Abstract
Diets high in fat or protein and extremely low in carbohydrate are frequently reported to result in weight loss in humans. We previously reported that rats maintained on a low carbohydrate-high fat diet (LC-HF) consumed similar kcals/day as chow (CH)-fed rats and did not differ in body weight after 7 weeks. LC-HF rats had a 45% decrease in POMC expression in the ARC, decreased plasma insulin, and increased plasma leptin and ghrelin. In the present study we assessed the effects of a low carbohydrate-high protein diet (HP: 30% fat, 65% protein, and 5% CHO) on body weight, caloric intake, plasma hormone levels and hypothalamic gene expression. Male rats (n=16) were maintained on CH or HP for 4 weeks. HP rats gained significantly less weight than CH rats (73.4 +/− 9.4 and 125.0 +/− 8.2 g) and consumed significantly less kcals/day (94.8 +/− 1.5 and 123.6 +/− 1.1). Insulin was significantly reduced in HP rats (HP: 1.8 +/− 0.6 vs. CH: 4.12 +/− 0.8 ng/ml), there were no differences between groups in plasma leptin and plasma ghrelin was significantly elevated in HP rats (HP: 127.5 +/− 45 vs. CH: 76.9 +/− 8 pg/ml). Maintenance on HP resulted in significantly increased ARC POMC (HP: 121 +/− 10.0 vs. 100 +/− 5.9) and DMH NPY (HP: 297 +/− 82.1 vs. CH: 100+/− 37.7) expression compared to CH controls. These data suggest that the macronutrient content of diets differentially influences hypothalamic gene expression in ways that can affect overall intake.
INTRODUCTION
Diets with varied macronutrient composition are frequently reported to influence total energy intake and long-term changes in body weight [1–8], however the mechanisms by which different macronutrient combinations influence food intake and body weight are unclear. Low carbohydrate, high protein low energy diets have been widely used in weight management programs. Compared with other diets, HP diets are reported to be effective in inducing weight loss while sparing lean body mass [3, 5, 9, 10]. It has been demonstrated that maintenance on these types of diets results in improved glycemic control in hyperinsulinemic, obese, or diabetic subjects [11, 12].
Although, low carbohydrate diets with elevated fat and/or protein content have been reported to result in weight loss in humans [13–18], the effects of these diets with regard to effects on feeding-related endocrine and hypothalamic systems have not been fully evaluated. We previously demonstrated that in the rat, a low-carbohydrate diet that was high in fat, (LC-HF: 80 % fat, 5% carbohydrate, 15% protein) did not alter body weight as compared to chow-fed rats, however it did result in alterations in plasma hormone profiles and hypothalamic gene expression levels. Despite consuming the same amount of kcals per day as chow-fed rat, rats on LC-HF had elevated ghrelin and decreased insulin and glucose levels. Additionally, LC-HF rats had elevated expression levels of NPY mRNA and decreased POMC mRNA expression levels in the hypothalamus[19]. While our characterization of the hypothalamic gene expression and endocrine profiles in rats maintained on LC-HF suggest mechanisms underlying the efficacy of low carbohydrate high fat diets, equivalent studies in rats maintained on high protein low carbohydrate diets have not been conducted. In these experiments we evaluate the effects of maintenance on a diet high in protein and limited to 5 % of the kcals derived from carbohydrate on body weight, body composition, food intake, hypothalamic gene expression, and endocrine profiles.
RESEARCH METHODS AND PROCEDURES
Animals and diets
Male Sprague Dawley rats (Charles River) weighing 250–300 g were individually housed in hanging wire cages and maintained on a 12-h light, 12-h dark schedule at constant temperature. All rats received ad libitum access to pelleted chow and tap water for one week after shipment to allow for acclimation to the new environment and stabilization of body weights. Rats were weighed daily and subsequently weight-matched for assignment to one of either chow (CH, n=8), or high protein (HP, n=8) diets. Chow was standard laboratory rodent chow (LabDiet, Prolab RMH 1000) consisting of 66% carbohydrate, 18% protein, and 16 % fat (% total kcals) and containing 3.4 kcal/g. The high protein diet, prepared by Research Diets (Diet # D03112401, New Briunswick, NJ), consisted of 5 % carbohydrate, 65% protein and 30% fat with 3.65 kcal/g. All procedures were approved by the institutional animal care and use committee at Johns Hopkins University. Except where noted, rats received ad libitum access to food and water for the duration of the experiments.
Food intake, body weight and plasma hormone profiles
Rats were maintained on their respective diets for 4 weeks. Twenty-four hour food consumption and body weight were measured one hour prior to the onset of the dark cycle each day. Three weeks after rats were placed on their respective diets, tail blood was collected in order to measure levels of β-hydroxybutyrate to determine if rats on HP were in ketosis. Fifty μl of blood from each rat was tested using the Statsite Ketone meter (GDS Technologies, Elkhart, IN). After 4 weeks of maintenance on the diets, food was removed 4 hours prior to the onset of the dark cycle and rats were sacrificed 2 hours later. Trunk blood was taken for evaluation of plasma levels leptin, insulin, and ghrelin (rat leptin and insulin RIA kits, active ghrelin RIA kit, Linco Research), as recommended by the manufacturer.
Brains were subsequently processed by in situ hybridization to measure expression levels of NPY, AgRP, and POMC mRNA in the ARC and NPY mRNA in the DMH.
Diet aversion and preference testing
Others have demonstrated that rats do not develop an aversion the high protein diets [20, 21]. Because previos examinations of high protein diets and aversion tested aversion to diets from which the protein source was different than that used in our diets, we employed a conditioned taste aversion (CTA) paradigm and a two-diet choice test. Naïve male rats (n=16) were trained on a water deprivation schedule during which they were allowed access to 2 water bottles for 1 h per day. Intake was measured and by day 10, each rat was consistently drinking at least 15ml/day (16.87 +/− 0.64) and drinking equivalently from both bottles. Rats were then weight-matched and given ad libitum access to either chow or HP for one week. During this time, rats had access to one flavor of unsweetened Kool-Aid (grape or cherry) presented in two bottles for one hour each day. This was the only fluid available to them during the week of diet exposure. For the second week of the study, rats were given access to the other diet (chow or HP) and given access to the other flavor of Kool-Aid (grape or cherry) for one hour each day. All diet and flavor presentations were done in a counter-balanced manner. On the 15th day, food was removed 4 hours prior to the onset of the dark cycle. When the lights went out, rats were given one bottle of grape and one bottle of cherry, presented on the left or right of the cage (counterbalanced). Intake after one hour was recorded, after which time water was placed on the cages.
In order to determine if rats prefer chow over HP, or vice versa, a two-diet choice test consisted of allowing naïve rats (n=8) ad libitum access to one feeder filled with chow and one feeder filled with HP diet. For one week, intake of both types of diet was recorded daily
Cryosections and riboprobes
Coronal sections (14 μm) through the arcuate nucleus (ARC) and dorsomedial hypothalamic nucleus (DMH) were taken via cryostat, mounted on superfrost/plus slides (Fisher Scientific), and fixed with 4% paraformaldehyde. Three sections per brain were anatomically matched among animals for each hybridization assay in the same condition. The plasmids of NPY, POMC and AgRP were linearized by appropriate restriction enzymes. Antisense riboprobes were labeled with [35S]UTP (Amersham Pharmacia Biotech) by using in vitro transcription systems with appropriate polymerases according to the manufacturer’s protocols (Promega) and purified by Quick Spin RNA columns (Roche Diagnostics) to yield a specific activity of 5 × 108 cpm/μg.
Sections were treated with acetic anhydride and incubated in hybridization buffer containing 50% formamide, 0.3 M NaCl, 10 mM Tris·Cl (pH 8.0), 1 nM EDTA (pH 8.0), 1 × Denhardt’s solution (Eppendorf), 10% dextran sulfate, 10 mM DTT, 500 μg/ml yeast tRNA, and 108 cpm/ml of [35S]UTP. NPY, POMC, and AgRP were incubated at 55° C overnight. After hybridization, sections were washed 3 times in 2 × SSC twice at 55° C, and washed twice for 15 minutes/wash in 0.1 × SSC at 55° C. Slides were dehydrated and exposed with BMR-2 film (Kodak). Exposure time for quantification of mRNA for NPY, AgRP, and POMC in the ARC was 1 day, and NPY in the DMH was 3 days.
In situ hybridization quantitative analysis
Images obtained by in situ hybridization were analyzed using National Institutes of Health Scion Image software. Autoradiographic images were first scanned by Epson Professional Scanner and then quantified with Scion Image software using autoradiographic 14C-microscales (Amersham Pharmacia Biotech) as a standard. Data for each animal were a mean of the product of hybridization area × density, with the background density subtracted, from 3 sections per animal that were anatomically matched among animals for each assay. Data for each animal were normalized to chow-fed controls as 100%, and all data are expressed as mean ± standard error of the mean.
Statistical analyses
Twenty-four hour food intake and body weight data were analyzed by one-way ANOVA. Student’s t tests were used to analyze effects of diet on levels of blood glucose and plasma leptin, insulin, and ghrelin, as well as hypothalamic neuropeptide gene expression. Values of p < 0.05 were taken to be statistically significant differences.
RESULTS
Food intake and body weights
Rats maintained on chow consumed significantly more kcals per day than HP rats beginning at the first day of diet access and continued to do so for the course of the 4 week study (Figure 1A). HP diet rats consumed significantly less on the first 6 days of the study, as compared to their caloric intake for the remainder of the study. By day 7, mean caloric intake was significantly greater than it was on days one through 6 (p<0.05 for the first 6 days compared to the seventh, Figure 1B), however despite the significant increase in caloric intake, intake remained depressed as compared to that of chow rats (p< 0.01).
Figure 1.
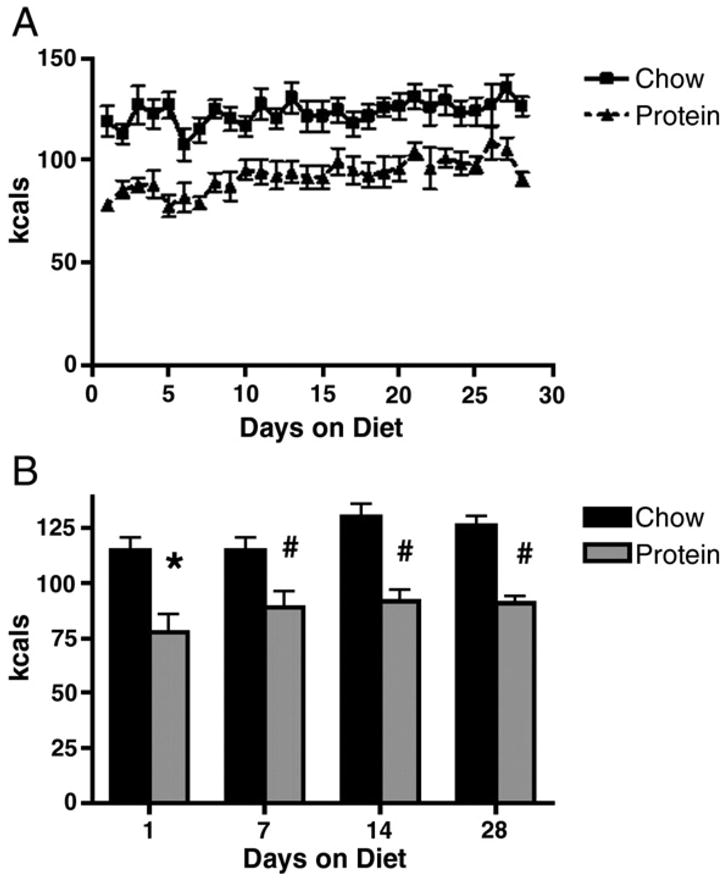
Caloric intakes (A) and mean caloric intake by week (B) for the 28 day study period. Rats maintained on HP diet consumed significantly less kcals/day (A). HP rats consumed significantly less kcals per day in the first week of maintenance on the diet than they did in the following 3 weeks, however despite increasing intake, total kcals per day remained significantly less than that of controls. Bars are means ± SEM; significant differences are denoted as * and #, the # symbol indicates differences in caloric intake by the HP group, as compared to first day on diet and a significant difference from CH at each time point.
The reduced caloric intake resulted in a decreased rate of weight gain in the rats on HP. As depicted in Figure 2, rats on HP lost weight after placement on the diet, and this weight was not recovered until they had been on the diet for one week. Rats on HP gained weight over the course of the 28 day study, but gained significantly less than chow-fed rats gained 125.0 +/− 8.4 g compared to 73.4 +/− 9.5 g gained by the rats on HP (p<0.01). Rats were sacrificed 28 days after placement on the assigned diets. Despite significant differences in body weights between CH and HP rats, there was no significant difference in epididymal fat weight (Figure 3).
Figure 2.
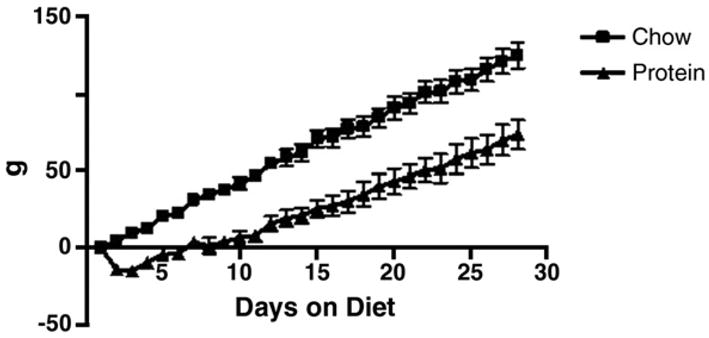
Body weight in chow versus high-protein, low-carbohydrate diet-fed rats. Rats on HP initially lost body weight after placement on HP. Body weight was recovered after one week on the diet, and HP rats continued to grow at a slower rate than CH rats. Values are means ± SEM.
Figure 3.
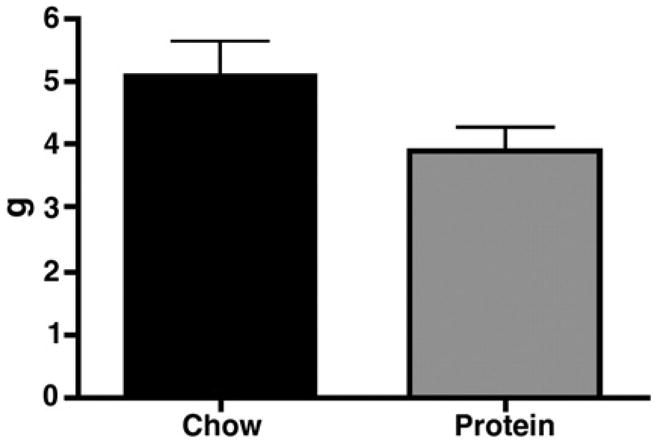
Epididymal fat weight. Rats maintained on HP did not have significantly decreased epididymal fat weight, despite reduced caloric intake and reduced rate of body weight gain, as compared to CH rats. Values are means ± SEM.
Preference and palatability
Rats were trained to consume a specific, novel flavor with chow and a different specific, novel flavor with HP. As shown in Figure 4, when given a two-bottle test such that the HP-paired flavor and the chow-paired flavor were presented simultaneously, rats consumed significantly more of the HP-paired flavor than the chow-paired flavor (p < 0.05).
Figure 4.
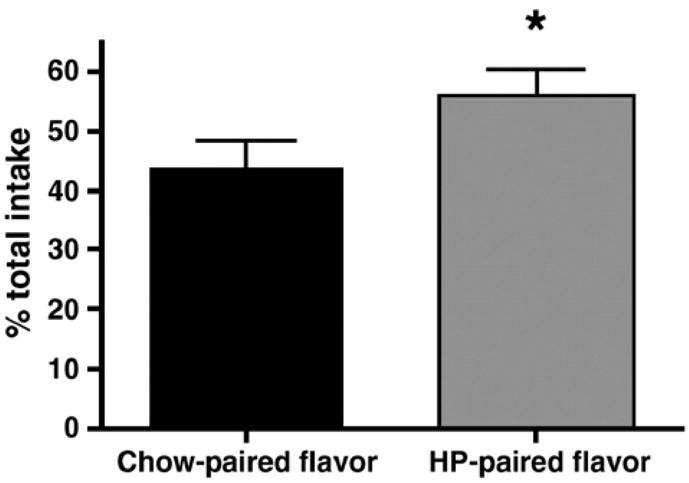
Aversion testing. Rats drank significantly more of the flavor that was paired with the HP diet than they did of the flavor paired with chow. Data are represented as percent total intake. * denotes significance at P < 0.05.
As depicted in Figure 5, when rats were allowed ad libitum access to both chow and HP, there were no differences in calories consumed in the form of either diet, suggesting the absence of preference for either diet.
Figure 5.
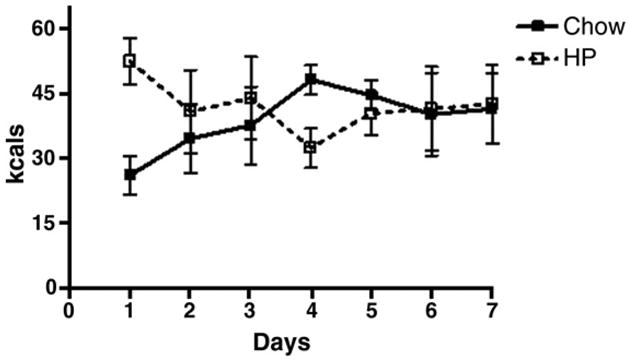
Preference testing. Rats were given access to chow and HP for one week. Caloric intake from each diet was measured daily. Rats consumed similar amounts of calories from chow and HP when given ad libitum access to both diets for one week.
β–hydroxybutyrate, insulin, leptin and ghrelin
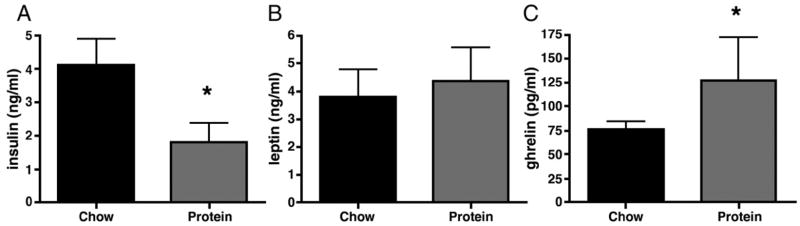
Despite the low level of carbohydrate in the HP diet, HP rats did not have elevated β– hydroxybutyrate levels in blood, as compared to controls (0.01279 +/− 0.03 and 0.01255 +/− 0.01 mM. respectively). Plasma insulin was significantly decreased in rats maintained on HP, as shown in Figure 6A (HP: 1.8 +/− 0.6 and CH:. 4.12 +/− 0.8 ng/ml, p<0.05). There were no differences in plasma leptin levels between CH and HP rats (Figure 6B). Plasma ghrelin levels were significantly elevated in HP rats as compared to CH such the mean plasma ghrelin level in HP rats was 127.5 +/− 45 and CH was 76.9 +/− 8 pg/ml (Figure 6C, p<0.05).
Figure 6.
Plasma insulin (A) leptin (B) and ghrelin (C) levels in rats on CH and HP rats. After 28 days on HP, rats had significantly lower plasma insulin(A) levels than CH rats (P < 0.05). Plasma leptin levels were indistinguishable between CH and HP rats (B), and HP rats had significantly elevated plasma ghrelin levels (C) as compared to their chow-fed counterparts (P < 0.05). Values are means ± SEM.
Patterns of hypothalamic mRNA expression in rats maintained on chow or low carbohydrate, high protein diet
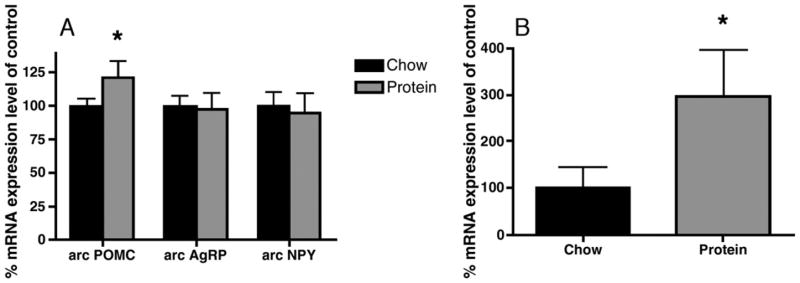
Diet type differentially affected patterns of hypothalamic gene expression. As depicted in Figure 7A, arcuate POMC mRNA expression in rats maintained on HP was increased 19% as compared to that of CH rats (P <0.05). There were no between group differences in levels of expression of NPY or AgRP in the ARC, In contrast to ARC NPY mRNA expression levels, NPY mRNA expression levels in the DMH were increased 198% in the HP rats as compared to CH (p <0.05, Figure 7B).
Figure 7.
Levels of expression of (A) POMC, AgRP, and NPY in the arcuate nucleus and (B) NPY in the dorsomedial of the hypothalamus of CH and HP diet rats after 4 weeks on the assigned diet. The amount of mRNA detected by in situ hybridization was quantified by computerized image analysis. Data for each animal were normalized to chow-fed controls as 100%, and all data are expressed as mean ± SEM. Significant differences from chow controls denoted as * p< 0.05.
DISCUSSION
Diets high in fat or protein and extremely low in carbohydrate are frequently reported to result in weight loss in humans. We previously reported that rats maintained on a low-carbohydrate, high fat diet (80% fat, 15% protein and 5% carbohydrate) consumed similar kcals per day as did chow-fed rats (16% fat, 19% protein, and 65% carbohydrate) and did not differ in body weight after 7 weeks on the diets. Despite similar body weights, the low carbohydrate high fat diet resulted in elevated fat mass in these rats as compared to chow-fed animals and alteration in plasma hormone profiles such that insulin was significantly reduced, and leptin and total ghrelin were significantly increased. In the brain, we measured a 45% decrease in POMC mRNA expression in the arcuate nucleus of the hypothalamus, and NPY mRNA levels were significantly increased in the ARC [19]. Here we assessed the effects of maintenance on a low carbohydrate diet in which the lack of carbohydrate is compensated for by protein.
Limiting dietary carbohydrate necessarily results in compensation with another macronutrient. We chose to evaluate a diet low in carbohydrate and high in protein in order to evaluate not only the effects of maintenance on this type of diet with respect to hypothalamic gene expression and endocrine profiles, but also to compare its effects of those of maintenance on LC-HF. Our current data demonstrate that maintenance on a low carbohydrate diet that is high in protein results in reduced food intake and body weight. Consistent with reduced body weight, rats on HP also had significantly decreased plasma insulin levels, as compared to controls. Despite the lower body weight, rats consuming HP for four weeks did not have significantly lower epididymal fat mass However, the percent difference in body weight gain in HP as compared to CH and the percent difference in epididymal fat pad weights are similar, suggesting that fat and lean tissue were decreased by similar proportions. Plasma leptin levels were indistinguishable between rats maintained on chow and those on the HP diet. Rats on HP had significantly elevated plasma ghrelin levels. Despite severely restricted carbohydrate, HP rats did not have elevated levels of β-hydroxybutyrate. Ketone bodies (acetoacetate, β-hydroxybutyrate, and acetone) are products of lipid metabolism and while HP rats were consuming a diet with greater fat content than CH, others have demonstrated that in order to achieve ketosis in rats, the diet must contain at least 80% of the kcals from fat, and 5% or less from carbohydrates [22–24]. While the carbohydrate content of the diet used in the current studies is sufficiently low, the fat content is likely not high enough.
Chronic food restriction and acute food deprivation appear to invoke different metabolic states which result in different endocrine and hypothalamic gene expression profiles. Chronic food restriction in rats maintained on laboratory chow results in significantly reduced plasma leptin, however insulin levels are indistinguishable from those of control rats, whereas acute food deprivation results in both reduced leptin and insulin [25]. Reduced plasma insulin levels in the animals maintained on the high protein low carbohydrate diet relative to the chow fed animals are likely due to a lack of dietary carbohydrate such that insulin release is not stimulated, in addition to overall weight loss. It has been demonstrated that plasma insulin and ghrelin levels have an inverse relationship such that when insulin levels rise, ghrelin levels fall [26–28]. Plasma ghrelin levels also fluctuate depending upon both short- and long-term nutritional status. Circulating ghrelin levels rise before a meal [29] and decrease rapidly following ingestion of a meal [29–31]. Additionally, ghrelin levels are responsive to long-term changes in nutritional status. Individuals who have lost weight by means of chronic caloric restriction and those who have lost weight due to illness-induced anorexia have elevated levels of plasma ghrelin [32–35]. Thus, elevated ghrelin levels in HP rats likely reflect the long-term caloric restriction in these rats as well as reduced insulin availability.
In the brain, we demonstrate that maintenance on HP resulted in significant increases in POMC expression over that of controls, with no effect on AgRP or NPY expression levels in the ARC, and increased DMH NPY expression. Recently, Bi and colleagues reported that hypothalamic gene expression levels were differentially affected by chronic food restriction as compared to acute food deprivation. For example, acute food deprivation (24 h) resulted in increased NPY and AgRP gene expression in the arcuate, and no changes in NPY mRNA levels in the DMH, as compared to controls. In contrast, two weeks of limited access to food (70% of the amount consumed by weight-matched controls) resulted in increased arcuate NPY mRNA, although to a significantly lesser degree than that of acute food deprivation and no differences in AgRP mRNA expression levels in the arcuate, as compared to non-restricted controls. Collectively, these data suggest differential regulation of hypothalamic gene expression in response to chronic, as compared to acute, food restriction. Our data are consistent with a role for DMH NPY in long-term energy homeostasis. Rats on HP consumed significantly less kcals than CH rats for 28 days, despite having ad libitum access to the diet, resulting in chronic food restriction. DMH NPY mRNA expression is significantly elevated in several models of obesity [36–39].
Ghrelin administration increases NPY and AgRP gene expression in the ARC and, as demonstrated by electrophysiological studies [40, 41], ghrelin directly activates NPY/AgRP neurons. Interestingly, despite significantly elevated plasma ghrelin levels in HP rats, these rats did not have elevated NPY or AgRP mRNA expression levels in the arcuate nucleus of the hypothalamus. Elevated POMC is seen in cases of obesity and high fat feeding [42] whereas high fat low carbohydrate feeding produces reduced POMC mRNA expression levels [19]. It may be that POMC expressing cells are sensitive to dietary macronutrient content and that the increase in POMC is in response to sufficient protein and fat in the HP diet. The relative influence of dietary macronutrients on POMC expression warrants further investigation.
Overall, our current data suggest the presence of macronutrient-specific effects on hypothalamic gene expression. Whereas maintenance on a high fat, low carbohydrate diet results in decreased POMC and increased NPY mRNA expression levels, increasing the level of protein in the diet produces increased POMC and no change in NPY, suggesting a mechanism by which caloric intake is reduced despite elevated ghrelin levels. Whereas the increased ghrelin and DMH NPY are consistent with the literature in that both are elevated in cases of calorie restriction [25, 34, 43–45], elevated POMC is not and may be a system through which decreased feeding in response to maintenance on a high protein diet is mediated. Elevated POMC is consistent with diminished food intake and body weight. Li and colleagues have recently demonstrated that central POMC gene delivery results in decreased food intake and attenuated weight gain in obese rats [46].
Our current data demonstrate that rats do not form an aversion to the HP diet utilized in these studies. They preferred a flavor paired with HP diet over a flavor paired with chow and when given the choice between the two diets, rats consume similar amounts of calories from each. The lack of increased food intake despite elevated plasma ghrelin levels coupled with increased levels of hypothalamic POMC mRNA expression in rats on high protein low carbohydrate diet indicate the possibility of dietary macronutrient influence on controls of feeding and regulation of body weight, and reveal mechanisms by which food intake and body weight are reduced in association with adherence to a low carbohydrate- high protein diet.
Acknowledgments
This research was supported by grants from the National Institutes of Health (DK070707 and MH15330).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Astrup A. Macronutrient balances and obesity: the role of diet and physical activity. Public Health Nutr. 1999;2:341–347. doi: 10.1017/s1368980099000464. [DOI] [PubMed] [Google Scholar]
- 2.Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: a dose-response relationship in adult male rats fed isocalorically. Am J Physiol. 1995;268:E546–550. doi: 10.1152/ajpendo.1995.268.4.E546. [DOI] [PubMed] [Google Scholar]
- 3.Eisenstein AB, Strack I, Steiner A. Increased hepatic gluconeogenesis without a rise of glucagon secretion in rats fed a high fat diet. Diabetes. 1974;23:869–875. doi: 10.2337/diab.23.11.869. [DOI] [PubMed] [Google Scholar]
- 4.Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002;60:189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- 5.Jean C, Fromentin G, Tome D, Larue-Achagiotis C. Wistar rats allowed to self-select macronutrients from weaning to maturity choose a high-protein, high-lipid diet. Physiol Behav. 2002;76:65–73. doi: 10.1016/s0031-9384(02)00676-5. [DOI] [PubMed] [Google Scholar]
- 6.Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2002;282:R46–54. doi: 10.1152/ajpregu.2002.282.1.R46. [DOI] [PubMed] [Google Scholar]
- 7.Pullar JD, Webster AJ. The energy cost of fat and protein deposition in the rat. Br J Nutr. 1977;37:355–363. doi: 10.1079/bjn19770039. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez I, Friedman MI. Dietary hyperphagia in rats: role of fat, carbohydrate, and energy content. Physiol Behav. 1990;47:1157–1163. doi: 10.1016/0031-9384(90)90367-d. [DOI] [PubMed] [Google Scholar]
- 9.Kettelhut IC, Foss MC, Migliorini RH. Glucose homeostasis in a carnivorous animal (cat) and in rats fed a high-protein diet. Am J Physiol. 1980;239:R437–444. doi: 10.1152/ajpregu.1980.239.5.R437. [DOI] [PubMed] [Google Scholar]
- 10.Makarios-Lahham L, Roseau SM, Fromentin G, Tome D, Even PC. Rats free to select between pure protein and a fat-carbohydrate mix ingest high-protein mixed meals during the dark period and protein meals during the light period. J Nutr. 2004;134:618–624. doi: 10.1093/jn/134.3.618. [DOI] [PubMed] [Google Scholar]
- 11.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003;78:31–39. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 13.Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med. 2002;113:30–36. doi: 10.1016/s0002-9343(02)01129-4. [DOI] [PubMed] [Google Scholar]
- 14.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Meckling KA, Gauthier M, Grubb R, Sanford J. Effects of a hypocaloric, low-carbohydrate diet on weight loss, blood lipids, blood pressure, glucose tolerance, and body composition in free-living overweight women. Can J Physiol Pharmacol. 2002;80:1095–1105. doi: 10.1139/y02-140. [DOI] [PubMed] [Google Scholar]
- 16.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142:253–258. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 17.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 18.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 19.Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Altered hypothalamic signaling and responses to food deprivation in rats fed a low-carbohydrate diet. Obes Res. 2005;13:1672–1682. doi: 10.1038/oby.2005.205. [DOI] [PubMed] [Google Scholar]
- 20.Bensaid A, Tome D, L’Heureux-Bourdon D, Even P, Gietzen D, Morens C, Gaudichon C, Larue-Achagiotis C, Fromentin G. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78:311–320. doi: 10.1016/s0031-9384(02)00977-0. [DOI] [PubMed] [Google Scholar]
- 21.L’Heureux-Bouron D, Tome D, Bensaid A, Morens C, Gaudichon C, Fromentin G. A very high 70%-protein diet does not induce conditioned taste aversion in rats. J Nutr. 2004;134:1512–1515. doi: 10.1093/jn/134.6.1512. [DOI] [PubMed] [Google Scholar]
- 22.Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35:21–28. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- 23.Likhodii SS, Musa K, Mendonca A, Dell C, Burnham WM, Cunnane SC. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia. 2000;41:1400–1410. doi: 10.1111/j.1528-1157.2000.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Taha AY, Ryan MA, Cunnane SC. Despite transient ketosis, the classic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabolism. 2005;54:1127–1132. doi: 10.1016/j.metabol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- 26.Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes. 2003;52:2923–2927. doi: 10.2337/diabetes.52.12.2923. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2003;284:E313–316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 28.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119:77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 30.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 31.Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 32.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 33.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 34.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673. [PubMed] [Google Scholar]
- 35.Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–3301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 36.Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998;9:3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- 37.Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 38.Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- 39.Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- 40.Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N Y Acad Sci. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 41.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 42.Clegg DJ, Benoit SC, Air EL, Jackman A, Tso P, D’Alessio D, Woods SC, Seeley RJ. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology. 2003;144:2941–2946. doi: 10.1210/en.2002-0218. [DOI] [PubMed] [Google Scholar]
- 43.Marks DL, Cone RD. Central melanocortins and the regulation of weight during acute and chronic disease. Recent Prog Horm Res. 2001;56:359–375. doi: 10.1210/rp.56.1.359. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu Y, Nagaya N, Isobe T, Imazu M, Okumura H, Hosoda H, Kojima M, Kangawa K, Kohno N. Increased plasma ghrelin level in lung cancer cachexia. Clin Cancer Res. 2003;9:774–778. [PubMed] [Google Scholar]
- 45.Hanada T, Toshinai K, Date Y, Kajimura N, Tsukada T, Hayashi Y, Kangawa K, Nakazato M. Upregulation of ghrelin expression in cachectic nude mice bearing human melanoma cells. Metabolism. 2004;53:84–88. doi: 10.1016/j.metabol.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Li G, Mobbs CV, Scarpace PJ. Central pro-opiomelanocortin gene delivery results in hypophagia, reduced visceral adiposity, and improved insulin sensitivity in genetically obese Zucker rats. Diabetes. 2003;52:1951–1957. doi: 10.2337/diabetes.52.8.1951. [DOI] [PubMed] [Google Scholar]