Abstract
The role of VEGF during development and in pathology is well known, but its function in normal adult tissues is poorly understood. Adverse effects associated with the use of anti-angiogenic therapies targeting VEGF in human pathologies have begun to reveal potential functions of VEGF in quiescent vasculature. Further clues from expression studies of VEGF and its receptors in the adult, from the disease preeclampsia, and from experimental neutralization studies, have suggested that VEGF is involved in endothelial cell survival and fenestration, as well as in the signaling and maintenance of non-endothelial cells. The various biochemical properties of VEGF, and its interaction with other growth factors, may be an important point in determining whether VEGF functions as a maintenance factor versus an angiogenic factor. A thorough understanding of the function of VEGF in the adult may lead to more efficacious pro- and anti-angiogenic therapies.
Keywords: VEGF, angiogenesis, preeclampsia, anti-angiogenic therapy, bevacizumab, endothelial cells, Avastin, fenestrations
Introduction
Vascular endothelial growth factor (VEGF, VEGF-A or VPF) was first described as a tumor-derived factor with potent ability to induce endothelial cell permeability [1], proliferation and angiogenesis [2, 3]. Since its initial discovery, VEGF’s action on endothelial cells (EC) has been expanded to include migration and invasion into the basement membrane, proliferation, survival and the formation of fenestrations, which has largely been elucidated using in vitro and in vivo tumor studies. VEGF is biologically active as a homodimer of approximately 40kD, belonging to a family of secreted glycoproteins, including VEGF- B, C, D and placenta growth factor (PlGF). During development, VEGF expression initiates prior to gastrulation [4], and is important in both vasculogenesis, the process by which blood vessels develop de novo from endothelial cell precursors, and angiogenesis, in which blood vessels sprout from existing blood vessels [5, 6]. Deletion of either a single or both alleles of the VEGF gene in mice results in embryonic lethality by E9.5 and E10.5 with severe vascular abnormalities [7, 8]. Furthermore, overexpression of VEGF also results in embryonic lethality [9]. These observations underscore the importance of proper regulation of VEGF expression for normal development to occur. VEGF signaling is mediated via two receptors: VEGFR1/Flt1 and VEGFR2/Flk1; homozygous mutation of either of these receptors results in embryonic lethality [10–12]. In addition, two co-receptors for VEGF, neuropilin-1 and 2 (Nrp-1 and Nrp-2) [13, 14], are also required embryonically [15, 16]. The relative contributions of the various VEGF receptors on VEGF signaling in different endothelial cell beds are still not well understood.
While extensive research has shown that VEGF is crucial for developmental, physiologic and pathologic angiogenesis, whether it is required in the adult is not well understood. Early studies of genetic VEGF targeting [8, 17] and neutralization studies in tumorigenic mice [18] suggested that VEGF is not required in the adult as these adult mice showed no obvious phenotype, making it an attractive target in many diseases. As a result, a wealth of pharmacologic agents have been designed to target either VEGF or its receptors. Though beneficial effects have been observed, the presence of consistent significant side effects suggests that VEGF is important in the maintenance of quiescent vasculature and non-vascular tissues. This review discusses the expression of VEGF and its receptors in adult tissue, VEGF association with fenestrated vasculature, VEGF effects on non-vascular cells, conditions and factors that may affect VEGF action, and implications for manipulating VEGF in disease treatment.
VEGF expression in the adult
VEGF is robustly expressed embryologically, and is critical for proper blood vessel formation, but its expression is also important in the adult in mediating physiologic angiogenesis during the female reproductive cycle in the uterus, ovary and breast [19, 20], in wound healing [21, 22], in bone repair [23] and in skeletal muscle in response to exercise [24]. VEGF is also upregulated and involved in pathophysiologic processes such as in rheumatoid arthritis [25, 26], psoriasis [27], atherosclerosis [28], amyloid lateral sclerosis (ALS) [29], age-related macular degeneration (AMD) [30, 31], diabetic retinopathy [32, 33], retinopathy of prematurity [34], sepsis [35] and tumor angiogenesis [36, 37]. Although VEGF is required for postnatal processes that involve angiogenesis, little is know about its potential role in quiescent vascular beds.
Some insight into VEGF’s potential role in the adult comes from the analysis of its expression pattern. VEGF is expressed in virtually every tissue in the adult [38–47]. A systematic study of VEGF expression in the adult using VEGF-lacZ mice [46], revealed that specific subsets of cells in each tissue express VEGF. Three general patterns of expression were observed, which may provide information to the role of VEGF in those tissues (Table 1). In regions with sparse cellular VEGF expression, VEGF was expressed primarily by pericytes and vascular stromal cells. During blood vessel maturation, pericytes are differentiated from mesenchyme and become tightly associated with EC, an event associated with blood vessel maturation. Coincident with their differentiation, pericytes begin to synthesize VEGF, which mediates, at least in part, capillary stabilization [48]. In tissues including the retina (Figure 1a), brain and testes, the vasculature is characterized as having barrier function (retinal-blood barrier, blood-brain barrier, testis-blood barrier); interestingly, relatively few cells express VEGF in these organs, and it is possible therefore that the relatively low expression of VEGF contributes to the impermeability of these vessels. In addition to its putative role as an endothelial survival factor, there is recent evidence to support a role for VEGF as a tropic factor for retinal neurons [49].
Table 1.
Pattern of VEGF expression in the adult
| Sparse cellular expression | Intermediate cellular expression | Dense cellular expression | |
|---|---|---|---|
| Cellular Source | Vascular stromal cells: pericytes and smooth muscle cells | Non-vascular mesenchymal cells | Epithelial cells directly abuting capillaries |
| Examples | Brain parenchymal cells, pericytes, retinal pericytes, Leydig cells | Skeletal myocytes, cardiac myocytes | Glomerular epithelium, choroid plexus epithelium, serous salivary epithelium, pancreatic β-islet epithelium, retinal pigmented epithelium |
| Microvasculature Characteristics | High resistance endothelium (blood-brain-barrier, retinal-blood-barrier, testis-blood-barrier) | Dynamic capillaries responsive to demands of tissue. | Highly leaky vessels- fenestrated vessels in endocrine organs, and sinusoidal vessels in liver |
| Postulated Function of VEGF on Endothelial cells | Endothelial cell survival | EC survival, dynamic angiogenesis | EC survival, maintenance of fenestrations and sinusoids |
Figure 1. Expression of VEGF and its receptors in the adult.
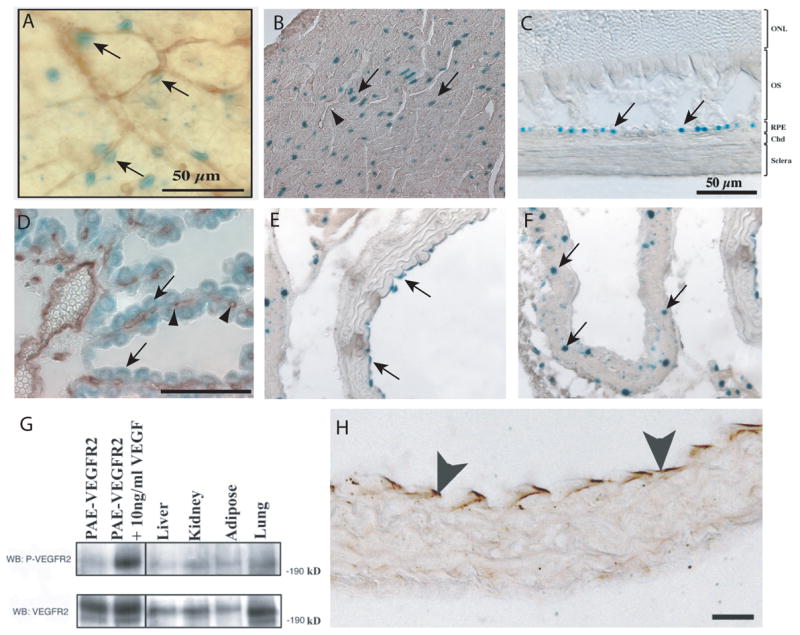
VEGF expression is shown in adult VEGF-lacZ mice, which make a nuclear localized β-galacotosidase (β-gal) protein wherever VEGF is expressed. Blood vessels, where shown, were identified by immunohistochemistry using the pan-endothelial cell marker- CD31 (arrowheads). VEGF expression in the pericytes in retina (A, arrows), cardiac myocytes (B), choroid RPE layer (C, arrows), choroid plexus epithelium (D, arrows) overlying fenestrated blood vessels (D, arrowheads), aortic endothelium (E, arrows), inferior vena cava medial layer cells (F, arrows). VEGFR2 expression and activation in adult tissues as identified by (G) western blot for VEGFR2 of protein lysates from liver, kidney, adipose and lung (bottom panel) and for phosphorylated VEGFR2 (top panel) and by (H) immunohistochemistry of aorta for phosphorylated VEGFR2 (arrows). Abbreviations (C): ONL, outer nuclear layer, OS, outer segment, RPE, retinal pigmented epithelium, Chd, choroid. Reprinted from IOVS 2006, 47: 3135–3142 (C), Am J Pathol 2006, 168: 639–658 with permission from the American Society of Investigative Pathology (B, D–H), unpublished data D’Amore lab (A).
In the tissues with an intermediate number of cells expressing VEGF, such as in cardiac and skeletal muscles, most myocytes appeared to express VEGF (Figure 1b). As these tissues are physiologically dynamic and have the capacity to hypertrophy with training, VEGF may act to support the rich vasculature of the muscle [50, 51], depending on the physiologic requirement of the muscle.
The highest density of VEGF expressing cells was found in tissues with fenestrated vasculature and was localized to the epithelia in contact with fenestrated vessels. Tissues, whose microvasculature is fenestrated, have secretory and/or filtration functions, for example the glomerulus (urine filtrate), choroid plexus (Figure 1d) (cerebrospinal fluid production), pancreas (exocrine and endocrine secretions) and liver (filtration and secretion). Thus, VEGF in these tissues may mediate both survival and maintenance of the fenestrated state of the EC.
Though ECs are the main target of VEGF, they do not generally express VEGF [52], but instead respond to VEGF secreted in a paracrine manner. It is speculated that expression of both the receptor and ligand in EC would lead to a dangerous positive feedback, resulting in overproliferation, such as is observed in hemangiomas [53] and in tumorigenesis [54]. One exception to this appears to be in the largest blood vessel, where aortic EC, but not inferior vena cava EC, express VEGF (Figure 1e, f, respectively)[46]. The mechanism or function of VEGF expression in the aortic EC is currently unknown.
Although VEGF binds to VEGFR1, VEGFR2, Nrp-1 and Nrp-2, its main signaling receptor in the endothelium is VEGFR2. VEGFR2 belongs to the family of receptor tyrosine kinases [55] (reviewed in [56]), and upon VEGF binding, there is dimerization and activation of the tyrosine kinase, resulting in phosphorylation of specific tyrosine residues on the cytoplasmic tail, which in turn promotes docking of signal transducing molecules. Several key tyrosine residues have been identified, and activation of specific residues leads to different downstream effects. Of these residues, Tyr 1175 is implicated in endothelial survival and permeability, though not exclusively [57], through the PI3-K/AKT pathway [56, 58]. Signaling via residue Tyr 951 is also involved in vascular permeability [59]. Although VEGFR1 is also expressed by EC, it is believed to act primarily to modulate VEGFR2 signaling. Evidence for this concept comes from observations that although targeted disruption of VEGFR1 signaling results in EC overgrowth and vascular disorganization [10, 60], mice in which the intracellular kinase domains of VEGFR1 was deleted have no obvious vascular phenotype [61]. Recent evidence has suggested that VEGFR1 can anchor VEGF to the cell-surface, allowing increased interaction with VEGFR2 [62].
For VEGF to exert an effect in the adult, its receptor also has to be expressed in close proximity to the VEGF source. Several recent reports have demonstrated that VEGFR2 is expressed and activated in adult tissue [46, 47, 63, 64] (Figure 1g, h), giving further evidence that VEGF plays a biologic role in the adult.
Implications of the role of VEGF in the adult from clinical observations
A. Side effects from clinical systemic VEGF neutralization
All cells require oxygen, and due to the limits of oxygen diffusion, cells more than 100 μm away from blood vessels become oxygen deprived. For this reason, solid tumors are restricted to a size of 3–4 mm in diameter without neovascularization. The discovery of a tumor-secreted, angiogenic factor [65], which was later identifed as VEGF [3], and was hypoxia-inducible [66], led to the concept that neutralization of VEGF could block tumor-induced blood vessel growth, thus inhibiting tumor growth and metastasis [67]. Early reports suggested that VEGF was not important in microvascular homeostasis [8, 17], and thus VEGF became an attractive target for anti-angiogenesis; it was believed that VEGF neutralization would specifically target the tumor microvasculature without affecting non-diseased vascular beds, thus circumventing side effects associated with standard chemotherapy. This model has now been also applied to other VEGF-mediated diseases, and has led to the implementation of therapies for ocular vascular proliferative diseases as well [68].
As an anti-tumor agent, clinical development of many agents that target either VEGF or its receptors (see [69] for a detailed list of agents) are in progress. The first FDA-approved anti-VEGF therapy, bevacizumab (Avastin™, Genentech) was introduced into the clinics in 2004 in combination with Irinotecan, fluorouracil, or leucovorin for the treatment of metastatic colorectal cancer (mCRC) [70] and more recently for the combination treatment of advanced non-small-cell lung cancer [71]. Subsequently, Avastin is now being clinically tested as a mono- or combination therapy agent for the treatment of cancers in almost every organ system [72], with many in either Phase III or IV. Clinical positive effects of Avastin treatment in mCRC and lung cancer include a marked tumor regression and increase in median survival as well as improvement in long-term survival in some patients [73].
Common side effects of Avastin treatment have been noted including hypertension, proteinuria, bleeding and impaired surgical wound healing [70, 71, 74]. Infrequent, but life-threatening complications have been reported as well, including, arterial thrombosis [75, 76], gastrointestinal perforation [77], and reversible focal posterior leukoencephalopathy (RFLP) [78–80] (Figure 2).
Figure 2.
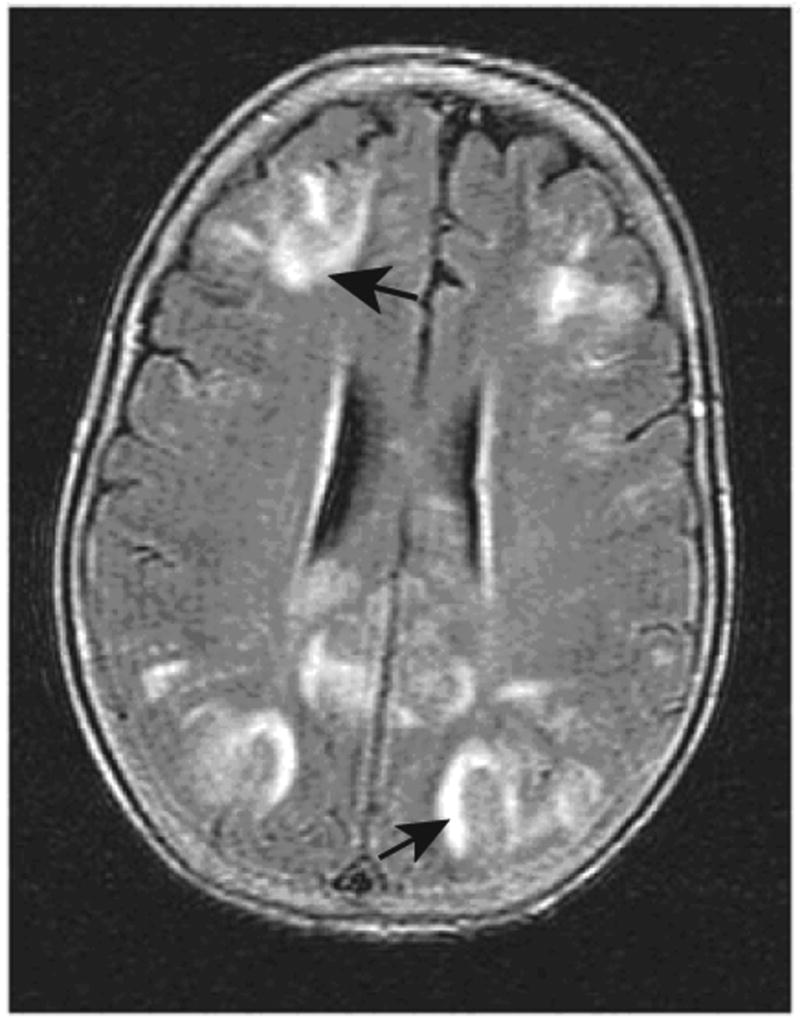
MRI FLAIR image showing focal lesions (arrows) associated with reversible focal leukoencephalopathy in a patient on Avastin. Taken with permission from N Engl J Med (2006) 354; 980–982, Copyright © 2006 Massachusetts Medical Society
In addition to targeting the VEGF ligand, another approach of anti-VEGF therapy is to target its receptors [81]. Several drugs targeting VEGF receptors have been FDA approved, including sunitinib (Sutent™, Pfizer) for the treatment of advanced renal cell carcinoma [82, 83] and gastrointestinal stromal tumors [84] and sorafenib (Nexavar, Bayer/Onyx Pharmaceuticals) for the treatment of clear-cell renal cell carcinoma [85]. Side effects similar to those noted with Avastin have been reported; however, these drugs have several additional side effects probably due to the effects on other receptor tyrosine kinases. Although these drugs selectively block VEGFR2 and VEGFR1, there is significant inhibition of other receptors as well, due to the homology of these receptors, including VEGFR3, PDGFRs, and receptor kinases associated with KIT, and RET receptor kinases [84, 85].
The reproducible side effects related with these drugs indicate that VEGF neutralization in quiescent tissue does in fact influence tissue function. Furthermore, the incidence and severity of side effects associated with VEGF blockage may provide insight to VEGF-sensitive tissues and VEGF-dependent processes in the adult.
B. VEGF neutralization in the pathogenesis of preeclampsia
Recent evidence from the disease preeclampsia has also begun to provide evidence concerning the role of VEGF in the adult. Preeclampsia (reviewed in [86]), a disease of pregnancy, affects 5–7% of women in their 2nd and 3rd trimester, and is marked by hypertension, edema and proteinuria. The pathology is caused by systemic endothelial dysfunction, and if left untreated, ascites, pleural edema, thrombocytopenia, headaches, disseminated intravascular coagulation, and blindness can occur. In severe cases, there is progression to eclampsia, characterized by seizures, and other neurologic manifestations including strokes.
Because delivery of the placenta results in immediate improvement and cessation of most symptoms, it was hypothesized that the placenta was the source of the insulting agent. Several factors were found to be over expressed by preeclamptic placentas, including soluble VEGFR1/sFlt1, which is found at high levels in the blood of preeclamptic mothers [87–89]. sFlt1 is produced by alternative splicing of the Flt1 transcript, resulting in a deletion of the intracellular and transmembrane domains of Flt1, rendering the truncated protein soluble. sFlt includes 85% of the extracellular domain, plus 30 amino acids from the 13th intron [90]. sFlt1 binds VEGF with an affinity 10-fold greater than VEGFR2, thus acting as a soluble trap of VEGF [91, 92]. sFlt1 can also heterodimerize with VEGFR2 acting in a dominant negative manner [93]. Systemic administration of sFlt1 to pregnant rats recapitulates several, but not all symptoms of preeclampsia, including hypertension and proteinuria [87]. In addition to proteinuria and hypertension, reversible posterior leukoencephalopathy, a severe complication of preeclampsia, has also been seen in patients on Avastin [94]. The parallel between these hallmarks of preeclampsia and the side effects of Avastin support the concept that VEGF is involved in vasculature maintenance. Although hypertension, which is common to both preeclamptic and Avastin patients, has been suggested to be the basis of the leukoencephalopathy, it has also been suggested that primary insult to brain endothelium may also contribute to this condition. However, this has not been studied, and remains an interesting, unanswered question.
Role of VEGF in the adult
A. Vascular stability
While therapeutic VEGF neutralization and preeclamspia have revealed potential roles for VEGF in the adult, experimental studies in rodents have begun to unravel the nature of VEGF function in quiescent tissues. Inhibition of VEGF or its receptors has been shown to lead to alteration of the microvasculature as well as vessel regression in a number of tissues. In the kidney this resulted in glomerular endotheliosis and proteinuria (Figure 3c, f) [87, 95], whereas in the lung alveolar apoptosis and enlarged airspaces were observed (Figure 3a, d) [96, 97]. Vessel regression was also noted in the pancreas [98, 99], trachea [98, 100], thyroid (Figure 3b, e) [98], and small intestine [98]. Interestingly, there were no apparent alterations in the microvasculature of heart, musculature of the tongue, or brain [98]. In addition to microvascular regression, hypertension was a common finding, and may be the direct result of disrupting vascular NO-mediated tone [87, 101]. Alternatively, it may be secondary to kidney injury or to destruction of microvasculature (rarefaction), resulting in increased systemic resistance.
Figure 3. Role of VEGF in vascular stability.
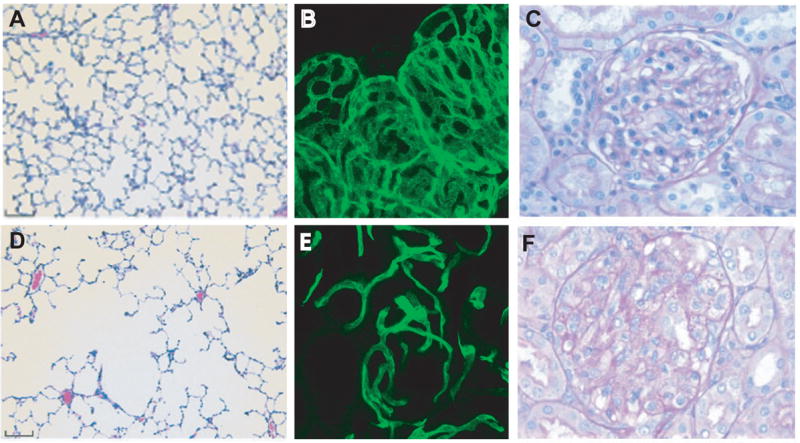
Administration of SU5416, a tyrosine kinase inhibitor with selective inhibition of VEGFR, to rats by bronchiolar delivery results in enlargement of air spaces, indicative of emphysema (D) compared to vehicle (A). Adenoviral delivery of sVEGFR1 to adult mice (B, E) results in decreased vascular density in the thyroid (E) compared to vehicle delivery (B), and endotheliosis in the glomerulus in the kidney in pregnant rats (F) compared to vehicle delivery (C). Taken with permission from J Clin Invest (2000) 106; 1311–1319 (A, D), Am J Physiol Heart Circ Physiol (2006): H560-H576 (B, E), and J Clin Invest (2003) 11; 649–658 (C, F).
The blood vessel regression and tissue dysfunction observed in these experiments are presumably due to interference with the trophic effect of VEGF on endothelial survival [100, 102]. The cascade of events likely begins with local thrombosis and decreased vascular perfusion, leading to endothelial cell apoptosis [102]. Basement membrane “sleeves” with pericytes devoid of EC [98] persist for a period of time, and can be recanalized by EC if neutralization is reversed. Neutralization of VEGF for seven days followed by seven days of reversal resulted in almost complete revascularization [103]. That short inhibition of VEGF may result in regrowth of vessels following cessation of VEGF inhibition, may provide a challenge in anti-VEGF therapies. Conversely, the vestigial presence of the basement membrane ghost in tissues such as infarcted brain and heart tissue, may allow targeted delivery of VEGF to efficiently stimulate new blood vessels.
B. Effects of VEGF on non-vascular cells
VEGF, like other growth factors (e.g. hepatocyte growth factor and fibroblast growth factor) was so named because of initial observations demonstrating EC as its target. However, VEGF may act on other cell types based on the presence of either VEGFR1 or VEGFR2 on those cells. In addition, several reports have demonstrated the action of VEGF on monocytes [104, 105], macrophages [106], mast cells [107], eosinophils [108], dendritic cells [109], megakaryocytes [110], lymphocytes [111], hematopoeitic stem cells/bone marrow-derived circulating cells [112–115], type II lung alveolar epithelial cells [116] and lens epithelium [117]. VEGF has been shown to mediate the survival of hematopoeitic stem cells, neuronal cells, neuronal stem cells and lymphocytes; the differentiation of megakaryocytes and dentritic cells; and, to mobilize bone marrow precursor cells. In addition to its direct effects on endothelial cells during angiogenesis, VEGF also influences angiogenesis via recruitment of bone marrow endothelial cell precursors and inflammatory cells.
Observations from the therapeutic use of anti-VEGF, as well as from preeclampsia, also provide evidence regarding the action of VEGF on non-endothelial cells. Findings of thrombocytopenia and neutropenia [69, 74, 118] are consistent with a role for VEGF in the regulation of platelet and leukocyte production. Gastroperforation and impaired wound healing likely result from impaired immune cell function and angiogenesis.
VEGF is also involved in lymphangiogenesis [119–121]. Although its function in lymphatic maintenance is unknown, edema in preeclamptic patients [86] may be due, at least in part, to lymphatic destruction besides decreased osmotic pressure, and warrants further study.
In addition to its action on vascular and immune cells, increasing evidence points to a role for VEGF in neuronal growth and survival. VEGF acts on neuronal cells [49, 122, 123] and neuronal stem cells [124] in vitro and in vivo. In the eye, both neuronal and glial cells express VEGF receptors [125]; addition of VEGF120 following ischemic/reperfusion injury resulted in neuroprotection of retinal ganglion cells and of cells in the inner nuclear layer (Figure 4) [126]. Pathologically, low levels of VEGF are associated with motor neuron degeneration [127], and are implicated as a modifier in amyotrophic lateral sclerosis in humans and mice [29, 128] and Alzheimer’s disease in humans [129]; experimental delivery of VEGF acts to prolong neuron survival in the ALS mice [130]. Although not examined experimentally, peripheral neuropathy associated with Avastin, reversible focal posterior leukoencephalopathy in both Avastin-treated and preeclamptic patients, as well as seizures in eclamptic patients, are consistent with a neuro-protective role for VEGF [131].
Figure 4. Neuroprotective role of VEGF in the eye.
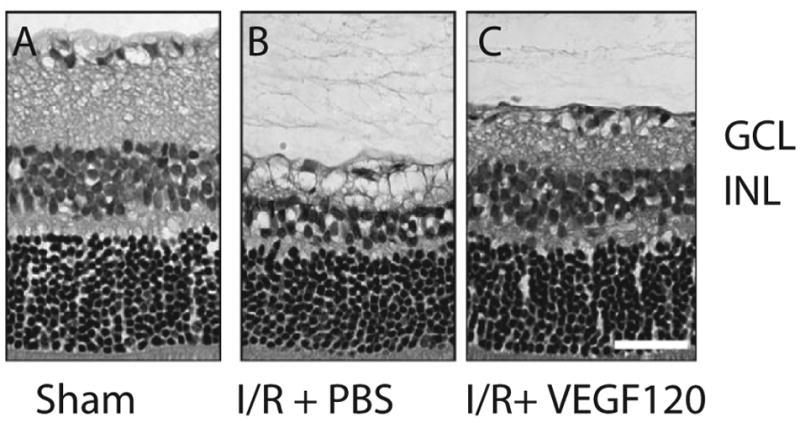
Addition of VEGF120 after 60 min of ischemic/reperfusion injury resulted in decreased cell death in the ganglion cell layer (GCL), and inner nuclear layer (INL) after 14 days (C) compared to addition of PBS only (B). Taken from Nishijima et al. AJP 2007 (IN PRESS) with permission from the American Society of Investigative Pathology.
C. VEGF and fenestrations
VEGF was first identified as a potent vascular permeability factor [1]. Permeability is important in many physiologic processes such as in glomerular filtration, cerebrospinal fluid production, liver blood filtration, and endocrine secretion into the blood stream [132], is central to wound healing [21], and exacerbates tumors by promoting ascites and edema. Endothelial cell permeability is mediated via both VEGFR1 and VEGFR2 [56], resulting in the formation of transcellular gaps, vesiculovacuolar organelle formation, and fenestrations [133]. Fenestrae are endothelial cell plasma membrane specializations that appear as circular discontinuities approximately 60 nm in diameter and facilitate movement of particles in and out of the circulation. Fenestrated EC are found in most endocrine tissues [132] as well as in the kidney glomerulus, choroid of the eye, choroid plexus, gastrointestinal tract and in tumor vessels. In each of these tissues VEGF expressing cells are found in close juxtaposition to the underlying fenestrated microvasculature (Figure 1d) [46].
VEGF has been shown to be involved in the induction of fenestrae formation in vitro [134–136], but the mechanism of fenestrae formation and maintenance in the adult is not well understood. One link that has been made between VEGF and fenestrae is the regulation of the caveolar protein plasmalemmal vesicle-associated PV-1 protein [137] [138]. However, not all fenestrations are diaphragmmed, so PV-1 may not be the only mediator of VEGF and fenestrations.
Although all fenestrated tissue contain VEGF expressing cells, not all tissue with VEGF expressing cells are fenestrated. There are several possible explanations for this observation. Although VEGF may be important for inducing and maintaining fenestration, other factors may be required along with VEGF for fenestrations. For instance, leptin has been shown to act synergistically with VEGF to induce fenestrations [139]. Whether leptin is expressed in all fenestrated tissues, is to be determined. These ‘co-factors’ may not be present in all tissue, even if VEGF is present, thus not allowing fenestrations. Conversely, some tissues may even produce factors to prevent permeability [140]. Another likely possibility is that the induction of fenestrations may simply be a dosage effect, with tissues that display fenestrated capillaries expressing higher levels of VEGF than tissues without fenestrated endothelium.
Several studies have demonstrated that VEGF neutralization in tumors in vivo leads to a diminution of fenestrations [102]. Tumors are often edematous, and it has been postulated that high interstitial pressure may make the delivery of chemotherapy to tumor cells inefficient. Therefore, reducing tumor vessel permeability may have therapeutic advantages in cancer treatment, allowing normalization of tumor vasculature and more efficient delivery of chemotherapy [69, 141]. However, systemic VEGF neutralization has also been reported to affect non-tumor fenestrated vasculature, including the thyroid, pancreas and glomerulus [98].
Biochemical, molecular and cellular factors affecting VEGF signaling in vivo
Although VEGF is constitutively expressed in the adult, and is a potent angiogenic factor, it does not result in widespread angiogenesis in resting tissue. The discovery of multiple pro-angiogenic and endogenous anti-angiogenic factors [142] with overlapping expression patterns indicates that angiogenesis is dependent on a balance between pro- and anti- angiogenic factors. In addition to countering the action of local angiogenic factors in quiescent tissue, endogenous factors maintain the avascularity, for example, chondromodulin-I acting in cardiac valves [143] and sFlt-1 in the cornea [144, 145]. VEGF signals via several receptors and co-receptors, and via multiple signaling pathways [56, 146]. Thus, whether VEGF signaling leads to permeability, proliferation, migration, or survival, may therefore depend on the signaling pathway components, effective tissue concentration as well as on the action of other factors.
VEGF expression in the adult is cell-type specific [45, 46] and is controlled at many levels from transcription [147] to translation [148], and is upregulated in tumors and in various pathologic states. One of the best-characterized stimuli of VEGF transcription is hypoxia, which acts by stabilization of the hypoxia-inducible factor-1 alpha (HIF1α) transcription factor [149]. Hypoxic regulation of VEGF also takes place post-transcriptionally via mRNA stabilization [150](reviewed in [151]). VEGF expression is induced by other growth factors and cytokines including IGF-1, Il-6, Il-1, PDGF, TNF-α, TGF-β and FGF-4 [152, 153]. In addition, VEGF expression is also stimulated by physical forces, including stretch [50, 154], with one putative transcription factor being the Kruppel like factor-2 [155]. Analysis of the VEGF promoter reveals many other potential transcription factor responsive elements [149], of which several pathways have been elucidated, for example EGF [156] and HGF signaling [157] via the SP1 responsive element (for an extensive list see [149]).
Alternative splicing of VEGF mRNA results in various isoforms, which include VEGF121, VEGF145, VEGF165, VEGF189 and VEGF206, in humans and VEGF120, VEGF164 and VEGF188 in mice. These isoforms display tissue-specific patterns of expression [45]. Studies of genetically engineered mice expressing only one VEGF isoform indicate that VEGF isoforms have distinct yet some overlapping roles in vascular development and function as evidenced by tissue-specific vascular defects in these mice [158]. The VEGF isoforms display differences in their biochemical properties, including receptor binding with VEGF165 and VEGF188 but not VEGF120 binding to neuropilins and heparan sulfate. The differential affinity to heparan sulfate is important in their binding to VEGFR1 and VEGF2 as heparan sulfate can mediate the binding and transactivation of these receptors [159]. Furthermore, differential binding to heparan sulfate is reported to lead to different VEGF actions, including endothelial cell survival, adhesion and vascular branch formation [160, 161]. Both VEGF164 and VEGF188 bind heparan sulfate, making them partially or fully cell-bound, respectively, whereas VEGF120 does not bind heparan sulfate, and is freely diffusible [162]. A newly identified splice variant of VEGF, VEGF165b, is postulated to have an inhibitory effect on angiogenesis [163].
VEGF, a secreted glycoprotein, is subject to a variety of extracellular modifications that have direct effects on its biochemistry and bioavailability. In addition to alternative splicing, VEGF variants arise from proteolytic processing (reviewed in [21]), which leads to differences in biologic effects. For instance, cleavage of VEGF189 by urokinase, allows it to more potently bind to VEGFR2 [148]; plasmin digestion of VEGF165 results in two fragments, one with and one without a heparin binding domain [164], while cleavage of VEGF by various metalloproteinases results in different cleavage products with different outcomes on vascular morphogenesis [165].
VEGF exerts its action via binding to VEGFR1, VEGFR2, Nrp-1 and Nrp-2. Whereas binding of VEGF to Nrps enhance VEGF’s action [166], binding to VEGFR1 results in a diminution of VEGF availability. Therefore, it is possible that the tissue ratios of VEGFR1:VEGFR2 determine the tissue’s angiogenic status. For instance, under hypoxic conditions, when VEGF is upregulated, concomitant upregulation of VEGFR1 [167], may act to modulate VEGF signaling.
Implications for therapeutic manipulation of VEGF
The role of VEGF in development and pathology is well known and emerging evidence points to a role for VEGF in the maintenance of quiescent tissues. Knowledge of adverse-effects associated with anti-VEGF therapy, observations from preeclampsia, and experimental blockade of VEGF in animal models have exposed tissues and vascular beds sensitive to VEGF neutralization and revealed potential limitations of current anti- therapy. One of the most frequently observed side effects of VEGF neutralization is hypertension [74, 87]. However, this issue can be pharmacologically managed, allowing continued patient treatment. Similarly, the knowledge of increased risks for other side effects such as impaired wound healing, gastrointestinal perforation, and arterial thrombosis allows careful monitoring of the patient to avoid serious consequences.
In addition to observations from experimental and clinical observations of VEGF, important information about additional roles for VEGF can be garnered from clinical observations in which low VEGF levels are associated with tissue dysfunction, including observation of alopecia in humans with loss of VEGF in hair follicles [168], decreased VEGF in the aged cochlea [169], and reduced VEGF associated with thyroid cartilage ossification [170]. Another interesting recent report that warrants careful clinical monitoring is an association of increased erythropoiesis with VEGF neutralization [171], as polycythaemia can lead to high blood viscosity and an increased risk for thrombosis. As studies reveal other functions of VEGF in the adult, clinicians should be able to incorporate this information into their clinical follow-up.
Understanding side effects and biologic effects of VEGF neutralization can also provide useful surrogate markers of therapeutic efficacy. Some of the markers currently being investigated include protein levels in plasma (e.g. VEGF) [172, 173] and urine [174], circulating endothelial progenitor cells [175, 176], and wound healing time [177].
Systemic side effects may also be minimized by local or targeted delivery of anti-VEGF therapies. VEGF-A is a major mediator of pathologic angiogenesis in the eye and is associated with diseases such as age-related macular degeneration (AMD) [31] and proliferative diabetic retinopathy [33]. As in tumors, VEGF is upregulated in these ocular diseases and is, thus, a useful potential therapeutic target (reviewed in [30, 68]). Two VEGF inhibitors, pegaptanib (Macugen™; OSI/Pfizer) [178] and ranibizumab (Lucentis™, Genentech) [179] have been approved by the FDA for intra-ocular use in AMD, and Avastin has been utilized intraocularly off-label for the treatment of AMD as well [180]. For this approach however, a primary consideration will be potential effects that may arise from high local delivery. As stated in Section B, VEGF is involved in lens signaling [117], choroid maintenance [47] and retinal neuronal maintenance [125].
As these drugs are delivered intravitreally, the potential for systemic side effects should be minimized, and have indeed resulted in minimal systemic side effects [68]. However, these medications appear to have the potential to inhibit VEGF systemically as intraocular injections of Lucentis in Rhesus monkeys have resulted in systemic serum levels of approximately 150 ng/ml following bilateral injection of 500 μl of the drug with a half life of 3.5 days [181]. This circulating level may be able to sequester plasma VEGF and act locally in tissue, and may be causal for increased incidences of arterial thromboembolic events in patients treated with Lucentis [182].
A better understanding of the role of VEGF in the adult may also lead to therapies aimed at stimulating blood vessel growth or tissue growth in damaged tissue. Pro-angiogenic therapy using VEGF may be useful in ischemic diseases such as stroke [183], myocardial ischemia and coronary artery disease [184–186], peripheral neuropathy [187], wound [21] and fracture healing [188], Alzheimer’s disease [129] and ALS [122, 123]. However, delivery of VEGF will have to be approached carefully to avoid a systemic VEGF increase [189] because shifting the balance between endogenous angiogenesis inhibitors and stimulators may lead to aberrant vessel growth and/or exacerbation of existing diseases. Support for this comes from studies in which overexpression of VEGF in mouse podocytes leads to collapsing glomerulopathy [95], and in which VEGF overexpression in the skin results in inflammation [163]. In clinical trials using VEGF therapy, lower extremity edema and hypotension was observed [190], likely attributable to increased vascular permeability.
The manipulation of VEGF has allowed for therapies that lead to significant improvement in survival in cancer, vision in AMD, cardiac function in heart disease, and is currently in clinical and experimental trials in the treatment of many more diseases. Elucidating the role of VEGF in the adult should permit the development of strategies that effectively target the disease, while minimizing side effects and morbidity.
Acknowledgments
We thank Dr. Janice Nagy, Dr. Robyn Loureiro and Ms. Christine Bagley for helpful discussions and critical review of this manuscript. This work was supported by NIH EY05318, EY15435 and CA45548 (P.A.D), and NIGMS minority pre-doctoral fellowship, NIH-F31-GM65079 (A.S.R.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–8. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 3.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–8. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212(2):307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 6.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 9.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127(18):3941–6. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 10.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 11.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 12.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255(5047):989–91. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 13.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165 [corrected] J Biol Chem. 2000;275(24):18040–5. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 14.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126(21):4895–902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 16.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99(6):3657–62. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98(8):4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otani N, Minami S, Yamoto M, Shikone T, Otani H, Nishiyama R, Otani T, Nakano R. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1999;84(10):3845–3851. doi: 10.1210/jcem.84.10.6025. [DOI] [PubMed] [Google Scholar]
- 20.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4(1):18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eming SA, Krieg T. Molecular Mechanisms of VEGF-A Action during Tissue Repair. J Invest Dermatol. 2006;126(Suppl):79–86. doi: 10.1038/sj.jidsymp.5650016. [DOI] [PubMed] [Google Scholar]
- 22.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111(1):1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 23.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mac Gabhann F, Ji JW, Popel AS. VEGF gradients, receptor activation, and sprout guidance in resting and exercising skeletal muscle. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.00800.2006. [DOI] [PubMed] [Google Scholar]
- 25.Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Angiogenesis in rheumatoid arthritis. Histol Histopathol. 2006;21(5):557–566. doi: 10.14670/HH-21.557. [DOI] [PubMed] [Google Scholar]
- 26.Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19(3):321–324. [PubMed] [Google Scholar]
- 27.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180(3):1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V. Neovascularization in human atherosclerosis. Circulation. 2006;113(18):2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- 29.Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, van Marion I, Al-Chalabi A, Bornes S, Musson R, Hansen V, Beckman L, Adolfsson R, Pall HS, Prats H, Vermeire S, Rutgeerts P, Katayama S, Awata T, Leigh N, Lang-Lazdunski L, Dewerchin M, Shaw C, Moons L, Vlietinck R, Morrison KE, Robberecht W, Van Broeckhoven C, Collen D, Andersen PM, Carmeliet P. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34(4):383–94. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to Treat Cancer and Age-Related Macular Degeneration. Annu Rev Med. 2006 doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- 31.Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. American Journal of Ophthalmology. 2004;137(3):504–510. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV, Campochiaro PA. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142(6):961–9. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 33.Tilton RG, Kawamura T, Chang KC, Ido Y, Bjercke RJ, Stephan CC, Brock TA, Williamson JR. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest. 1997;99(9):2192–2202. doi: 10.1172/JCI119392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024–8. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 35.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203(6):1447–58. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 37.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 38.Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108(1–2):45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- 39.Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A. 1998;95(26):15809–14. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monacci WT, Merrill MJ, Oldfield EH. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol. 1993;264(4 Pt 1):C995–1002. doi: 10.1152/ajpcell.1993.264.4.C995. [DOI] [PubMed] [Google Scholar]
- 41.Bernini GP, Moretti A, Bonadio AG, Menicagli M, Viacava P, Naccarato AG, Iacconi P, Miccoli P, Salvetti A. Angiogenesis in human normal and pathologic adrenal cortex. J Clin Endocrinol Metab. 2002;87(11):4961–4965. doi: 10.1210/jc.2001-011799. [DOI] [PubMed] [Google Scholar]
- 42.Katoh R. Angiogenesis in endocrine glands: special reference to the expression of vascular endothelial growth factor. Microsc Res Tech. 2003;60(2):181–185. doi: 10.1002/jemt.10256. [DOI] [PubMed] [Google Scholar]
- 43.Pammer J, Weninger W, Mildner M, Burian M, Wojta J, Tschachler E. Vascular endothelial growth factor is constitutively expressed in normal human salivary glands and is secreted in the saliva of healthy individuals. J Pathol. 1998;186(2):186–191. doi: 10.1002/(SICI)1096-9896(1998100)186:2<186::AID-PATH148>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 44.Taichman NS, Cruchley AT, Fletcher LM, Hagi-Pavli EP, Paleolog EM, Abrams WR, Booth V, Edwards RM, Malamud D. Vascular endothelial growth factor in normal human salivary glands and saliva: a possible role in the maintenance of mucosal homeostasis. Lab Invest. 1998;78(7):869–875. [PubMed] [Google Scholar]
- 45.Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220(2):112–21. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 46.Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168(2):639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saint-Geniez M, Maldonado AE, D’Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47(7):3135–42. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- 48.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264(1):275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Kilic U, Kilic E, Jarve A, Guo Z, Spudich A, Bieber K, Barzena U, Bassetti CL, Marti HH, Hermann DM. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci. 2006;26(48):12439–46. doi: 10.1523/JNEUROSCI.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Hampton T, Morgan JP, Simons M. Stretch-induced VEGF expression in the heart. J Clin Invest. 1997;100(1):18–24. doi: 10.1172/JCI119510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milkiewicz M, Haas T. Mechanical stimuli differentially modulate the expression of HIF-2 alpha, HIF-1 alpha and Ets-1 in rat skeletal muscle endothelial cells. FASEB Journal. 2005;19(5):A1661. [Google Scholar]
- 52.Couffinhal T, Kearney M, Witzenbichler B, Chen D, Murohara T, Losordo DW, Symes J, Isner JM. Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in normal and atherosclerotic human arteries. Am J Pathol. 1997;150(5):1673–1685. [PMC free article] [PubMed] [Google Scholar]
- 53.Hamlat A, Adn M, Pasqualini E, Brassier G, Askar B. Pathophysiology of capillary haemangioma growth after birth. Med Hypotheses. 2005;64(6):1093–1096. doi: 10.1016/j.mehy.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6(5):485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 55.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49(3):568–81. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 56.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 57.Holmqvist K, Cross MJ, Rolny C, Hagerkvist R, Rahimi N, Matsumoto T, Claesson-Welsh L, Welsh M. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004;279(21):22267–75. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- 58.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274(23):16349–54. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. Embo J. 2005;24(13):2342–53. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126(13):3015–25. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 61.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95(16):9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiratsuka S, Nakao K, Nakamura K, Katsuki M, Maru Y, Shibuya M. Membrane fixation of vascular endothelial growth factor receptor 1 ligand-binding domain is important for vasculogenesis and angiogenesis in mice. Mol Cell Biol. 2005;25(1):346–354. doi: 10.1128/MCB.25.1.346-354.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang SZ, Zhang LM, Huang YL, Sun FY. Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol. 2003;274(1):851–6. doi: 10.1002/ar.a.10103. [DOI] [PubMed] [Google Scholar]
- 64.Witmer AN, Dai J, Weich HA, Vrensen GF, Schlingemann RO. Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J Histochem Cytochem. 2002;50(6):767–777. doi: 10.1177/002215540205000603. [DOI] [PubMed] [Google Scholar]
- 65.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133(2):275–88. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 67.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 68.van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. Jama. 2005;293(12):1509–13. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 69.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 70.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 71.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 72. http://www.cancer.gov/clinicaltrials.
- 73.Hurwitz HI, Honeycutt W, Haley S, Favaro J. Long-term treatment with bevacizumab for patients with metastatic colorectal cancer: case report. Clin Colorectal Cancer. 2006;6(1):66–9. doi: 10.3816/CCC.2006.n.023. [DOI] [PubMed] [Google Scholar]
- 74.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006;33(5 Suppl 10):S26–34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Yoon S, Schmassmann-Suhijar D, Zuber M, Konietzny P, Schmassmann A. Chemotherapy with bevacizumab, irinotecan, 5-fluorouracil and leucovorin (IFL) associated with a large, embolizing thrombus in the thoracic aorta. Ann Oncol. 2006;17(12):1851–2. doi: 10.1093/annonc/mdl140. [DOI] [PubMed] [Google Scholar]
- 76.Roncalli J, Delord JP, Galinier M, Massabuau P, Lescure M, Fauvel JM, Azria D. Bevacizumab in metastatic colorectal cancer: a left intracardiac thrombotic event. Ann Oncol. 2006;17(7):1177–8. doi: 10.1093/annonc/mdl025. [DOI] [PubMed] [Google Scholar]
- 77.Heinzerling JH, Huerta S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: incidence, etiology, and management. Curr Surg. 2006;63(5):334–7. doi: 10.1016/j.cursur.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354(9):980–2. 980–2. [PubMed] [Google Scholar]
- 79.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354(9):980–982. 980–2. doi: 10.1056/NEJMc052954. [DOI] [PubMed] [Google Scholar]
- 80.Allen JA, Adlakha A, Bergethon PR. Reversible posterior leukoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metastatic colon cancer. Arch Neurol. 2006;63(10):1475–8. doi: 10.1001/archneur.63.10.1475. [DOI] [PubMed] [Google Scholar]
- 81.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11(7):753–64. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 82.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295(21):2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 83.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 84.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 85.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 86.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 87.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191(4):1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 90.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000;80(4):443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 91.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90(22):10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004;164(5):1531–5. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226(2):324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217(2):371–376. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 95.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. 2000;106(11):1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative Stress and Apoptosis Interact and Cause Emphysema Due to Vascular Endothelial Growth Factor Receptor Blockade. Am J Respir Cell Mol Biol. 2003;29(1):88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- 98.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien M, Davis SRB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 99.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13(12):1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 100.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290(2):H547–559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 101.Horowitz JR, Rivard A, van der Zee R, Hariawala M, Sheriff DD, Esakof DD, Chaudhry GM, Symes JF, Isner JM. Vascular endothelial growth factor/vascular permeability factor produces nitric oxide-dependent hypotension. Evidence for a maintenance role in quiescent adult endothelium. Arterioscler Thromb Vasc Biol. 1997;17(11):2793–800. doi: 10.1161/01.atv.17.11.2793. [DOI] [PubMed] [Google Scholar]
- 102.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87(8):3336–43. [PubMed] [Google Scholar]
- 105.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271(30):17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 106.Duyndam MC, Hilhorst MC, Schluper HM, Verheul HM, van Diest PJ, Kraal G, Pinedo HM, Boven E. Vascular endothelial growth factor-165 overexpression stimulates angiogenesis and induces cyst formation and macrophage infiltration in human ovarian cancer xenografts. Am J Pathol. 2002;160(2):537–48. doi: 10.1016/s0002-9440(10)64873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Norrby K. Mast cells and angiogenesis. Apmis. 2002;110(5):355–71. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 108.Feistritzer C, Kaneider NC, Sturn DH, Mosheimer BA, Kahler CM, Wiedermann CJ. Expression and function of the vascular endothelial growth factor receptor FLT-1 in human eosinophils. Am J Respir Cell Mol Biol. 2004;30(5):729–35. doi: 10.1165/rcmb.2003-0314OC. [DOI] [PubMed] [Google Scholar]
- 109.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174(1):215–22. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 110.Casella I, Feccia T, Chelucci C, Samoggia P, Castelli G, Guerriero R, Parolini I, Petrucci E, Pelosi E, Morsilli O, Gabbianelli M, Testa U, Peschle C. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood. 2003;101(4):1316–23. doi: 10.1182/blood-2002-07-2184. [DOI] [PubMed] [Google Scholar]
- 111.Farahani M, Treweeke AT, Toh CH, Till KJ, Harris RJ, Cawley JC, Zuzel M, Chen H. Autocrine VEGF mediates the antiapoptotic effect of CD154 on CLL cells. Leukemia. 2005;19(4):524–30. doi: 10.1038/sj.leu.2403631. [DOI] [PubMed] [Google Scholar]
- 112.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417(6892):954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 113.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 114.Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30(8):967–72. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 115.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105(6):732–8. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 116.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8(7):702–10. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 117.Shui YB, Wang X, Hu JS, Wang SP, Garcia CM, Potts JD, Sharma Y, Beebe DC. Vascular endothelial growth factor expression and signaling in the lens. Invest Ophthalmol Vis Sci. 2003;44(9):3911–3919. doi: 10.1167/iovs.02-1226. [DOI] [PubMed] [Google Scholar]
- 118.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 119.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196(11):1497–506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y. Vascular Endothelial Growth Factor-A Promotes Peritumoral Lymphangiogenesis and Lymphatic Metastasis. Cancer Res. 2005;65(20):9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 121.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201(7):1089–99. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–7. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27(2):304–7. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14(3):237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Famiglietti EV, Stopa EG, McGookin ED, Song P, LeBlanc V, Streeten BW. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Res. 2003;969(1–2):195–204. doi: 10.1016/s0006-8993(02)03766-6. [DOI] [PubMed] [Google Scholar]
- 126.Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson S, Robinson G, Adamis AP, Shima DT. VEGF-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007 doi: 10.2353/ajpath.2007.061237. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–8. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 128.Devos D, Moreau C, Lassalle P, Perez T, De Seze J, Brunaud-Danel V, Destee A, Tonnel AB, Just N. Low levels of the vascular endothelial growth factor in CSF from early ALS patients. Neurology. 2004;62(11):2127–2129. doi: 10.1212/01.wnl.0000129913.44351.a3. [DOI] [PubMed] [Google Scholar]
- 129.Del Bo R, Scarlato M, Ghezzi S, Martinelli Boneschi F, Fenoglio C, Galbiati S, Virgilio R, Galimberti D, Galimberti G, Crimi M, Ferrarese C, Scarpini E, Bresolin N, Comi GP. Vascular endothelial growth factor gene variability is associated with increased risk for AD. Ann Neurol. 2005;57(3):373–80. doi: 10.1002/ana.20390. [DOI] [PubMed] [Google Scholar]
- 130.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 131.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26(9):943–54. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 132.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140(4):947–59. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200(6):581–97. doi: 10.1046/j.1469-7580.2002.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94(5):664–70. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 135.Yokomori H, Oda M, Yoshimura K, Nagai T, Ogi M, Nomura M, Ishii H. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int. 2003;23(6):467–475. doi: 10.1111/j.1478-3231.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 136.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 137.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A. 1999;96(23):13203–7. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Strickland LA, Jubb AM, Hongo JA, Zhong F, Burwick J, Fu L, Frantz GD, Koeppen H. Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF) J Pathol. 2005;206(4):466–75. doi: 10.1002/path.1805. [DOI] [PubMed] [Google Scholar]
- 139.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98(11):6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006;290(1):H107–18. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- 141.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 142.Folkman J. Endogenous angiogenesis inhibitors. Apmis. 2004;112(7–8):496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 143.Yoshioka M, Yuasa S, Matsumura K, Kimura K, Shiomi T, Kimura N, Shukunami C, Okada Y, Mukai M, Shin H, Yozu R, Sata M, Ogawa S, Hiraki Y, Fukuda K. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12(10):1151–9. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 144.Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal anti-angiogenic barrier. Br J Ophthalmol. 2006 doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–7. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9(4):777–94. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. Exs, 2005. (94):209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 148.Prats AC, Prats H. Translational control of gene expression: role of IRESs and consequences for cell transformation and angiogenesis. Prog Nucleic Acid Res Mol Biol. 2002;72:367–413. doi: 10.1016/s0079-6603(02)72075-8. [DOI] [PubMed] [Google Scholar]
- 149.Loureiro RM, D’Amore PA. Transcriptional regulation of vascular endothelial growth factor in cancer. Cytokine Growth Factor Rev. 2005;16(1):77–89. doi: 10.1016/j.cytogfr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 150.Shima DT, Deutsch U, D’Amore PA. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370(3):203–8. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- 151.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18(6):3112–9. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90(2):649–52. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- 153.Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269(9):6271–6274. [PubMed] [Google Scholar]
- 154.Seko Y, Fujikura H, Pang J, Tokoro T, Shimokawa H. Induction of vascular endothelial growth factor after application of mechanical stress to retinal pigment epithelium of the rat in vitro. Invest Ophthalmol Vis Sci. 1999;40(13):3287–3291. [PubMed] [Google Scholar]
- 155.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr, Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116(1):49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Loureiro RM, Maharaj AS, Dankort D, Muller WJ, D’Amore PA. ErbB2 overexpression in mammary cells upregulates VEGF through the core promoter. Biochem Biophys Res Commun. 2005;326(2):455–465. doi: 10.1016/j.bbrc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 157.Gille J, Khalik M, Konig V, Kaufmann R. Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor (VPF/VEGF) expression by cultured keratinocytes. J Invest Dermatol. 1998;111(6):1160–5. doi: 10.1046/j.1523-1747.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 158.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109(3):327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Selleck SB. Signaling from across the way: transactivation of VEGF receptors by HSPGs. Mol Cell. 2006;22(4):431–2. doi: 10.1016/j.molcel.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16(20):2684–98. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ashikari-Hada S, Habuchi H, Kariya Y, Kimata K. Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J Biol Chem. 2005;280(36):31508–15. doi: 10.1074/jbc.M414581200. [DOI] [PubMed] [Google Scholar]
- 162.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114(Pt 5):853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 163.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102(1):161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 164.Lauer G, Sollberg S, Cole M, Krieg T, Eming SA. Generation of a novel proteolysis resistant vascular endothelial growth factor165 variant by a site-directed mutation at the plasmin sensitive cleavage site. FEBS Lett. 2002;531(2):309–13. doi: 10.1016/s0014-5793(02)03545-7. [DOI] [PubMed] [Google Scholar]
- 165.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169(4):681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132(5):941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 167.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272(38):23659–67. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 168.Goldman CK, Tsai JC, Soroceanu L, Gillespie GY. Loss of vascular endothelial growth factor in human alopecia hair follicles. J Invest Dermatol. 1995;104(5 Suppl):18S–20S. doi: 10.1038/jid.1995.40. [DOI] [PubMed] [Google Scholar]
- 169.Picciotti P, Torsello A, Wolf FI, Paludetti G, Gaetani E, Pola R. Age-dependent modifications of expression level of VEGF and its receptors in the inner ear. Exp Gerontol. 2004;39(8):1253–8. doi: 10.1016/j.exger.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 170.Pufe T, Mentlein R, Tsokos M, Steven P, Varoga D, Goldring MB, Tillmann BN, Paulsen FP. VEGF expression in adult permanent thyroid cartilage: implications for lack of cartilage ossification. Bone. 2004;35(2):543–52. doi: 10.1016/j.bone.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 171.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, Jiang S, Niyak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12(7):793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 172.Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, Weinerman A, Emmenegger U, Ma L, Thorpe P, Davidoff A, Huber J, Hicklin DJ, Kerbel RS. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64(18):6616–25. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 173.Lissoni P, Rovelli F, Malugani F, Brivio F, Fumagalli L, Gardani GS. Changes in circulating VEGF levels in relation to clinical response during chemotherapy for metastatic cancer. Int J Biol Markers. 2003;18(2):152–5. doi: 10.1177/172460080301800209. [DOI] [PubMed] [Google Scholar]
- 174.Chan LW, Moses MA, Goley E, Sproull M, Muanza T, Coleman CN, Figg WD, Albert PS, Menard C, Camphausen K. Urinary VEGF and MMP levels as predictive markers of 1-year progression-free survival in cancer patients treated with radiation therapy: a longitudinal study of protein kinetics throughout tumor progression and therapy. J Clin Oncol. 2004;22(3):499–506. doi: 10.1200/JCO.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 175.Beaudry P, Force J, Naumov GN, Wang A, Baker CH, Ryan A, Soker S, Johnson BE, Folkman J, Heymach JV. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11(9):3514–3522. doi: 10.1158/1078-0432.CCR-04-2271. [DOI] [PubMed] [Google Scholar]
- 176.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mundhenke C, Thomas JP, Wilding G, Lee FT, Kelzc F, Chappell R, Neider R, Sebree LA, Friedl A. Tissue examination to monitor antiangiogenic therapy: a phase I clinical trial with endostatin. Clin Cancer Res. 2001;7(11):3366–3374. [PubMed] [Google Scholar]
- 178.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 179.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 180.Rich RM, Rosenfeld PJ, Puliafito CA, Dubovy SR, Davis JL, Flynn HW, Jr, Gonzalez S, Feuer WJ, Lin RC, Lalwani GA, Nguyen JK, Kumar G. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006;26(5):495–511. doi: 10.1097/01.iae.0000225766.75009.3a. [DOI] [PubMed] [Google Scholar]
- 181.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46(2):726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 182. http://www.lucentis.com/lucentis/prescribing.jsp.
- 183.Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stewart DJ, Hilton JD, Arnold JM, Gregoire J, Rivard A, Archer SL, Charbonneau F, Cohen E, Curtis M, Buller CE, Mendelsohn FO, Dib N, Page P, Ducas J, Plante S, Sullivan J, Macko J, Rasmussen C, Kessler PD, Rasmussen HS. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13(21):1503–11. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 185.Freedman SB, Isner JM. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med. 2002;136(1):54–71. doi: 10.7326/0003-4819-136-1-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 186.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2(11):863–71. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 187.Schratzberger P, Schratzberger G, Silver M, Curry C, Kearney M, Magner M, Alroy J, Adelman LS, Weinberg DH, Ropper AH, Isner JM. Favorable effect of VEGF gene transfer on ischemic peripheral neuropathy. Nat Med. 2000;6(4):405–13. doi: 10.1038/74664. [DOI] [PubMed] [Google Scholar]
- 188.Simpson AH, Mills L, Noble B. The role of growth factors and related agents in accelerating fracture healing. J Bone Joint Surg Br. 2006;88(6):701–5. doi: 10.1302/0301-620X.88B6.17524. [DOI] [PubMed] [Google Scholar]
- 189.Freedman SB, Vale P, Kalka C, Kearney M, Pieczek A, Symes J, Losordo D, Isner JM. Plasma vascular endothelial growth factor (VEGF) levels after intramuscular and intramyocardial gene transfer of VEGF-1 plasmid DNA. Hum Gene Ther. 2002;13(13):1595–603. doi: 10.1089/10430340260201680. [DOI] [PubMed] [Google Scholar]
- 190.Baumgartner I, Rauh G, Pieczek A, Wuensch D, Magner M, Kearney M, Schainfeld R, Isner JM. Lower-extremity edema associated with gene transfer of naked DNA encoding vascular endothelial growth factor. Ann Intern Med. 2000;132(11):880–884. doi: 10.7326/0003-4819-132-11-200006060-00005. [DOI] [PubMed] [Google Scholar]


