OVERVIEW
We propose a model based on the risk for detecting CIN3/(treatable) cancer before the next scheduled examination for clinical decision making regarding how often and how intensively to follow or treat patients such that comparable risk leads to comparable interventions.
Introduction
With the introduction of multiple new, promising screening and diagnostic tests, we are more capable of preventing cervical cancer and of more precisely characterizing a woman’s risk for cervical cancer. However, we must devise new rational management strategies to avoid confusion among clinicians facing unfamiliar combinations of test results and to provide optimal consistent care for women.
Background
Traditionally, the Pap smear or other cytologic method has been used as the primary test for cervical cancer screening programs. A positive cytologic result led to colposcopic evaluation and directed biopsy of apparent lesions. In turn, a biopsy diagnosed as CIN2 or worse (≥CIN2), or sometimes CIN1, led to treatment. Repetition of the cytology/colposcopy-based program has led to substantial decreases in cervical cancer rates in countries that have sufficient resources to sustain a high-quality, organized program.
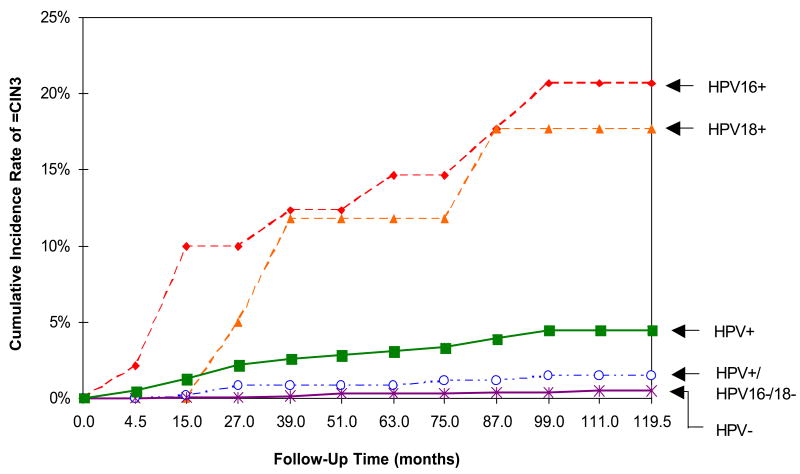
Each step in this process—cytology, colposcopy, and histology—has limitations in terms of sensitivity and specificity for our target for treatment: CIN3/cancer. (We will refer to CIN3 as the best surrogate of invasive cancer risk.) To compensate for such test limitations, we have relied on repeated screening on an annual or biannual basis. In addition, we have used CIN2 as a threshold for treatment in order to provide an additional margin of safety, even though many CIN2 lesions are destined to regress. The addition of more accurate methods of screening and diagnosis could increase both the sensitivity and efficiency of the process by decreasing the number of repetitions needed to achieve programmatic efficacy. For example, HPV testing has been introduced to increase the efficiency of cervical cancer screening by better “risk” stratification than cytology alone. However, the addition of new tests will raise questions about the appropriate management of a positive result.
Risk stratification
It is rational to make clinical decisions based on knowledge of risk of precancer (ie, CIN3) as the surrogate for cancer risk, which can be estimated for various test results and combinations of test results in prospective studies and clinical trials. Clinical algorithms, with agreed-upon a priori thresholds, would then apply for any new clinically validated test or diagnostic assay with well-documented risk associated with a positive or negative result. Careful emphasis on risk, rather than procedure, would clarify many points of confusion.
Risk thresholds
Professional clinical societies and cancer prevention experts together will make the decisions regarding the proper clinical responses to risk levels, considering benefits as well as total cost, which includes both financial and negative health consequences. In general, we believe that because screening is inherently a populationwide intervention among predominantly well women, the costs and benefits of a screening test or program should be described on a population basis. Evaluation of women with abnormal screening results, decisions on treatment, and proper posttreatment follow-up require increasing degrees of individualization and clinical judgment as risk becomes more clearly defined.
Basically, there are currently 4 options for management: 1) routine screening interval; 2) more intensive follow-up with shorter interval to screening; 3) colposcopy and biopsy; and 4) treatment. To promote discussion, we can provide some possible thresholds for risk of CIN3/cancer, as illustrated in the Figure, based on examples from currently used management strategies. Given that there is no such thing as zero risk, the first principle is to acknowledge that there is an acceptable low level of risk that does not trigger more intensive follow-up.
Women with a <2% risk of precancer within the subsequent 2–3 years (eg, women who test carcinogenic HPV negative in routine screening) may be at an acceptable low risk to remain in regular interval screening. Regular screening may include 1- to 2-year screening with cytology alone or every 3 years with cytology and carcinogenic HPV co-testing for women 30 and over. Women with a 2% to <10% risk (eg, women with negative cytology who test carcinogenic HPV positive in screening or women with atypical cells of undetermined significance [ASCUS] cytology unqualified by carcinogenic HPV testing) might warrant rescreening in a year. Women with a ≥10% risk (eg, women with low-grade squamous intraepithelial lesion [LSIL] cytology or carcinogenic HPV-positive ASCUS) may warrant immediate colposcopic evaluation, including multiple biopsies to maximize the sensitivity of colposcopy in high-risk women.
More controversial is the suggestion that women identified with a risk of precancer above a certain threshold may warrant treatment even if an initial colposcopic evaluation and directed biopsy, because of imperfect sensitivity, does not identify a precancerous lesion on that day. We should recognize that the current treatment threshold of CIN2 results in significant overtreatment of many women whose lesions would regress spontaneously; we already accept this overtreatment in order to provide a margin of safety against misclassification of diagnosis and risk.
With informed decision making between patient and clinician that takes into account patient well-being, it may be more cost effective and less invasive to immediately treat women with very high-risk profiles than to require 2 or more clinical visits, including an intensive follow-up with multiple biopsies to find the precancerous lesion that is probably present before it invades, followed by treatment.
Final Comments
In summary, we have unprecedented capability to identify women at the greatest and lowest risk of cervical precancer and cancer using current and soon-to-be-available prevention tools. Our capacity to accomplish this is much more robust than the extensively cited and used Gail model for breast cancer risk as we can now or will soon be able to ascribe risks of cervical precancer ranging from virtually zero risk to almost 100% risk. A risk management model for cervical cancer prevention, based on clinical actions corresponding to risk stratum, can result in better allocation of resources to and increased safety for women at the greatest risk and increased well-being for women at the lowest risk.
CLINICAL IMPLICATIONS
A risk management model for cervical cancer prevention, based on clinical actions corresponding to risk of CIN3/(treatable) cancer, can result in better allocation of resources to and increased safety for women at greatest risk and increased well-being for women at lowest risk.
Figure 1.
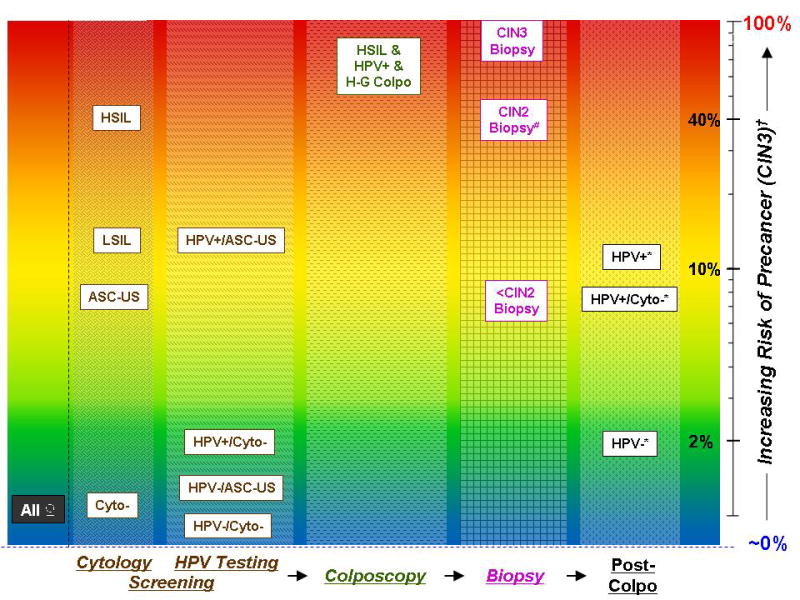
A graphical representation: the risk of cervical precancer at different stages and results of screening and clinical management for cervical cancer prevention. The risks for each stage and result are approximate risks for CIN3 within a screening interval. The axis to the left of the figure represents increasing risk, from nearly 0% (blue) to 100% (red), of cervical precancer on a log scale. Each stage of screening and clinical management is represented by a different pattern, with the arrows indicating the sequence of the stages. #Less than half of the cases of CIN2 on biopsy are subsequently diagnosed as CIN3 on excisional tissue (precancer). †Within a screening interval. *Test results at the next follow-up visit (≥6 months).
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.