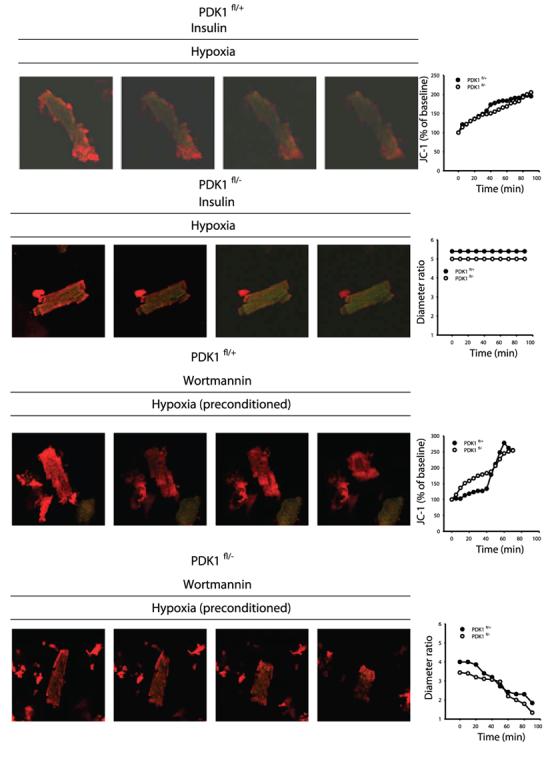
Insulin protects PDK1fl/+ and PDK1fl/− cells against hypoxia, while wortmannin abolishes any difference between PDK1fl/+ and PDK1fl/− cells in the effect of preconditioning. Original images of cardiomyocytes loaded with JC-1 under depicted conditions. Each frame in each row corresponds to 0 min, 30 min, 60 min, and 90 min, respectively. Graphs on the right show time course of diameter ratio or JC-1 ratio (essentially the same result have been obtained in two more independent experiments).
DISCUSSION
PDK1 was uncovered as a master kinase that mediates hormone- and growth factor-induced activation of AGC kinases by phosphorylating these enzymes at their activation loop (1). A total lack of PDK1 in the heart results in heart failure and a dramatic decrease in average life span of mice to only 6 wk. The underlying mechanism of heart failure and premature death in these mice has yet to be fully understood, but one possibility is that cardiomyocytes from PDK1 knockout mice are more sensitive to metabolic stress (5). The heart is continuously subjected to the high metabolic demand required to sustain myocardial beating and it is quite possible that an increased sensitivity to stress could lead to cardiomyocyte cell death and the development of heart failure. Such an effect of PDK1 knockout would suggest an involvement of PDK1 in cardioprotective signaling. PDK1fl/− mice that were used in the present study express ∼20% of normal PDK1 activity and are free from any adverse cardiac conditions. The susceptibility of hearts/cells from PDK1fl/− to ischemia-reperfusion/hypoxia was not different from those found in the wild-type (WT), which suggests that even the partial presence of PDK1 is enough to preserve physiological cardiac resistance to metabolic stress.
However, preconditioning could not be conferred in PDKf1/− mice, which suggests that intact PDK1 levels are required for preconditioning-induced cardioprotection. It has been previously suggested that the endefector of preconditioning in the heart is the opening of sarcolemmal KATP channels (16). Taking this into consideration, it was quite possible that PDK1 is involved in regulation of the activity of these ion channels. Therefore, to test this particular possibility, we have examined action membrane potential in cardiomyocytes from PDK1fl/+ and PDK1fl/− mice under hypoxia without and with preconditioning. It is well established that severe and long-lasting ischemia/hypoxia induces shortening of action membrane potential which is mediated by the opening of sarcolemmal KATP channels (24, 25). However, action potential shortening is known to be preceded by an initial prolongation due to the inhibition of transient outward current while L-type Ca2+ and steady-state outward current remained unchanged (26). We have previously shown that during first 15 min of hypoxia sarcolemmal KATP channels remain mostly closed in single beating nonpreconditioned cardiomyocytes while sarcolemmal KATP channels are activated in the first few minutes of hypoxia in preconditioned cardiomyocytes, which should lead to a shortening of action potential duration (16). In the present study, hypoxia was associated with an initial prolongation of action membrane potential duration in both types of mice and this would be expected based on previous findings (16, 26). In preconditioned cells, prolongation of action membrane potential was inhibited, which is in accord with the predicted activation of sarcolemmal KATP channels. The observed effect of preconditioning on action potential duration was indistinguishable between PDK1fl/+ and PDK1fl/− mice, which suggests that the changes in behavior of sarcolemmal KATP channels or any other ion channel in the sarcolemma do not mediate preconditioning-induced cardioprotection by PDK1.
The other target of the preconditioning-induced signaling in the heart is reported to be mitochondria (27). In these regards, it has been recently suggested that the inhibition of the opening of mitochondrial permeability transition pore (mPTP) mediates preconditioning-induced cardioprotection (27-29). The mPTP is a nonspecific megachannel in the inner mitochondrial membrane. The opening of mPTP results in collapse of the inner membrane potential, uncoupling of respiratory chain, efflux of cytochrome c and other molecules which, all together, lead to cell death (19, 30). As the opening of mPTP is associated with mitochondrial membrane depolarization, we have measured mitochondrial membrane potential using JC-1. JC-1 is a cationic dye that exhibits potential-dependent accumulation in mitochondria as indicated by a fluorescence emission shift from green (525 nm) to red (590 nm). Mitochondrial membrane depolarization is manifest as an increase in green/red fluorescent ratio (20). In the present study, mitochondrial membrane potential remained steady during exposure to 20 min hypoxia in both PDK1fl/+ and PDK1fl/− cells. When cardiomyocytes from PDK1fl/+ mice were preconditioned, exposure to sustained hypoxia (15–20 min) resulted in a gradual mitochondrial membrane depolarization, which was associated with an increased cell viability. This preconditioning-induced, gradual mitochondrial membrane depolarization was absent in PDK1fl/− cardiomyocytes. In contrast, in preconditioned PDK1fl/− cells, mitochondrial membrane potential remained constant during hypoxia until a sudden and rapid mitochondrial membrane depolarization occurred, which was associated with irreversible hyper-contracture and cell death. In previous studies, it has been shown in Girardi cells and C2C12 myotubes that ischemic preconditioning induces mitochondrial membrane depolarization and results in mitochondrial uncoupling, which improves cellular tolerance to metabolic stress (31). Mitochondrial membrane depolarization was ascribed to the opening of putative mitochondrial KATP channels (31, 32), and recently it has been suggested that other types of mitochondrial K+ channels may also be involved (33). However, it has been suggested that the inhibition of MPTP and mitochondrial membrane depolarization mediate ischemic preconditioning (27). At first sight, it seems that the two concepts of cardioprotection involving either induction or inhibition of mitochondrial membrane depolarization contradict each other. It is yet to be fully understood why/how the changes of mitochondrial membrane potential act in a cardioprotective way. It has been proposed that the activation of putative mitochondrial KATP channels leads to moderate mitochondrial membrane depolarization, which allows further extrusion of H+ ions, inhibits elevation of intramitochondrial Ca2+, and prevents high-energy phosphate depletion by creating a more favorable electrochemical gradient for ATP synthesis (reviewed in 34). However, the opening of MPTP, caused by high intramitochondrial concentration of Ca2+, induces a series of events leading to rupture of mitochondrial membranes, uncoupling oxidative phosphorylation, releasing numerous proteins, and activating of degradative enzymes that lead ultimately to cell death (19). The results of the present study suggest that early, but moderate, mitochondrial membrane depolarization (that is compatible with the opening of a K+ conductance; ref. 32) could be associated with the inhibition of late, but dramatic, mitochondrial membrane depolarization (that is compatible with the opening of MPTP; ref. 19) and cell death. A particular property of preconditioning to regulate mitochondrial membrane potential was lost in PDK1fl− mice. This finding suggests that the PDK1-mediated regulation of mitochondrial membrane potential mediates preconditioning and plays a significant role in controlling cellular resistance to ischemia/hypoxia.
It has been previously reported that PDK1 is substrate of PI3K and that PKB, an enzyme that has been recently associated with preconditioning and cardioprotection (12), is a substrate for PDK1 (1). Full activation of PKB is a multistep process and phosphorylation of PKB at Thr308 and Ser-473 are necessary for full activation of PKB (35, 36). Preconditioning has induced phosphorylation of PKB in PDK1fl/+, but not PDK1fl/−, mice, suggesting that the levels of PDK1 in the hypomorphic mice are not sufficient to activate PKB in response to preconditioning stimulus. Note that although we have found basal levels of phosphorylated PKB lower in PDK1fl/− mice than in PDK1fl/+ mice, this has not affected the myocardial resistance to ischemia-reperfusion. However, a lack of PKB phosphorylation in preconditioning was associated with the inhibition of preconditioning-induced cardioprotection. This could be explained by the fact that preconditioning profoundly changes intracellular signaling setting (9, 37), which, in turn, could result in PKB being more important for cardioprotection in preconditioning than under basal state. GSK-3 was the first physiological target of PKB to be identified, although numerous other substrates have since been described. There are two isoforms of GSK-3, termed β and α, which are encoded by distinct genes (36, 38). It has recently been suggested that distinct signaling pathways converge to mediate cardioprotection by phosphorylation of GSK-3β (39). Phosphorylation inhibits GSK-3β, which leads to inhibition of MPTP and cardioprotection (29, 39). In the present study, phosphorylation of GSK-3β remained constant in PDK1fl/+ mice throughout all experimental protocols. However, in PDK1fl/− hearts, phosphorylation of GSK-3β was reduced below basal levels following exposure to preconditioning. Therefore, the results obtained here in PDK1fl/− mice would be in accord with previous studies and would imply that a fully intact PDK1/PKB/GSK-3β cascade is crucial for preconditioning-induced cardioprotection. Indeed, our experiments have shown that insulin, an activator of PI3K/PDK1/PKB/GSK-3β signaling pathway (22), protect both PDK1fl/+ and PDK1fl/− cells against hypoxia, confirming that the activation of this signaling pathway is cardioprotective. The fact that protection with insulin was efficient in PDK1fl/− phenotype is not surprising when it is known that 20% of PDK1 activity is enough for this hormone to fully activate PKB and GSK-3β (14; see also this study). Furthermore, as the use of wortmannin, a PI3K inhibitor (23), has abolished any difference that preconditioning made in response to hypoxia between PDK1fl/+ and PDK1fl/− phenotype. The activation of PI3K is known to mediate preconditioned-induced cardioprotection (reviewed in 40), PDK1 is a substrate for PI3K, and the observed lack of difference between PDK1fl/+ and PDK1fl/− in the presence of wortmannin would suggest that PI3K targets PDK1 to mediate preconditioning. Taking into consideration that PKB is a PDK1 substrate and that GSK-3β, an enzyme that might target both MPTP and KATP channels (29, 41) is a PKB substrate, it is logical to conclude that signaling cascade PI3K/PDK1/PKB/GSK-3β mediates preconditioning. The obtained results further suggest that this pathway does so by regulating of mitochondrial membrane potential, which, in turn, results in increased cellular resistance to ischemia/hypoxia.
Acknowledgments
We thank the Association for International Cancer Research (D.R.A.), Biotechnology and Biological Sciences Research Council (A. J.), British Heart Foundation (D.R.A. and A.J.), Diabetes UK (D.R.A.), the Medical Research Council UK (D.R.A. and A.J.), Wellcome Trust (A.J.), as well as the pharmaceutical companies that support the Division of Signal Transduction Therapy (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck & Co. Inc., Merck KGaA and Pfizer) for financial support.
REFERENCES
- 1.Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Biochem. Soc Trans. 2001;29:1–14. doi: 10.1042/0300-5127:0290001. [DOI] [PubMed] [Google Scholar]
- 2.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell. Dev. Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 4.Wick MJ, Ramos FJ, Chen H, Quon MJ, Dong LQ, Liu F. Mouse 3′-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J. Biol. Chem. 2003;278:42913–42919. doi: 10.1074/jbc.M304172200. [DOI] [PubMed] [Google Scholar]
- 5.Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanović S, Mouton V, Kahn RC, Lucocq JM, Gray GA, et al. Deficiency of PDK1 in heart results in heart failure and increased sensitivity to hypoxia. EMBO J. 2003;22:4666–4676. doi: 10.1093/emboj/cdg469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugden PH. Signalling pathways in cardiac myocyte hypertrophy. Ann. Med. 2001;33:611–622. [PubMed] [Google Scholar]
- 7.Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN (+/−) mice. Curr. Biol. 2005;15:1839–1846. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 8.Murray CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Yellon DM, Downey JM. Preconditioning of the myocardium: from cellular physiology to clinical cardiology. Physiol. Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Ytrehus K, Downey JM. Evidence that Translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. J. Mol. Cell. Cardiol. 1994;26:661–668. doi: 10.1006/jmcc.1994.1078. [DOI] [PubMed] [Google Scholar]
- 11.Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ. Res. 1994;75:586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- 12.Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ. Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 13.Uchiyama T, Engelman RM, Maulik N, Dipak K, Das DK. Role of Akt signaling in mitochondrial survival pathway triggered by hypoxic preconditioning. Circulation. 2004;109:3042–3049. doi: 10.1161/01.CIR.0000130647.29030.90. [DOI] [PubMed] [Google Scholar]
- 14.Lawlor MA, Mora A, Ashby PR, Williams MR, Murray-Tait V, Malone L, Prescott AR, Lucocq JM, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21:3728–3738. doi: 10.1093/emboj/cdf387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Q, Jovanović S, Clelland A, Sukhodub A, Budas GR, Phelan K, Murray-Tait V, Malone L, Jovanović A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischaemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budas GR, Jovanović S, Crawford RM, Jovanović A. Hypoxia-induced preconditioning in adult stimulated cardiomyocytes is mediated by the opening and trafficking of sarcolemmal KATP channels. FASEB J. 2004;18:1046–1048. doi: 10.1096/fj.04-1602fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am. J. Physiol. Heart. Circ. Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 19.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc. Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 20.Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 21.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 22.Farese RV, Sajan MP, Standaert ML. Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): actions and defects in obesity and type II diabetes. Exp. Biol. Med. (Maywood) 2005;230:593–605. doi: 10.1177/153537020523000901. [DOI] [PubMed] [Google Scholar]
- 23.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billman GE. Role of ATP-sensitive potassium channel in extracellular potassium accumulation and cardiac arrhythmias during myocardial ischaemia. Cardiovasc. Res. 1994;28:762–769. doi: 10.1093/cvr/28.6.762. [DOI] [PubMed] [Google Scholar]
- 25.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J. Mol. Cell. Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verkerk AO, Veldkamp MW, van Ginneken AC, Bouman LN. Biphasic response of action potential duration to metabolic inhibition in rabbit and human ventricular myocytes: role of transient outward current and ATP-regulated potassium current. J. Mol. Cell. Cardiol. 1996;28:2443–2456. doi: 10.1006/jmcc.1996.0237. [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning. Cardiovasc. Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 28.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the perfused rat heart. J. Physiol. (Lond.) 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 2003;93:1385–1394. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 31.Minners J, Lacerda L, McCarthy J, Meiring JJ, Yellon DM, Sack MN. Ischemic and pharmacological preconditioning in Girardi cells and C2C12 myotubes induce mitochondrial uncoupling. Circ. Res. 2002;89:787–792. doi: 10.1161/hh2101.098372. [DOI] [PubMed] [Google Scholar]
- 32.Hanley PJ, Daut J. KATP channels and preconditioning: a re-examination of the role of mitochondrial KATP channels and an overview of alternative mechanisms. J. Mol. Cell. Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 33.O'Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology. 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuo ML, Huang Y, Liu DP, Liang CC. KATP channel: relation with cell metabolism and role in cardiovascular system. Int. J. Biochem. Cell Biol. 2005;37:751–764. doi: 10.1016/j.biocel.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 36.Hanada M, Feng J, Hemmings B. Structure, regulation and function of PKB/AKT-a major therapeutic target. Biochim. Biophys. Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Sukhodub A, Jovanović S, Du Q, Budas GR, Clelland A, Shen M, Sakamoto K, Tian R, Jovanović A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J. Cell. Physiol. 2006 doi: 10.1002/jcp.20862. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodgett JR. Recent advances in the protein kinase B signalling pathway. Curr. Opinion Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3β during preconditioning through a phoaphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ. Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 40.Oudit GY, Sun H, Kerfent BG, Crackower MA, Penninger MA, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J. Mol. Cell. Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Mirshamis S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford MLJ. Stimulation of PI3K by leptin and insulin leads to actin reorganisation and KATP activation of arcuate nucleus neurons. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]